Himalayan Sources of Anthocyanins and Its Multifunctional Applications: A Review
Abstract
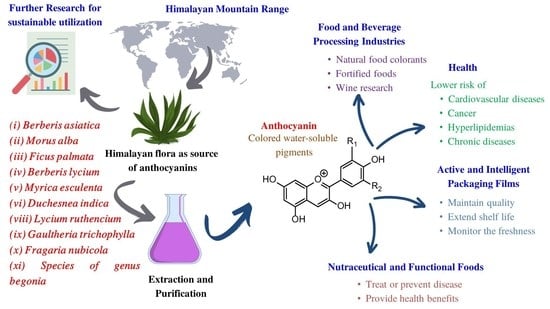
:1. Introduction
2. Chemistry of Anthocyanins
3. Extraction of Anthocyanins
4. Himalayan Sources of Anthocyanins
4.1. Berberis asiatica
4.2. Morus alba
4.3. Ficus palmata
4.4. Berberis lycium
4.5. Myrica esculenta
4.6. Duchesnea indica
4.7. Lycium ruthencium
4.8. Gaultheria trichophylla
4.9. Species of Genus Begonia
4.10. Fragaria nubicola
| Species | Anthocyanin Composition | Concentration | References |
|---|---|---|---|
| Berberis lycium |
| 35.32% | [61] |
| 0.08% | ||
| 47.24% | ||
| 0.53% | ||
| 1.62% | ||
| 2.26% | ||
| 4.21% | ||
| 0.58% | ||
| 1.05% | ||
| 6.12% | ||
| 0.77% | ||
| 0.22% | ||
| Morus alba |
| 1. 301.75 mg/g MAE | [98] |
| 2. 108.79 mg/g MAE | ||
| 3. NA | ||
| 4. NA | ||
| 5. 270 mg/g CMA | ||
| 6. 57 mg/g CMA | ||
| 7. 233 mg/g CMA | ||
| 8. 33 mg/g CMA | ||
| 9. 33 mg/g CMA | ||
| Myrica esculen. |
| 0.205 ± 0.4 mg CGE/100 g FW’ | [68] |
| 0.342 ± 0.4 mg CGE/100 g FW’ | ||
| 0.421 ± 0.4 mg CGE/100 g FW’ | ||
| 0.421 ± 0.4 mg CGE/100 g FW’ | ||
| Duchesnea indica |
| 125 mg/g | [75] |
| 70 mg/g | ||
| 10 mg/g | ||
| Lycium ruthencium |
| 10 mg CGE/g DW | [77] |
| 5.71 mg CGE/g DW | ||
| 0.47 mg CGE/g DW | ||
| 0.29 mg CGE/g DW |
5. Physical and Functional Properties
6. Applications of Anthocyanins
6.1. Food and Beverage Processing Industries
6.2. Health
6.3. Active and Intelligent Packaging Films
6.4. Nutraceuticals and Functional Foods
7. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Schaal, B. Plants and People: Our Shared History and Future. Plants People Planet 2019, 1, 14–19. [Google Scholar] [CrossRef]
- Raskin, I.; Ribnicky, D.M.; Komarnytsky, S.; Ilic, N.; Poulev, A.; Borisjuk, N.; Brinker, A.; Moreno, D.A.; Ripoll, C.; Yakoby, N.; et al. Plants and Human Health in the Twenty-First Century. Trends Biotechnol. 2002, 20, 522–531. [Google Scholar] [CrossRef]
- Kumar, V.; Chopra, A.K. Impact of Climate Change on Biodiversity of India with Special Reference to Himalayan Region-An Overview. J. Appl. Nat. Sci. 2009, 1, 117–122. [Google Scholar] [CrossRef]
- Delgoda, R.; Murray, J.E. Evolutionary Perspectives on the Role of Plant Secondary Metabolites. In Pharmacognosy; Elsevier: Amsterdam, The Netherlands, 2017; pp. 93–100. [Google Scholar] [CrossRef]
- Oz, A.T.; Kafkas, E. Phytochemicals in Fruits and Vegetables. In Superfood and Functional Food—An Overview of Their Processing and Utilization; InTech: Sydney, Australia, 2017; pp. 175–184. [Google Scholar] [CrossRef]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An Overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef]
- Hurst, R.D.; Lyall, K.A.; Wells, R.W.; Sawyer, G.M.; Lomiwes, D.; Ngametua, N.; Hurst, S.M. Daily Consumption of an Anthocyanin-Rich Extract Made from New Zealand Blackcurrants for 5 Weeks Supports Exercise Recovery Through the Management of Oxidative Stress and Inflammation: A Randomized Placebo Controlled Pilot Study. Front. Nutr. 2020, 7, 6. [Google Scholar] [CrossRef]
- Roy, S.; Rhim, J.-W. Preparation of gelatin/carrageenan-based color-indicator film integrated with shikonin and propolis for smart food packaging applications. ACS Appl. Bio Mater. 2021, 4, 770–779. [Google Scholar] [CrossRef]
- Roy, S.; Rhim, J.-W. Fabrication of cellulose nanofiber-based functional color indicator film incorporated with shikonin extracted from root. Food Hydrocoll. 2021, 114, 106566. [Google Scholar] [CrossRef]
- Ghosh, S.; Sarkar, T.; Das, A.; Chakraborty, R. Micro and Nanoencapsulation of Natural Colors: A Holistic View. Appl. Biochem. Biotechnol. 2021, 193, 3787–3811. [Google Scholar] [CrossRef] [PubMed]
- Ren, S.; Jiménez-Flores, R.; Giusti, M.M. The Interactions between Anthocyanin and Whey Protein: A Review. Compr. Rev. Food Sci. Food Saf. 2021, 20, 5992–6011. [Google Scholar] [CrossRef] [PubMed]
- Cruz, L.; Fernandes, I.; Guimarães, M.; de Freitas, V.; Mateus, N. Enzymatic Synthesis, Structural Characterization and Antioxidant Capacity Assessment of a New Lipophilic Malvidin-3-Glucoside–Oleic Acid Conjugate. Food Funct. J. 2016, 7, 2754–2762. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.-L.; Zhou, Q.; Yang, Z.-W.; Wang, R.-D.; Zhang, J.-L. Evaluation of Structure and Bioprotective Activity of Key High Molecular Weight Acylated Anthocyanin Compounds Isolated from the Purple Sweet Potato (Ipomoea Batatas L. Cultivar Eshu No. 8). Food Chem. 2018, 241, 23–31. [Google Scholar] [CrossRef]
- Yang, W.; Kortesniemi, M.; Ma, X.; Zheng, J.; Yang, B. Enzymatic Acylation of Blackcurrant (Ribes nigrum) Anthocyanins and Evaluation of Lipophilic Properties and Antioxidant Capacity of Derivatives. Food Chem. 2019, 281, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Matsufuji, H.; Kido, H.; Misawa, H.; Yaguchi, J.; Otsuki, T.; Chino, M.; Takeda, M.; Yamagata, K. Stability to Light, Heat, and Hydrogen Peroxide at Different PH Values and DPPH Radical Scavenging Activity of Acylated Anthocyanins from Red Radish Extract. J. Agric. Food Chem. 2007, 55, 3692–3701. [Google Scholar] [CrossRef]
- Cavalcanti, R.N.; Santos, D.T.; Meireles, M.A.A. Non-Thermal Stabilization Mechanisms of Anthocyanins in Model and Food Systems—An Overview. Food Res. Int. 2011, 44, 499–509. [Google Scholar] [CrossRef]
- Ursu, M.S.; Aprodu, I.; Milea, Ș.A.; Enachi, E.; Râpeanu, G.; Bahrim, G.E.; Stănciuc, N. Thermal Degradation Kinetics of Anthocyanins Extracted from Purple Maize Flour Extract and the Effect of Heating on Selected Biological Functionality. Foods 2020, 9, 1593. [Google Scholar] [CrossRef] [PubMed]
- Sadilova, E.; Carle, R.; Stintzing, F.C. Thermal Degradation of Anthocyanins and Its Impact on Color Andin Vitro Antioxidant Capacity. Mol. Nutr. Food Res. 2007, 51, 1461–1471. [Google Scholar] [CrossRef]
- Cisse, M.; Vaillant, F.; Acosta, O.; Dhuique-Mayer, C.; Dornier, M. Thermal Degradation Kinetics of Anthocyanins from Blood Orange, Blackberry, and Roselle Using the Arrhenius, Eyring, and Ball Models. J. Agric. Food Chem. 2009, 57, 6285–6291. [Google Scholar] [CrossRef] [PubMed]
- Rashwan, A.K.; Karim, N.; Xu, Y.; Xie, J.; Cui, H.; Mozafari, M.R.; Chen, W. Potential Micro-/Nano-Encapsulation Systems for Improving Stability and Bioavailability of Anthocyanins: An Updated Review. Crit. Rev. Food Sci. Nutr. 2021, 1–24. [Google Scholar] [CrossRef]
- Tarone, A.G.; Cazarin, C.B.B.; Marostica Junior, M.R. Anthocyanins: New Techniques and Challenges in Microencapsulation. Food Res. Int. 2020, 133, 109092. [Google Scholar] [CrossRef]
- Farooq, S.; Shah, M.A.; Siddiqui, M.W.; Dar, B.N.; Mir, S.A.; Ali, A. Recent Trends in Extraction Techniques of Anthocyanins from Plant Materials. J. Food Meas. Charact. 2020, 14, 3508–3519. [Google Scholar] [CrossRef]
- Weber, F.; Boch, K.; Schieber, A. Influence of Copigmentation on the Stability of Spray Dried Anthocyanins from Blackberry. LWT-Food Sci. Technol. 2017, 75, 72–77. [Google Scholar] [CrossRef]
- Ongkowijoyo, P.; Luna-Vital, D.A.; Gonzalez de Mejia, E. Extraction Techniques and Analysis of Anthocyanins from Food Sources by Mass Spectrometry: An Update. Food Chem. 2018, 250, 113–126. [Google Scholar] [CrossRef] [PubMed]
- del Pilar Garcia-Mendoza, M.; Espinosa-Pardo, F.A.; Baseggio, A.M.; Barbero, G.F.; Maróstica Junior, M.R.; Rostagno, M.A.; Martínez, J. Extraction of Phenolic Compounds and Anthocyanins from Juçara (Euterpe Edulis Mart.) Residues Using Pressurized Liquids and Supercritical Fluids. J. Supercrit. Fluids 2017, 119, 9–16. [Google Scholar] [CrossRef]
- Chand, R.; Singh, A.N.; Nirmala, C. Ethnoecological Survey of Underutilized Plant Diversity of Hamirpur District, Himachal Pradesh, India: An Edibility Assessment. Environ. Ecol. Res. 2017, 5, 13–29. [Google Scholar] [CrossRef]
- Muhammad, F.; Javed, I.; Akhtar, M.; Khaliq, T.; Aslam, B.; Waheed, A.; Yasmin, R.; Zafar, H.; Zafar, M. White Mulberry (Morus alba): A Brief Phytochemical and Pharmacological Evaluations Account. Int. J. Agric. Biol. 2013, 15, 612–620. [Google Scholar]
- Parra, S.; Gaur, K.; Ranawat, S.; Rather, M. An Overview on Various Aspects of Plant Berberis Lycium Royale. Am. J. Pharmacol. Sci. 2018, 6, 19–24. [Google Scholar] [CrossRef]
- Shri, R.; Sood, P. A Review on Ethnomedicinal, Phytochemical and Pharmacological Aspects of Myrica esculenta. Indian J. Pharm. Sci. 2018, 80, 2–13. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, B.; Wen, H.; Tao, Y.; Shao, Y. Phytochemical Profiles, Nutritional Constituents and Antioxidant Activity of Black Wolfberry (Lycium Ruthenicum Murr.). Ind. Crops Ad Prod. 2020, 154, 112692. [Google Scholar] [CrossRef]
- Bhutia, P.O.; Kewlani, P.; Pandey, A.; Rawat, S.; Bhatt, I.D. Physico-Chemical Properties and Nutritional Composition of Fruits of the Wild Himalayan Strawberry (Fragaria nubicola Lindle.) in Different Ripening Stages. J. Berry Res. 2021, 11, 481–496. [Google Scholar] [CrossRef]
- Alam, F.; Najum us Saqib, Q. Pharmacognostic Standardization and Preliminary Phytochemical Studies of Gaultheria trichophylla. Pharm. Biol. 2015, 53, 1711–1718. [Google Scholar] [CrossRef]
- Iwashina, T.; Saito, Y.; Kokubugata, G.; Peng, C.-I. Flavonoids in the Leaves of Hillebrandia and Begonia Species (Begoniaceae). Biochem. Syst. Ecol. 2020, 90, 104040. [Google Scholar] [CrossRef]
- Ahrendt, L.W.A. Berberis and Mahonia. Bot. J. Linn. Soc. 1961, 57, 1–410. [Google Scholar] [CrossRef]
- Srivastava, S.K.; Singh Rawat, A.K.; Mehrotra, S. Pharmacognostic Evaluation of the Root of Berberis asiatica. Pharm. Biol. 2004, 42, 467–473. [Google Scholar] [CrossRef]
- Leopoldini, M.; Russo, N.; Toscano, M. The Molecular Basis of Working Mechanism of Natural Polyphenolic Antioxidants. Food Chem. 2011, 125, 288–306. [Google Scholar] [CrossRef]
- Potdar, D.; Hirwani, R.R.; Dhulap, S. Phyto-Chemical and Pharmacological Applications of Berberis aristata. Fitoterapia 2012, 83, 817–830. [Google Scholar] [CrossRef]
- Belwal, T.; Pandey, A.; Bhatt, I.D.; Rawal, R.S.; Luo, Z. Trends of Polyphenolics and Anthocyanins Accumulation along Ripening Stages of Wild Edible Fruits of Indian Himalayan Region. Sci. Rep. 2019, 9, 5894. [Google Scholar] [CrossRef]
- Cicero, A.F.G.; Baggioni, A. Berberine and Its Role in Chronic Disease. In Anti-Inflammatory Nutraceuticals and Chronic Diseases; Advances in Experimental Medicine and Biology; Springer: Cham, Switzerland, 2016; pp. 27–45. [Google Scholar] [CrossRef]
- Bhatt, I.D.; Rawat, S.; Badhani, A.; Rawal, R.S. Nutraceutical Potential of Selected Wild Edible Fruits of the Indian Himalayan Region. Food Chem. 2017, 215, 84–91. [Google Scholar] [CrossRef]
- Srivastava, S.; Kapoor, R.; Thathola, A.; Srivastava, R.P. Nutritional Quality of Leaves of Some Genotypes of Mulberry (Morus alba). Int. J. Food Sci. Nutr. 2006, 57, 305–313. [Google Scholar] [CrossRef]
- Awasthi, A.K.; Nagaraja, G.; Naik, G.; Kanginakudru, S.; Thangavelu, K.; Nagaraju, J. Genetic Diversity and Relationships in Mulberry (Genus Morus) as Revealed by RAPD and ISSR Marker Assays. BMC Genet. 2004, 5, 1. [Google Scholar] [CrossRef]
- Yadav, S.; Nair, N.; Biharee, A.; Prathap, V.M.; Majeed, J. Updated Ethnobotanical Notes, Phytochemistry and Phytopharmacology of Plants Belonging to the Genus Morus (Family: Moraceae). Phytomed. Plus 2022, 2, 100120. [Google Scholar] [CrossRef]
- Chan, E.W.-C.; Lye, P.-Y.; Wong, S.-K. Phytochemistry, Pharmacology, and Clinical Trials of Morus alba. Chin. J. Nat. Med. 2016, 14, 17–30. [Google Scholar] [CrossRef] [PubMed]
- Ercisli, S.; Orhan, E. Chemical Composition of White (Morus alba), Red (Morus rubra) and Black (Morus nigra) Mulberry Fruits. Food Chem. 2007, 103, 1380–1384. [Google Scholar] [CrossRef]
- Kumar, R.; And, R.; Chauhan, S. Mulberry: Life Enhancer. J. Med. Plants Res. 2008, 2, 271–278. [Google Scholar]
- Gryn-Rynko, A.; Bazylak, G.; Olszewska-Slonina, D. New Potential Phytotherapeutics Obtained from White Mulberry (Morus alba L.) Leaves. Biomed. Pharmacother. 2016, 84, 628–636. [Google Scholar] [CrossRef]
- Kimura, T.; Nakagawa, K.; Kubota, H.; Kojima, Y.; Goto, Y.; Yamagishi, K.; Oita, S.; Oikawa, S.; Miyazawa, T. Food-Grade Mulberry Powder Enriched with 1-Deoxynojirimycin Suppresses the Elevation of Postprandial Blood Glucose in Humans. J. Agric. Food Chem. 2007, 55, 5869–5874. [Google Scholar] [CrossRef]
- Kumari, K.; Sharma, S.; Kaushik, R. Wild Himalayan Fig: A Nutraceutical under exploited fruit of Western Himalayan region—A Review. Int. J. Adv. Res. 2017, 5, 833–839. [Google Scholar] [CrossRef]
- Joshi, Y.; Joshi, A.K.; Prasad, N.; Juyal, D. A Review on Ficus palmata (Wild Himalayan Fig). J. Phytopharm. 2014, 3, 374–377. [Google Scholar] [CrossRef]
- Kothiyal, S.C.; Saklani, S. Isolation, and Identification of Ficus palmata leaves and their antimicrobial activities. J. Sci. Res. 2017, 9, 193–200. [Google Scholar] [CrossRef]
- Saklani, S.; Kothiyal, S. Phytochemical Screening of Garhwal Himalaya Wild Edible Fruit Ficus palmata. Int. J. Pharm. Tech Res. 2012, 4, 1185–1191. [Google Scholar]
- Shabbir, A. Berberis lycium Royle: A Review of Its Traditional Uses, Phytochemistry and Pharmacology. Afr. J. Pharm. Pharmacol. 2012, 6, 2346–2353. [Google Scholar] [CrossRef]
- Anjum, N.; Ridwan, Q.; Akhter, F.; Hanief, M. Phytochemistry and Therapeutic Potential of Berberis lycium Royle; an Endangered Species of Himalayan Region. Acta Ecol. Sin. 2022, 43, 577–584. [Google Scholar] [CrossRef]
- Kaur, C.; Kapoor, H.C. Antioxidants in Fruits and Vegetables—The Millennium’s Health. Int. J. Food Sci. Technol. 2008, 36, 703–725. [Google Scholar] [CrossRef]
- Gupta, M.; Singh, A.; Joshi, H. Berberis lycium Multipotential Medicinal Application: An Overview. Int. J. Chem. Stud. 2015, 10, 10–13. [Google Scholar]
- Bhardwaj, D.; Kaushik, N. Phytochemical and Pharmacological Studies in Genus Berberis. Phytochem. Rev. 2012, 11, 523–542. [Google Scholar] [CrossRef]
- Gulfraz, M.; Mehmood, S.; Ahmad, A.; Fatima, N.; Praveen, Z.; Williamson, E.M. Comparison of the Antidiabetic Activity of Berberis lycium Root Extract and Berberine in Alloxan-Induced Diabetic Rats. Phytother. Res. 2008, 22, 1208–1212. [Google Scholar] [CrossRef] [PubMed]
- Nazir, N.; Rahman, A.; Uddin, F.; Khan Khalil, A.A.; Zahoor, M.; Nisar, M.; Ullah, S.; Ullah, R.; Ezzeldin, E.; Mostafa, G.A.E. Quantitative Ethnomedicinal Status and Phytochemical Analysis of Berberis lycium Royle. Agronomy 2021, 11, 130. [Google Scholar] [CrossRef]
- Sendri, N.; Bhandari, P. Polyphenolic Composition and Antioxidant Potential of Underutilized Himalayan Wild Edible Berries by High-performance Liquid Chromatography Coupled with Electrospray Ionization Quadrupole Time-of-flight Mass Spectrometry. J. Sep. Sci. 2021, 44, 4237–4254. [Google Scholar] [CrossRef]
- Pradhan, P.C.; Saha, S. Anthocyanin Profiling of Berberis lycium Royle Berry and Its Bioactivity Evaluation for Its Nutraceutical Potential. J. Food Sci. Technol. 2016, 53, 1205–1213. [Google Scholar] [CrossRef]
- Yanthan, M.; Biate, D.; Misra, A.K. Taxonomic Resolution of Actinorhizal Myrica Species from Meghalaya (India) through Nuclear RDNA Sequence Analyses. Funct. Plant Biol. 2011, 38, 738. [Google Scholar] [CrossRef]
- Gusain, Y.S.; Khanduri, V.P. Myrica esculenta Wild Edible Fruit of Indian Himalaya: Need a Sustainable Approach for Indigenous Utilization. Ecol. Environ. Conserv. J. 2016, 22, S267–S270. [Google Scholar]
- Rawat, S.; Jugran, A.; Giri, L.; Bhatt, I.D.; Rawal, R.S. Assessment of Antioxidant Properties in Fruits of Myrica esculenta: A Popular Wild Edible Species in Indian Himalayan Region. Evid.-Based Complement. Altern. Med. 2011, 2011, 51278. [Google Scholar] [CrossRef]
- Kabra, A.; Martins, N.; Sharma, R.; Kabra, R.; Baghel, U.S. Myrica esculenta Buch.-Ham. Ex D. Don: A Natural Source for Health Promotion and Disease Prevention. Plants 2019, 8, 149. [Google Scholar] [CrossRef] [PubMed]
- Nhiem, N.X.; Van Kiem, P.; Van Minh, C.; Tai, B.H.; Cuong, N.X.; Thu, V.K.; Anh, H.L.T.; Jo, S.-H.; Jang, H.-D.; Kwon, Y.-I.; et al. A New Monoterpenoid Glycoside from Myrica esculenta and the Inhibition of Angiotensin I-Converting Enzyme. Chem. Pharm. Bull. 2010, 58, 1408–1410. [Google Scholar] [CrossRef] [PubMed]
- Bahuguna, D.P.; London, H.K.; Kharwal, H.; Joshi, D. Myrica Nagi: A Review on Active Constituents, Biological and Therapeutic Effects. Int. J. Pharm. Pharm. Sci. 2012, 4, 38–42. [Google Scholar]
- Kumar, T.; Pande, K.K.; Sharma, H.; Koranga, M.; Pande, L. HRLC-ESI-MS Based Separation, and Identification of Anthocyanins Extracted from Popular Wild Edible Fruit of Himalaya: Myrica esculenta (Himalayan Bayberry). J. Adv. Sci. Res. 2020, 11, 269–275. [Google Scholar]
- Kakar, M.; Kakar, I.; Akram, M. Antimicrobial and Phytochemical Exploration of Duchesnea indica Plant. Plant Cell Biotechnol. Mol. Biol. 2021, 22, 74–85. [Google Scholar]
- Faghir, M.B.; Pourebrahim, S.; Shahi Shavvon, R. New Insight into the Molecular and Micromorphological Characteristics of Potentilla indica and Potentilla reptans (Rosaceae). Iran. J. Bot. 2022, 28, 77–95. [Google Scholar] [CrossRef]
- Kar, T.; Nayak, A.K.N.A.K.; Dash, B.; Mandal, K.K.M.K.K. Duchesnea indica (Rosaceae): An Addition to the Flora of Odisha, India. Biosci. Discov. 2014, 5, 202–203. [Google Scholar]
- Ahmad, I.; Ibrar, M.; Barkatullah; Ali, N. Ethnobotanical Study of Tehsil Kabal, Swat District, KPK, Pakistan. J. Bot. 2011, 2011, 368572. [Google Scholar] [CrossRef]
- Peng, B.; Chang, Q.; Wang, L.; Hu, Q.; Wang, Y.; Tang, J.; Liu, X. Suppression of Human Ovarian SKOV-3 Cancer Cell Growth by Duchesnea Phenolic Fraction Is Associated with Cell Cycle Arrest and Apoptosis. Gynecol. Oncol. 2008, 108, 173–181. [Google Scholar] [CrossRef]
- Zhu, M.; Dong, X.; Guo, M. Phenolic Profiling of Duchesnea indica Combining Macroporous Resin Chromatography (MRC) with HPLC-ESI-MS/MS and ESI-IT-MS. Molecules 2015, 20, 22463–22475. [Google Scholar] [CrossRef] [PubMed]
- Qin, C.; Li, Y.; Zhang, R.; Niu, W.; Ding, Y. Separation and Elucidation of Anthocyanins in the Fruit of Mockstrawberry (Duchesnea indica Focke). Nat. Prod. Res. 2009, 23, 1589–1598. [Google Scholar] [CrossRef]
- Wang, H.; Li, J.; Tao, W.; Zhang, X.; Gao, X.; Yong, J.; Duan, J.A. Lycium ruthenicum studies: Molecular biology, phytochemistry and pharmacology. Food Chem. 2018, 240, 759–766. [Google Scholar] [CrossRef]
- Sharma, R.; Raghuvanshi, R.; Kumar, R.; Thakur, M.S.; Kumar, S.; Patel, M.K.; Chaurasia, O.P.; Saxena, S. Current Findings and Future Prospective of High-Value Trans Himalayan Medicinal Plant Lycium ruthenicum Murr: A Systematic Review. Clin. Phytosci. 2022, 8, 3. [Google Scholar] [CrossRef]
- Chaurasia, O.; Ballabh, B. Herbal Formulations from Cold Desert Plants Used for Gynecological Disorders. Ethnobot. Res. Appl. 2011, 9. [Google Scholar] [CrossRef]
- Proksch, P. Chinese Marine Materia Medica. By Huashi Guan and Shuguang Wang. Shanghai Scientific and Technical Publishers, China Ocean Press, and Chemical Industry Press: Shanghai, Beijing, China, 2009. Mar. Drugs 2014, 12, 193–195. [Google Scholar] [CrossRef]
- Ballabh, B.; Chaurasia, O.P.; Ahmed, Z.; Singh, S.B. Traditional Medicinal Plants of Cold Desert Ladakh—Used against Kidney and Urinary Disorders. J. Ethnopharmacol. 2008, 118, 331–339. [Google Scholar] [CrossRef]
- Chopra, R.N. Glossary of Indian Medicinal Plants; Council of Scientific & Industrial Research: New Delhi, India, 1956. [Google Scholar]
- Gairola, S.; Sharma, J.; Bedi, Y.S. A Cross-Cultural Analysis of Jammu, Kashmir and Ladakh (India) Medicinal Plant Use. J. Ethnopharmacol. 2014, 155, 925–986. [Google Scholar] [CrossRef]
- Yun, D.; Yan, Y.; Liu, J. Isolation, structure and biological activity of polysaccharides from the fruits of Lycium ruthenicum murr: A review. Carbohydr. Polym. 2022, 291, 119618. [Google Scholar] [CrossRef]
- Liu, W.-R.; Qiao, W.-L.; Liu, Z.-Z.; Wang, X.-H.; Jiang, R.; Li, S.-Y.; Shi, R.-B.; She, G.-M. Gaultheria: Phytochemical and Pharmacological Characteristics. Molecules 2013, 18, 12071–12108. [Google Scholar] [CrossRef]
- Alam, F.; Saqib, Q.N.; Ashraf, M. Gaultheria trichophylla (Royle): A Source of Minerals and Biologically Active Molecules, Its Antioxidant and Anti-Lipoxygenase Activities. BMC Complement. Altern. Med. 2017, 17, 3. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Liu, R.; Sun, L.; Huang, C.; Wang, C.; Zhang, D.-M.; Zhang, T.-T.; Du, G.-H. Anti-Inflammatory Activity of Methyl Salicylate Glycosides Isolated from Gaultheria yunnanensis (Franch.) Rehder. Molecules 2011, 16, 3875–3884. [Google Scholar] [CrossRef] [PubMed]
- Bahukh, A.; Aseesh, P.; Sekar, K.C.; Bhatt, I.D. Polyphenolics, Nutrients and Antioxidant Activity of Gaultheria trichophylla Royle: A High Value Wild Edible Plant of Trans Himalaya. Hortic. Int. J. 2017, 1, 39–43. [Google Scholar]
- Moonlight, P.W.; Ardi, W.H.; Padilla, L.A.; Chung, K.-F.; Fuller, D.; Girmansyah, D.; Hollands, R.; Jara-Muñoz, A.; Kiew, R.; Leong, W.-C.; et al. Dividing and Conquering the Fastest–Growing Genus: Towards a Natural Sectional Classification of the Mega–Diverse Genus Begonia (Begoniaceae). Taxon 2018, 67, 267–323. [Google Scholar] [CrossRef]
- Taram, M.; Borah, D.; Hughes, M. Two New Records of Begonia for the Flora of India from Arunachal Pradesh. Phytotaxa 2023, 584, 2. [Google Scholar] [CrossRef]
- Bhattarai, B.; Rana, M. Diversified Morphological and Phytochemical Screening of Wild Begonia of Sikkim Himalaya. Ecol. Environ. Conserv. 2020, 26, S129–S138. [Google Scholar]
- Roshan, R.; Ahmed, S.; ul Hassan, M.M. Fragaria nubicola (Rosaceae): A Review of Medicinal Uses, Phytochemistry and Pharmacology. J. Pharmacogn. Phytochem. 2019, 8, 3390–3393. [Google Scholar]
- Staudt, G. Himalayan Species of Fragaria (Rosaceae). Bot. JahrbÜCher Syst. Pflanzengesch. Pflanzengeogr. 2006, 126, 483–508. [Google Scholar] [CrossRef]
- Chakraborty, T.; Saha, S.; Bisht, N. First Report on the Ethnopharmacological Uses of Medicinal Plants by Monpa Tribe from the Zemithang Region of Arunachal Pradesh, Eastern Himalayas, India. Plants 2017, 6, 13. [Google Scholar] [CrossRef]
- Antonio, R.L.; Kozasa, E.H.; Galduróz, J.C.F.; Dawa; Dorjee, Y.; Kalsang, T.; Norbu, T.; Tenzin, T.; Rodrigues, E. Formulas Used by Tibetan Doctors at Men-Tsee-Khang in India for the Treatment of Neuropsychiatric Disorders and Their Correlation with Pharmacological Data. Phytother. Res. 2013, 27, 552–563. [Google Scholar] [CrossRef]
- Thakur, P. Sarika, Ethno-Medicinal Uses of Some Plants of Potter’s Hill in Shimla (Himachal Pradesh, India). Proc. Biol. Forum 2016, 8, 417–422. [Google Scholar]
- Rakhunde, P.B.; Ali, S.A. Antioxidant and Cytoprotective Effect of Fragaria nubicola on Ischemia Reperfusion Induced Brain Injury. Ann. Exp. Biol. 2014, 2, 33–38. [Google Scholar]
- Bahukhandi, A.; Barola, A.; Sekar, K.C. Antioxidant Activity and Polyphenolics of Fragaria nubicola: A Wild Edible Fruit Species of Himalaya. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2020, 90, 761–767. [Google Scholar] [CrossRef]
- Yuan, Q.; Zhao, L. The Mulberry (Morus alba L.) Fruit—A Review of Characteristic Components and Health Benefits. J. Agric. Food Chem. 2017, 65, 10383–10394. [Google Scholar] [CrossRef] [PubMed]
- Miguel, M. Anthocyanins: Antioxidant and/or Anti-Inflammatory Activities. J. Appl. Pharm. Sci. 2011, 1, 7–15. [Google Scholar]
- Tena, N.; Martín, J.; Asuero, A.G. State of the Art of Anthocyanins: Antioxidant Activity, Sources, Bioavailability, and Therapeutic Effect in Human Health. Antioxidants 2020, 9, 451. [Google Scholar] [CrossRef]
- Liang, T.; Sun, G.; Cao, L.; Li, J.; Wang, L. A pH and NH3 Sensing Intelligent Film Based on Artemisia sphaerocephala Krasch. Gum and Red Cabbage Anthocyanins Anchored by Carboxymethyl Cellulose Sodium Added as a Host Complex. Food Hydrocoll. 2019, 87, 858–868. [Google Scholar] [CrossRef]
- Liu, S.; Fu, Y.; Nian, S. Buffering Colour Fluctuation of Purple Sweet Potato Anthocyanins to Acidity Variation by Surfactants. Food Chem. 2014, 162, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Sasaki, N.; Ohmiya, A. Biosynthesis of Plant Pigments: Anthocyanins, Betalains and Carotenoids. Plant J. 2008, 54, 733–749. [Google Scholar] [CrossRef]
- Pietrini, F.; Iannelli, M.A.; Massacci, A. Anthocyanin Accumulation in the Illuminated Surface of Maize Leaves Enhances Protection from Photo-Inhibitory Risks at Low Temperature, without Further Limitation to Photosynthesis. Plant Cell Environ. 2002, 25, 1251–1259. [Google Scholar] [CrossRef]
- Chalker-Scott, L. Environmental Significance of Anthocyanins in Plant Stress Responses. Photochem. Photobiol. 1999, 70, 1–9. [Google Scholar] [CrossRef]
- Feild, T.S.; Lee, D.W.; Holbrook, N.M. Why Leaves Turn Red in Autumn. The Role of Anthocyanins in Senescing Leaves of Red-Osier Dogwood. Plant Physiol. 2001, 127, 566–574. [Google Scholar] [CrossRef]
- Hoch, W.A.; Zeldin, E.L.; McCown, B.H. Physiological Significance of Anthocyanins during Autumnal Leaf Senescence. Tree Physiol. 2001, 21, 1–8. [Google Scholar] [CrossRef]
- Ross, J.A.; Kasum, C.M. Dietary Flavonoids: Bioavailability, Metabolic Effects, and Safety. Annu. Rev. Nutr. 2002, 22, 19–34. [Google Scholar] [CrossRef]
- Wu, H.; Oliveira, G.; Lila, M.A. Protein-binding Approaches for Improving Bioaccessibility and Bioavailability of Anthocyanins. Compr. Rev. Food Sci. Food Saf. 2023, 22, 333–354. [Google Scholar] [CrossRef]
- Kamonpatana, K.; Giusti, M.M.; Chitchumroonchokchai, C.; MorenoCruz, M.; Riedl, K.M.; Kumar, P.; Failla, M.L. Susceptibility of Anthocyanins to Ex Vivo Degradation in Human Saliva. Food Chem. 2012, 135, 738–747. [Google Scholar] [CrossRef]
- Fernandes, I.; Faria, A.; Calhau, C.; de Freitas, V.; Mateus, N. Bioavailability of Anthocyanins and Derivatives. J. Fuctional Foods 2014, 7, 54–66. [Google Scholar] [CrossRef]
- Lila, M.A.; Burton-Freeman, B.; Grace, M.; Kalt, W. Unraveling Anthocyanin Bioavailability for Human Health. Annu. Rev. Food Sci. Technol. 2016, 7, 375–393. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Zhang, N.; Tian, J.; Xin, G.; Liu, L.; Sun, X.; Li, B. Advanced Approaches for Improving Bioavailability and Controlled Release of Anthocyanins. J. Control Release 2022, 341, 285–299. [Google Scholar] [CrossRef] [PubMed]
- Giusti, M.M.; Wrolstad, R.E. Acylated Anthocyanins from Edible Sources and Their Applications in Food Systems. Biochem. Eng. J. 2003, 14, 217–225. [Google Scholar] [CrossRef]
- Oplatowska-Stachowiak, M.; Elliott, C.T. Food Colors: Existing and Emerging Food Safety Concerns. Crit. Rev. Food Sci. Nutr. 2017, 57, 524–548. [Google Scholar] [CrossRef]
- McCann, D.; Barrett, A.; Cooper, A.; Crumpler, D.; Dalen, L.; Grimshaw, K.; Kitchin, E.; Lok, K.; Porteous, L.; Prince, E.; et al. Food Additives and Hyperactive Behaviour in 3-Year-Old and 8/9-Year-Old Children in the Community: A Randomised, Double-Blinded, Placebo-Controlled Trial. Lancet 2007, 370, 1560–1567. [Google Scholar] [CrossRef]
- Cevallos-Casals, B.A.; Cisneros-Zevallos, L. Stability of Anthocyanin-Based Aqueous Extracts of Andean Purple Corn and Red-Fleshed Sweet Potato Compared to Synthetic and Natural Colorants. Food Chem. 2004, 86, 69–77. [Google Scholar] [CrossRef]
- Amchova, P.; Kotolova, H.; Ruda-Kucerova, J. Health Safety Issues of Synthetic Food Colorants. Regul. Toxicol. Pharmacol. 2015, 73, 914–922. [Google Scholar] [CrossRef]
- Bakowska-Barczak, A. Acylated Anthocyanins as Stable, Natural Food Colorants—A Review. Pol. J. Food Nutr. Sci. 2005, 14, 107–116. [Google Scholar]
- Kim, I.; Lee, J. Variations in Anthocyanin Profiles and Antioxidant Activity of 12 Genotypes of Mulberry (Morus spp.) Fruits and Their Changes during Processing. Antioxidants 2020, 9, 242. [Google Scholar] [CrossRef]
- Natić, M.M.; Dabić, D.Č.; Papetti, A.; Fotirić Akšić, M.M.; Ognjanov, V.; Ljubojević, M.; Tešić, Ž.L. Analysis and Characterisation of Phytochemicals in Mulberry (Morus alba L.) Fruits Grown in Vojvodina, North Serbia. Food Chem. 2015, 171, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Mohamad Razali, U.H.; Saikim, F.H.; Mahyudin, A.; Mohd Noor, N.Q.I. Morus alba L. Plant: Bioactive Compounds and Potential as a Functional Food Ingredient. Foods 2021, 10, 689. [Google Scholar] [CrossRef]
- Cortez, R.; Luna-Vital, D.A.; Margulis, D.; Gonzalez de Mejia, E. Natural Pigments: Stabilization Methods of Anthocyanins for Food Applications. Compr. Rev. Food Sci. Food Saf. 2017, 16, 180–198. [Google Scholar] [CrossRef]
- Echegaray, N.; Munekata, P.E.S.; Gullón, P.; Dzuvor, C.K.O.; Gullón, B.; Kubi, F.; Lorenzo, J.M. Recent Advances in Food Products Fortification with Anthocyanins. Crit. Rev. Food Sci. Nutr. 2022, 62, 1553–1567. [Google Scholar] [CrossRef]
- Ruta, L.L.; Farcasanu, I.C. Anthocyanins and Anthocyanin-Derived Products in Yeast-Fermented Beverages. Antioxidants 2019, 8, 182. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Ma, Z.; Luo, X.; Li, X. Effects of Mulberry Fruit (Morus alba L.) Consumption on Health Outcomes: A Mini-Review. Antioxidants 2018, 7, 69. [Google Scholar] [CrossRef] [PubMed]
- Salehi, B.; Selamoglu, Z.; Sener, B.; Kilic, M.; Kumar Jugran, A.; de Tommasi, N.; Sinisgalli, C.; Milella, L.; Rajkovic, J.; Flaviana, B.; et al. Berberis Plants—Drifting from Farm to Food Applications, Phytotherapy, and Phytopharmacology. Foods 2019, 8, 522. [Google Scholar] [CrossRef]
- Sui, X.; Zhang, Y.; Zhou, W. Bread Fortified with Anthocyanin-Rich Extract from Black Rice as Nutraceutical Sources: Its Quality Attributes and in Vitro Digestibility. Food Chem. 2016, 196, 910–916. [Google Scholar] [CrossRef]
- Albuquerque, B.R.; Pinela, J.; Barros, L.; Oliveira, M.B.P.P.; Ferreira, I.C.F.R. Anthocyanin-Rich Extract of Jabuticaba Epicarp as a Natural Colorant: Optimization of Heat- and Ultrasound-Assisted Extractions and Application in a Bakery Product. Food Chem. 2020, 316, 126364. [Google Scholar] [CrossRef] [PubMed]
- López, C.J.; Caleja, C.; Prieto, M.A.; Sokovic, M.; Calhelha, R.C.; Barros, L.; Ferreira, I.C.F.R. Stability of a Cyanidin-3-O-Glucoside Extract Obtained from Arbutus unedo L. and Incorporation into Wafers for Colouring Purposes. Food Chem. 2019, 275, 426–438. [Google Scholar] [CrossRef] [PubMed]
- Maner, S.; Sharma, A.K.; Banerjee, K. Wheat Flour Replacement by Wine Grape Pomace Powder Positively Affects Physical, Functional and Sensory Properties of Cookies. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2017, 87, 109–113. [Google Scholar] [CrossRef]
- Papillo, V.A.; Locatelli, M.; Travaglia, F.; Bordiga, M.; Garino, C.; Arlorio, M.; Coïsson, J.D. Spray-Dried Polyphenolic Extract from Italian Black Rice (Oryza sativa L., Var. Artemide) as New Ingredient for Bakery Products. Food Chem. 2018, 269, 603–609. [Google Scholar] [CrossRef]
- Karaaslan, M.; Ozden, M.; Vardin, H.; Turkoglu, H. Phenolic Fortification of Yogurt Using Grape, and Callus Extracts. LWT-Food Sci. Technol. 2011, 44, 1065–1072. [Google Scholar] [CrossRef]
- Montibeller, M.J.; de Lima Monteiro, P.; Tupuna-Yerovi, D.S.; Rios, A.; de Oliveira Rios, V. Stability Assessment of Anthocyanins Obtained from Skin Grape Applied in Kefir and Carbonated Water as a Natural Colorant. J. Food Preserv. 2018, 42, e13698. [Google Scholar] [CrossRef]
- Wallace, T.C.; Giusti, M.M. Determination of Color, Pigment, and Phenolic Stability in Yogurt Systems Colored with Nonacylated Anthocyanins from Berberis boliviana L. as Compared to Other Natural/Synthetic Colorants. J. Food Sci. 2008, 73, C241–C248. [Google Scholar] [CrossRef]
- de Pascual-Teresa, S.; Sanchez-Ballesta, M.T. Anthocyanins: From Plant to Health. Phytochem. Rev. 2008, 7, 281–299. [Google Scholar] [CrossRef]
- Gonçalves, A.C.; Nunes, A.R.; Falcão, A.; Alves, G.; Silva, L.R. Dietary Effects of Anthocyanins in Human Health: A Comprehensive Review. Pharmaceuticals 2021, 14, 690. [Google Scholar] [CrossRef]
- He, X.; Fang, J.; Ruan, Y.; Wang, X.; Sun, Y.; Wu, N.; Huang, L. Structures, bioactivities and future prospective of polysaccharides from Morus alba (white mulberry): A review. Food Chem. 2018, 245, 899–910. [Google Scholar] [CrossRef]
- Prashar, S.; Sharma, S.; Kumar, N.; Kaushik, R.; Chawla, P. Formulation, Characterization, and In Vitro Mineral Absorption of Ficus palmata Fruit Extract Nanoemulsion. J. Am. Nutr. Assoc. 2022, 41, 291–300. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Alonso, M.; Rimbach, G.; Sasai, M.; Nakahara, M.; Matsugo, S.; Uchida, Y.; Rivas-Gonzalo, J.C.; De Pascual-Teresa, S. Electron Spin Resonance Spectroscopy Studies on the Free Radical Scavenging Activity of Wine Anthocyanins and Pyranoanthocyanins. Mol. Nutr. Food Res. 2005, 49, 1112–1119. [Google Scholar] [CrossRef]
- Derosa, G.; Romano, D.; D’Angelo, A.; Maffioli, P. Berberis aristata Combined with Silybum Marianum on Lipid Profile in Patients Not Tolerating Statins at High Doses. Atherosclerosis 2015, 239, 87–92. [Google Scholar] [CrossRef]
- Derosa, G.; Romano, D.; D’Angelo, A.; Maffioli, P. Berberis aristata/Silybum marianum Fixed Combination (Berberol®) Effects on Lipid Profile in Dyslipidemic Patients Intolerant to Statins at High Dosages: A Randomized, Placebo-Controlled, Clinical Trial. Phytomedicine 2015, 22, 231–237. [Google Scholar] [CrossRef]
- Derosa, G.; D’Angelo, A.; Maffioli, P. The Role of a Fixed Berberis aristata/Silybum marianum Combination in the Treatment of Type 1 Diabetes Mellitus. Clin. Nutr. 2016, 35, 1091–1095. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.A.; Islam, P.; Subhan, N.; Rahman, M.M.; Khan, F.; Burrows, G.E.; Nahar, L.; Sarker, S.D. Potential Health Benefits of Anthocyanins in Oxidative Stress Related Disorders. Phytochem. Rev. 2021, 20, 705–749. [Google Scholar] [CrossRef]
- García-Alonso, M.; Rimbach, G.; Rivas-Gonzalo, J.C.; de Pascual-Teresa, S. Antioxidant and Cellular Activities of Anthocyanins and Their Corresponding Vitisins A-Studies in Platelets, Monocytes, and Human Endothelial Cells. J. Agric. Food Chem. 2004, 52, 3378–3384. [Google Scholar] [CrossRef]
- Kamei, H.; Hashimoto, Y.; Koide, T.; Kojima, T.; Hasegawa, M. Anti-Tumor Effect of Methanol Extracts from Red and White Wines. Cancer Biother. Radiopharm. 1998, 13, 447–452. [Google Scholar] [CrossRef] [PubMed]
- Shih, P.-H.; Yeh, C.-T.; Yen, G.-C. Effects of Anthocyanidin on the Inhibition of Proliferation, and Induction of Apoptosis in Human Gastric Adenocarcinoma Cells. Food Chem. Toxicol. 2005, 43, 1557–1566. [Google Scholar] [CrossRef] [PubMed]
- Tsuda, T. Dietary Anthocyanin-Rich Plants: Biochemical Basis and Recent Progress in Health Benefits Studies. Mol. Nutr. Food Res. 2012, 56, 159–170. [Google Scholar] [CrossRef] [PubMed]
- Nakaishi, H.; Matsumoto, H.; Tominaga, S.; Hirayama, M. Effects of Black Currant Anthocyanoside Intake on Dark Adaption and VDT Work-Induced Transient Refractive Alteration in Healthy Humans. Altern. Med. Rev. 2001, 5, 553–562. [Google Scholar]
- Pojer, E.; Mattivi, F.; Johnson, D.; Stockley, C.S. The Case for Anthocyanin Consumption to Promote Human Health: A Review. Compr. Rev. Food Sci. Food Saf. 2013, 12, 483–508. [Google Scholar] [CrossRef]
- Ghosh, D.; Konishi, T. Anthocyanins and Anthocyanin-Rich Extracts: Role in Diabetes and Eye Function. Asia Pac. J. Clin. Nutr. 2007, 16, 200–208. [Google Scholar]
- Smeriglio, A.; Barreca, D.; Bellocco, E.; Trombetta, D. Chemistry, Pharmacology and Health Benefits of Anthocyanins. Phytother. Res. 2016, 30, 1265–1286. [Google Scholar] [CrossRef]
- Roy, S.; Rhim, J.-W. Anthocyanin food colorant and its application in pH-responsive color change indicator films. Crit. Rev. Food Sci. Nutr. 2021, 61, 2297–2325. [Google Scholar] [CrossRef]
- Kim, H.J.; Roy, S.; Rhim, J.-W. Gelatin/agar-based color-indicator film integrated with Clitoria ternatea flower anthocyanin and zinc oxide nanoparticles for monitoring freshness of shrimp. Food Hydrocoll. 2022, 124, 107294. [Google Scholar] [CrossRef]
- Gupta, V.; Biswas, D.; Roy, S. A comprehensive review of biodegradable polymer-based films and coatings and their food packaging applications. Materials 2022, 15, 5899. [Google Scholar] [CrossRef]
- Roy, S.; Priyadarshi, R.; Ezati, P.; Rhim, J.-W. Curcumin and its uses in active and smart food packaging applications-A comprehensive review. Food Chem. 2022, 375, 131885. [Google Scholar] [CrossRef] [PubMed]
- Oun, A.A.; Roy, S.; Shin, G.H.; Yoo, S.; Kim, J.T. pH-sensitive smart indicators based on cellulose and different natural pigments for tracing kimchi ripening stages. Int. J. Biol. Macromol. 2023, 242, 124905. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Roy, S.; Ezati, P.; Yang, D.P.; Rhim, J.-W. Tannic acid: A green crosslinker for biopolymer-based food packaging films. Trends Food Sci. Technol. 2023, 136, 11–23. [Google Scholar] [CrossRef]
- Santhosh, R.; Nath, D.; Sarkar, P. Novel Food Packaging Materials Including Plant-Based Byproducts: A Review. Trends Food Sci. Technol. 2021, 118, 471–489. [Google Scholar] [CrossRef]
- Roy, S.; Kim, H.J.; Rhim, J.-W. Effect of blended colorants of anthocyanin and shikonin on carboxymethyl cellulose/agar-based smart packaging film. Int. J. Biol. Macromol. 2021, 183, 305–315. [Google Scholar] [CrossRef]
- Ma, Q.; Wang, L. Preparation of a Visual PH-Sensing Film Based on Tara Gum Incorporating Cellulose and Extracts from Grape Skins. Sens. Actuators B Chem. 2016, 235, 401–407. [Google Scholar] [CrossRef]
- Mushtaq, M.; Gani, A.; Gani, A.; Punoo, H.A.; Masoodi, F.A. Use of Pomegranate Peel Extract Incorporated Zein Film with Improved Properties for Prolonged Shelf Life of Fresh Himalayan Cheese (Kalari/Kradi). Innov. Food Sci. Emerg. Technol. 2018, 48, 25–32. [Google Scholar] [CrossRef]
- Halliwell, B.; Murcia, M.A.; Chirico, S.; Aruoma, O.I. Free Radicals and Antioxidants in Food and in Vivo: What They Do and How They Work. Crit. Rev. Food Sci. Nutr. 1995, 35, 7–20. [Google Scholar] [CrossRef]
- Ebrahimzadeh, S.; Biswas, D.; Roy, S.; McClements, D.J. Incorporation of essential oils in edible seaweed-based films: A comprehensive review. Trends Food Sci. Technol. 2023, 135, 43–56. [Google Scholar] [CrossRef]
- Yan, J.; Zhang, H.; Yuan, M.; Qin, Y.; Chen, H. Effects of Anthocyanin-Rich Kadsura coccinea Extract on the Physical, Antioxidant, and pH-Sensitive Properties of Biodegradable Film. Food Biophys. 2022, 17, 375–385. [Google Scholar] [CrossRef]
- Kaya, M.; Ravikumar, P.; Ilk, S.; Mujtaba, M.; Akyuz, L.; Labidi, J.; Salaberria, A.M.; Cakmak, Y.S.; Erkul, S.K. Production and Characterization of Chitosan Based Edible Films from Berberis crataegina’s Fruit Extract and Seed Oil. Innov. Food Sci. Emerg. Technol. 2018, 45, 287–297. [Google Scholar] [CrossRef]
- Ma, Y.; Ding, S.; Fei, Y.; Liu, G.; Jang, H.; Fang, J. Antimicrobial Activity of Anthocyanins and Catechins against Foodborne Pathogens Escherichia coli and Salmonella. Food Control 2019, 106, 106712. [Google Scholar] [CrossRef]
- Campos, C.A.; Gerschenson, L.N.; Flores, S.K. Development of Edible Films and Coatings with Antimicrobial Activity. Food Bioprocess Technol. 2011, 4, 849–875. [Google Scholar] [CrossRef]
- Qin, Y.; Xu, F.; Yuan, L.; Hu, H.; Yao, X.; Liu, J. Comparison of the Physical and Functional Properties of Starch/Polyvinyl Alcohol Films Containing Anthocyanins and/or Betacyanins. Int. J. Biol. Macromol. 2020, 163, 898–909. [Google Scholar] [CrossRef]
- Choi, H.-J.; Choi, S.-W.; Lee, N.; Chang, H.-J. Antimicrobial Activity of Chitosan/Gelatin/Poly(Vinyl Alcohol) Ternary Blend Film Incorporated with Duchesnea indica Extract in Strawberry Applications. Foods 2022, 11, 3963. [Google Scholar] [CrossRef] [PubMed]
- Santos, L.G.; Alves-Silva, G.F.; Martins, V.G. Active-Intelligent and Biodegradable Sodium Alginate Films Loaded with Clitoria ternatea Anthocyanin-Rich Extract to Preserve and Monitor Food Freshness. Int. J. Biol. Macromol. 2022, 220, 866–877. [Google Scholar] [CrossRef] [PubMed]
- Ansari, S.; Chauhan, B.; Kalam, N.; Kumar, G. Current Concepts and Prospects of Herbal Nutraceutical: A Review. J. Adv. Pharm. Technol. Res. 2013, 4, 4. [Google Scholar] [CrossRef]
- Alappat, B.; Alappat, J. Anthocyanin Pigments: Beyond Aesthetics. Molecules 2020, 25, 5500. [Google Scholar] [CrossRef]
- Mattioli, R.; Francioso, A.; Mosca, L.; Silva, P. Anthocyanins: A Comprehensive Review of Their Chemical Properties and Health Effects on Cardiovascular and Neurodegenerative Diseases. Molecules 2020, 25, 3809. [Google Scholar] [CrossRef]
- Garzón, G.A.; Wrolstad, R.E. Major Anthocyanins and Antioxidant Activity of Nasturtium Flowers (Tropaeolum majus). Food Chem. 2009, 114, 44–49. [Google Scholar] [CrossRef]
- Azuma, K.; Ohyama, A.; Ippoushi, K.; Ichiyanagi, T.; Takeuchi, A.; Saito, T.; Fukuoka, H. Structures and Antioxidant Activity of Anthocyanins in Many Accessions of Eggplant and Its Related Species. J. Agric. Food Chem. 2008, 56, 10154–10159. [Google Scholar] [CrossRef] [PubMed]
- Szymanowska, U.; Złotek, U.; Karaś, M.; Baraniak, B. Anti-Inflammatory and Antioxidative Activity of Anthocyanins from Purple Basil Leaves Induced by Selected Abiotic Elicitors. Food Chem. 2015, 172, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Sapian, S.; Taib, I.S.; Katas, H.; Latip, J.; Zainalabidin, S.; Hamid, Z.A.; Anuar, N.N.M.; Budin, S.B. The Role of Anthocyanin in Modulating Diabetic Cardiovascular Disease and Its Potential to Be Developed as a Nutraceutical. Pharmaceuticals 2022, 15, 1344. [Google Scholar] [CrossRef]
- Strugała, P.; Dzydzan, O.; Brodyak, I.; Kucharska, A.Z.; Kuropka, P.; Liuta, M.; Kaleta-Kuratewicz, K.; Przewodowska, A.; Michałowska, D.; Gabrielska, J.; et al. Antidiabetic and Antioxidative Potential of the Blue Congo Variety of Purple Potato Extract in Streptozotocin-Induced Diabetic Rats. Molecules 2019, 24, 3126. [Google Scholar] [CrossRef]
- Sarikaphuti, A.; Nararatwanchal, T.; Hashiguchi, T.; Ito, T.; Thaworanunta, S.; Kikuchi, K.; Omaya, Y.; Maruyama, I.; Tancharoen, S. Preventive Effects of Morus alba L. Anthocyanins on Diabetes in Zucker Diabetic Fatty Rats. Exp. Ther. Med. 2013, 6, 689–695. [Google Scholar] [CrossRef]
- Lin, B.-W.; Gong, C.-C.; Song, H.-F.; Cui, Y.-Y. Effects of Anthocyanins on the Prevention and Treatment of Cancer. Br. J. Pharmacol. 2017, 174, 1226–1243. [Google Scholar] [CrossRef]
- Bontempo, P.; De Masi, L.; Carafa, V.; Rigano, D.; Scisciola, L.; Iside, C.; Grassi, R.; Molinari, A.M.; Aversano, R.; Nebbioso, A.; et al. Anticancer Activities of Anthocyanin Extract from Genotyped Solanum tuberosum L. “Vitelotte”. J. Funct. Foods 2015, 19, 584–593. [Google Scholar] [CrossRef]
- Joshi, R.; Rana, A.; Kumar, V.; Kumar, D.; Padwad, Y.S.; Yadav, S.K.; Gulati, A. Anthocyanins Enriched Purple Tea Exhibits Antioxidant, Immunostimulatory and Anticancer Activities. J. Food Sci. Technol. 2017, 54, 1953–1963. [Google Scholar] [CrossRef]
- Kapoor, B.B.S.; Sood, P.; Modgil, R.; Sood, M. Berberis lycium a Medicinal Plant with Immense Value. Indian J. Pharm. Biol. Res. 2013, 1, 27. [Google Scholar] [CrossRef]
- Gaur, P.; Bhatia, S.; Andola, H.; Gupta, R. In Vitro Radical Scavenging Activity and Antimicrobial Potential of Berberis asiatica Roxb. Ex DC. Fruit Extracts in Four Different Processed Forms. Indian J. Tradit. Knowl. 2017, 16, 706–713. [Google Scholar]
- Tewari, D.; Zengin, G.; Ak, G.; Sinan, K.I.; Cziáky, Z.; Mishra, S.T.; Jekő, J. Phenolic Profiling, Antioxidants, Multivariate, and Enzyme Inhibitory Properties of Wild Himalayan Fig (Ficus palmata Forssk.): A Potential Candidate for Designing Innovative Nutraceuticals and Related Products. Anal. Lett. 2021, 54, 1439–1456. [Google Scholar] [CrossRef]



| Name of the Species | Family | Common Name | References |
|---|---|---|---|
| Berberis asiatica | Berberidaceae | H: Chitra/Chotra E: Indian Barberry, Tree Turmeric | [26] |
| Morus alba | Moraceae | H: Shahtoot E: Mul berry/Silkworm Mulberry | [27] |
| Berberis lycium | Berberidaceae | E: Indian Barberry, H: Kashmal | [28] |
| Myrica esculenta | Myricaceae | E: Box berry H: Kaphal | [29] |
| Duchesnea indica | Rosaceae | H: Kiphaliya E: Indian strawberry/Mock strawberry | [26] |
| Lycium ruthencium | Solanaceae | E: Black wolfberry | [30] |
| Fragaria nubicola | Rosaceae | E: Himalayan strawberry | [31] |
| Ficus palmata | Moraceae | H: Abjiri/Bedu E: Wild Fig | [26] |
| Gaultheria trichophylla | Ericaeae | E: Himalayan snowberry | [32] |
| Genus begonia | Begoniacece | NA | [33] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmed, M.; Bose, I.; Goksen, G.; Roy, S. Himalayan Sources of Anthocyanins and Its Multifunctional Applications: A Review. Foods 2023, 12, 2203. https://doi.org/10.3390/foods12112203
Ahmed M, Bose I, Goksen G, Roy S. Himalayan Sources of Anthocyanins and Its Multifunctional Applications: A Review. Foods. 2023; 12(11):2203. https://doi.org/10.3390/foods12112203
Chicago/Turabian StyleAhmed, Mustafa, Ipsheta Bose, Gulden Goksen, and Swarup Roy. 2023. "Himalayan Sources of Anthocyanins and Its Multifunctional Applications: A Review" Foods 12, no. 11: 2203. https://doi.org/10.3390/foods12112203