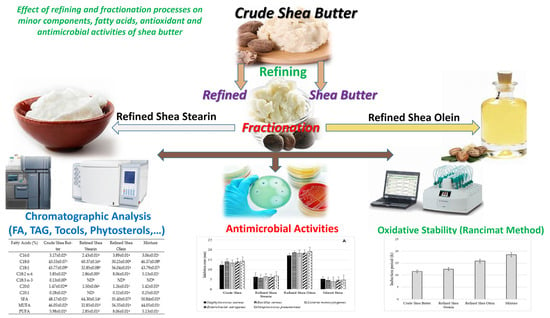
Effect of Refining and Fractionation Processes on Minor Components, Fatty Acids, Antioxidant and Antimicrobial Activities of Shea Butter
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Shea Butter and Its Fractions
2.1.2. Standards and Reagents
2.2. Methods
2.2.1. Fatty Acids Composition
2.2.2. Triacylglycerols Composition (TAG)
2.2.3. Physicochemical Parameters
2.2.4. Carotenoids Content
2.2.5. Tocopherol Content
2.2.6. Phytosterols Content
2.2.7. Oxidative Stability
2.2.8. Antimicrobial Activity
2.2.9. Antifungal Activity
2.3. Statistical Analysis
3. Results and Discussion
3.1. Fatty Acid Composition
3.2. Triacylglycerol Composition
3.3. Free Fatty Acids
3.4. Unsaponifiable Matter
3.5. Total Phenolic Content
3.6. Total Flavonoid Content
3.7. Peroxide Value
3.8. Oxidative Stability Parameters
3.9. Melting Point
3.10. Total Carotenoid Content
3.11. Tocopherols
3.12. Phytosterol Composition
3.13. Induction Period (IP)
3.14. Antimicrobial Activity of Shea Butter Samples
3.14.1. Antibacterial Activity
3.14.2. Antifungal Activity
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Honfo, F.G.; Linnemann, A.R.; Akissoe, N.; Soumanou, M.M.; Van Boekel, M. Characteristics of traditionally processed shea kernels and butter. Int. J. Food Sci. Technol. 2013, 48, 1714–1721. [Google Scholar] [CrossRef]
- Davrieux, F.; Allal, F.; Piombo, G.; Kelly, B.; Okulo, J.B.; Thiam, M.; Diallo, O.B.; Bouvet, J.M. Near infrared spectroscopy for high-throughput characterization of shea tree (Vitellaria paradoxa) nut fat profiles. J. Agric. Food Chem. 2010, 58, 7811–7819. [Google Scholar] [CrossRef] [PubMed]
- Maanikuu PM, I.; Peker, K. Medicinal and nutritional benefits from the Shea tree (Vitellaria paradoxa). J. Biol. Agric. Healthc. 2017, 22, 51–57. [Google Scholar]
- Lovett, P.N. Shea butter: Properties and processing for use in food. In Specialty Oils and Fats in Food and Nutrition, 1st ed.; Talbot, G., Ed.; Woodhead Publishing: Sawston, UK, 2015; Volume 5, pp. 125–158. [Google Scholar]
- Perederic, O.A.; Mansouri, S.S.; Appel, S.; Sarup, B.; Gani, R.; Woodley, J.M.; Kontogeorgis, G.M. Process analysis of shea butter solvent fractionation using a generic systematic approach. Ind. Eng. Chem. Res. 2020, 59, 9152–9164. [Google Scholar] [CrossRef]
- Shehata, M.; Badr, A.; Abdel-Razek, A.; Hassanein, M.; Amra, H. Oil-bioactive Films as an Antifungal Application to Save Post-harvest Food Crops. Annu. Res. Rev. Biol. 2017, 16, 1–16. [Google Scholar] [CrossRef]
- Badr, A.N.; Shehata, M.G.; Abdel-Razek, A.G. Antioxidant activities and potential impacts to reduce aflatoxins utilizing jojoba and jatropha oils and extracts. Int. J. Pharmacol. 2017, 13, 1103–1114. [Google Scholar] [CrossRef] [Green Version]
- AOCS Official Method Ce 1k-07—Direct Methylation of Lipids for the Determination of Total Fat, Saturated, Cis-Monounsaturated, Cis-Polyunsaturated, and Trans Fatty Acids by Chromatography, 7th ed.; American Oil Chemists’ Society: Urbana, IL, USA, 2017; pp. 1–4.
- Ciftci, O.N.; Przybylski, R.; Rudzińska, M.; Acharya, S. Characterization of Fenugreek (Trigonella foenum-graecum) Seed Lipids. J. Am. Oil Chem. Soc. 2011, 88, 1603–1610. [Google Scholar] [CrossRef]
- Idrus, S.I.S.; Latiff, A.A.; Ismail, M.N. Determination of triacylglycerols in food by high-performance liquid chromatography. Instrum. Sci. Technol. 2017, 5, 577–591. [Google Scholar] [CrossRef]
- AOCS Official Method Ca 5a-40—Free Fatty Acids, 7th ed.; American Oil Chemists’ Society: Urbana, IL, USA, 2017; pp. 1–2.
- AOCS Official Method Ca 6b-53—Unsaponifiable Matter, 7th ed.; American Oil Chemists’ Society: Urbana, IL, USA, 2017; pp. 1–3.
- Gutfinger, T. Polyphenols in olive oils. J. Am. Oil Chem. Soc. 1981, 58, 966–968. [Google Scholar] [CrossRef]
- Mohamed, D.A.; Hassanein, M.M.; El-Messery, T.M.; Fouad, M.T.; El-Said, M.M.; Fouda, K.A.; Abdel-Raze, A.G. Amelioration of diabetes in a rat model through yoghurt supplemented with probiotics and olive pomace extract. J. Biol. Sci. 2017, 17, 320–333. [Google Scholar] [CrossRef] [Green Version]
- Hassan, H.; Attia, E.; Desoukey, S.; Mohamed, K.; Kamel, M. Quantitative Analysis of Total Phenolic and Total Flavonoid Constituents of some Ficus species. J. Adv. Biomed. Pharm. Sci. 2019, 2, 38–40. [Google Scholar] [CrossRef] [Green Version]
- AOCS Official Method Cd 8b-90—Peroxide Value Acetic Acid–Isooctane Method, 7th ed.; American Oil Chemists’ Society: Urbana, IL, USA, 2017; pp. 1–3.
- Ramadan, M.F.; Kroh, L.W.; Mörsel, J.T. Radical Scavenging Activity of Black Cumin (Nigella sativa L.), Coriander (Coriandrum sativum L.), and Niger (Guizotia abyssinica Cass.) Crude Seed Oils and Oil Fractions. J. Agric. Food Chem. 2003, 51, 6961–6969. [Google Scholar] [CrossRef] [PubMed]
- AOCS Official Method Cc 1-25—Melting Point: Capillary Tube Method, 7th ed.; American Oil Chemists’ Society: Urbana, IL, USA, 2017; pp. 1–3.
- Hart, D.J.; Scott, K.J. Development and evaluation of an HPLC method for the analysis of carotenoids in foods, and the measurement of the carotenoid content of vegetables and fruits commonly consumed in the UK. Food Chem. 1995, 54, 101–111. [Google Scholar] [CrossRef]
- Gawrysiak-Witulska, M.; Siger, A.; Rudzińska, M.; Bartkowiak-Broda, I. The effect of drying on the native tocopherol and phytosterol content of Sinapis alba L. seeds. J. Sci. Food Agric. 2020, 100, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Hassanein, M.M.; Abdel-Razek, A.G.; Affifi, S.M.; Qian, Y.; Radziejewska-Kubzdela, E.; Siger, A.; Rudzińska, M.; Abo-Elwafa, G.A.; Grygier, A. Characterization of New Egyptian Linseed Varieties and the Effects of Roasting on Their Pigments, Tocochromanols, Phytosterols, Omega-3 Fatty Acids, and Stability. Molecules 2022, 27, 8526. [Google Scholar] [CrossRef] [PubMed]
- AOCS Official Method Ch 6-91—Determination of the Composition of the Sterol Fraction of Animal and Vegetable Oils and Fats by TLC and Capillary GLC, 7th ed.; American Oil Chemists’ Society: Urbana, IL, USA, 2017; pp. 1–4.
- Kowalski, B.; Ratusz, K.; Kowalska, D.; Bekas, W. Determination of the oxidative stability of vegetable oils by differential scanning calorimetry and Rancimat measurements. Eur. J. Lipid Sci. Technol. 2004, 106, 165–169. [Google Scholar] [CrossRef]
- Shoaib, A.; Saeed, G.; Ahmad, S. Antimicrobial activity and chemical analysis of some edible oils (Clove, Kalonji and Taramira). Afr. J. Biotechnol. 2014, 13, 4347–4354. [Google Scholar] [CrossRef]
- Badr, A.N.; El-Said, M.M.; Elmessery, T.M.; Abdel-Razek, A.G. Non-traditional Oils Encapsulation as Novel Food Additive Enhanced Yogurt Safety against Aflatoxins. Pak. J. Biol. Sci. 2019, 22, 51–58. [Google Scholar] [CrossRef] [Green Version]
- Calder, P.C. Polyunsaturated fatty acids and inflammation. Prostaglandins Leukot. Essent. Fat. Acids 2006, 75, 197–202. [Google Scholar] [CrossRef] [Green Version]
- CXS 325R-2017; International Food Standards, Codex Standards for Named Vegetable Oils. Codex Alimentarius Commission: Geneva, Switzerland, 2017.
- Chibor, B.; Kiin-Kabari, D.; Ejiofor, J. Physicochemical properties and fatty acid profile of shea butter and fluted pumpkin seed oil, a suitable blend in bakery fat seed oil, a suitable blend in bakery fat production. Int. J. Nutr. Food Sci. 2017, 6, 122–128. [Google Scholar] [CrossRef] [Green Version]
- Rudzińska, M.; Hassanein, M.M.; Abdel-Razek, A.G.; Kmiecik, D.; Siger, A.; Ratusz, K. Influence of composition on degradation during repeated deep-fat frying of binary and ternary blends of palm, sunflower and soybean oils with health-optimised saturated-to-unsaturated fatty acid ratios. Int. J. Food Sci. Technol. 2018, 53, 1021–1029. [Google Scholar] [CrossRef]
- Goumbri, B.W.; da Silva, T.L.T.; Marini, R.D.; Semdé, R.; Somé, T.I.; Danthine, S. African Shea Butter Properties Related to Common Extraction Technologies: A Review. Food Bioprocess Technol. 2021, 15, 231–248. [Google Scholar] [CrossRef]
- Maranz, S.; Wiesman, Z.; Bisgaard, J.; Bianchi, G. Germplasm resources of Vitellaria paradoxa based on variations in fat composition across the species distribution range. Agrofor. Syst. 2004, 60, 71–76. [Google Scholar] [CrossRef]
- Womeni, H.M.; Ndjouenkeu, R.; Kapseu, C.; Parmentier, M.; Fanni, J. Application of drying-frying processing of shea kernels: Influence on chemical quality indices and melting properties of the butter. OCL—Oilseeds Fats Crops Lipids 2006, 13, 297–302. [Google Scholar]
- Nkouam, G.B.; Barth, D.; Dirand, M.; Tchatcheng, J.B. Oil extraction from shea kernel (Vitellaria paradoxa, Gaertn.) and Canarium pulp (Canarium schweinfurthii, Engl.) using supercritical CO2 and hexane: A comparative study. Res. J. Appl. Sci. 2007, 2, 646–652. [Google Scholar]
- Okullo JB, L.; Omujal, F.; Agea, J.; Vuzi, P.; Namutebi, A.; Okello, J.; Nyanzi, S. Physico-chemical characteristics of Shea butter (Vitellaria paradoxa CF Gaertn.) oil from the Shea district of Uganda. Afr. J. Food Agric. Nutr. Dev. 2010, 10, 1–15. [Google Scholar]
- Estiasih, T.; Ahmadi, K.G.S.; Widyaningsih, T.D.; Maligan, J.M.; Mubarok, A.Z.; Zubaidah, E.; Mukhlisiyyah, J.; Puspitasari, R. Bioactive compounds of palm fatty acid distillate (PFAD) from several palm oil refineries. Adv. J. Food Sci. Technol. 2013, 5, 1153–1159. [Google Scholar] [CrossRef]
- Zhang, Z.; Ma, X.; Huang, H.; Wang, Y. Shea olein based specialty fats: Preparation, characterization and potential application. LWT—Food Sci. Technol. 2017, 86, 492–500. [Google Scholar] [CrossRef]
- Nahm, H.S. Quality Characteristics of West African Shea Butter (Vitellaria paradoxa) and Approaches to Extend Shelf-Life. Master’s Thesis, The State University of New Jersey, New Brunswick, NJ, USA, 2011. Available online: https://rucore.libraries.rutgers.edu/rutgers-lib/33959/PDF/1/play/ (accessed on 6 January 2023).
- Masters, E.T.; Yidane, J.A.; Loveth, P.N. Reinforcing Sound Management through Trade: Shea Tree Products in Africa. Unasylva 2004, 219, 46–52. [Google Scholar]
- Zacchi, P.; Eggers, R. High-temperature pre-conditioning of rapeseed: A polyphenol-enriched oil and the effect of refining. Eur. J. Lipid Sci. Technol. 2008, 110, 111–119. [Google Scholar] [CrossRef]
- Abbas, A.M.; Azizul, I.M.; Hossen, J.; Ibrahim, M. Antioxidative evaluation of solvent extracts and fractions of oil refining steps from rice bran. Int. Food Res. J. 2019, 26, 791–799. [Google Scholar]
- Njoku, O.U.; Eneh, F.U.; Ononogbu, I.C.; Adikwu, M.U. Compositional and toxicological studies on shea butter. J. Nutraceuticals Funct. Med. Foods 2000, 2, 33–39. [Google Scholar] [CrossRef]
- Dandjouma AK, A.; Adjia, H.Z.; Kameni, A.; Tchiegang, C. Traditionnal production and commercialization of shea butter in North Cameroon. Tropicultura 2009, 27, 3–7. [Google Scholar]
- Ghohestani, E.; Tashkhourian, J.; Hemmateenejad, B. Colorimetric determination of peroxide value in vegetable oils using a paper based analytical device. Food Chem. 2023, 403, 134345. [Google Scholar] [CrossRef]
- Honfo, F.G.; Akissoe, N.; Linnemann, A.R.; Soumanou, M.; Van Boekel, M.A. Nutritional composition of shea products and chemical properties of shea butter: A review. Crit. Rev. Food Sci. 2014, 54, 673–686. [Google Scholar] [CrossRef]
- Letchamo, W.; Khoo, B.K.; Hartman, T.G. Evaluation of the quality of West African shea butter (Vitellaria paradoxa). Acta Hortic. 2007, 756, 273–288. [Google Scholar] [CrossRef]
- Mbah, J.B.B.; Kamga, R.; Nguetnkam, J.P.; Fanni, J. Adsorption of pigments and free fatty acids from shea butter on activated Cameroonian clays. Eur. J. Lipid Sci. Technol. 2005, 107, 387–398. [Google Scholar] [CrossRef]
- Allal, F.; Piombo, G.; Kelly, B.A.; Okullo, J.B.; Thiam, M.; Diallo, O.B.; Nyarko, G.; Davrieux, F.; Lovett, P.N.; Bouvet, J.M. Fatty acid and tocopherol patterns of variation within the natural range of the shea tree (Vitellaria paradoxa). Agrofor. Syst. 2013, 87, 1065–1082. [Google Scholar] [CrossRef]
- Wong, M.L.; Timms, R.E.; Goh, E.M. Colorimetric determination of total tocopherols in palm oil, olein and stearin. J. Am. Oil Chem. Soc. 1988, 65, 258–261. [Google Scholar] [CrossRef]
- Kamal-Eldin, A.; Appelqvist, L.A. The chemistry and antioxidant properties of tocopherols and tocotrienols. Lipids 1996, 31, 671–701. [Google Scholar] [CrossRef]
- Di Vincenzo, D.; Maranz, S.; Serraiocco, A.; Vito, R.; Wiesman, Z.; Bianchi, G. Regional variation in shea butter lipid and triterpene composition in four African countries. J. Agric. Food Chem. 2005, 53, 7473–7479. [Google Scholar] [CrossRef] [PubMed]
- Méndez, E.; Sanhueza, J.; Speisky, H.; Valenzuela, A. Validation of the Rancimat test for the assessment of the relative stability of fish oils. J. Am. Oil Chem. Soc. 1996, 73, 1033–1037. [Google Scholar] [CrossRef]
- Patra, J.K.; Lee, S.W.; Park, J.G.; Baek, K.H. Antioxidant and antibacterial properties of essential oil extracted from an edible seaweed Undaria pinnatifida. J. Food Biochem. 2017, 41, 12278. [Google Scholar] [CrossRef]
- Cosmulescu, S.; Trandafir, I.; Nour, V. Phenolic acids and flavonoids profiles of extracts from edible wild fruits and their antioxidant properties. Int. J. Food Prop. 2017, 20, 3124–3134. [Google Scholar] [CrossRef] [Green Version]
- Połeć, K.; Wójcik, A.; Flasiński, M.; Wydro, P.; Broniatowski, M.; Hąc-Wydro, K. The influence of terpinen-4-ol and eucalyptol—The essential oil components-on fungi and plant sterol monolayers. Biochim. Biophys. Acta (BBA)-Biomembr. 2019, 1861, 1093–1102. [Google Scholar] [CrossRef] [PubMed]
- Ornella, T.T.J.; Flore, T.N.; Tiencheu, B.; Tenyang, N.; Ashu, A.O.E.; Marie, M.E.; Ufuan, A.A. Chemical and Antibacterial Properties of Lipids Extracted from Some Plant Seeds and Fruits Commonly Used in Cosmetics. Am. J. Food Technol. 2022, 10, 10–19. [Google Scholar] [CrossRef]
- Zengin, H.; Baysal, A.H. Antibacterial and Antioxidant Activity of Essential Oil Terpenes against Pathogenic and Spoilage-Forming Bacteria and Cell Structure-Activity Relationships Evaluated by SEM Microscopy. Molecules 2014, 19, 17773–17798. [Google Scholar] [CrossRef] [Green Version]
- Čmolík, J.; Pokorný, J. Physical refining of edible oils. Eur. J. Lipid Sci. Technol. 2000, 102, 472–486. [Google Scholar] [CrossRef]
- Cao, J.H.; Zhou, Z.H.; Xu, Z.C.; Zhang, Q.; Li, S.H.; Cui, H.B.; Zhang, L.; Zhang, L. Synergism/antagonism between crude oil fractions and novel betaine solutions in reducing interfacial tension. Energy Fuels 2016, 30, 924–932. [Google Scholar] [CrossRef]
- Giaconia, M.A.; dos Passos Ramos, S.; Pereira, C.F.; Lemes, A.C.; De Rosso, V.V.; Braga, A.R. Overcoming restrictions of bioactive compounds biological effects in food using nanometer-sized structures. Food Hydrocoll. 2020, 107, 105939. [Google Scholar] [CrossRef]
- Cert, A.; Moreda, W.; Pérez-Camino, M.C. Chromatographic analysis of minor constituents in vegetable oils. J. Chromatogr. A 2000, 881, 131–148. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Razek, A.G.; Badr, A.N.; El-Messery, T.M.; El-Said, M.M.; Hussein, A.M.S. Micro-nano encapsulation of black seed oil ameliorate its characteristics and its mycotoxin inhibition. Biosci. Res. 2018, 15, 2591–2601. [Google Scholar]
- Badr, A.N.; Ali, H.S.; Abdel-Razek, A.G.; Shehata, M.G.; Albaridi, N.A. Bioactive components of pomegranate oil and their influence on mycotoxin secretion. Toxins 2020, 12, 748. [Google Scholar] [CrossRef]
- Zhao, S.; Baik, O.D.; Choi, Y.J.; Kim, S.M. Pretreatments for the efficient extraction of bioactive compounds from plant-based biomaterials. Crit. Rev. Food Sci. Nutr. 2014, 54, 1283–1297. [Google Scholar] [CrossRef]
- Kitahara, T.; Koyama, N.; Matsuda, J.; Aoyama, Y.; Hirakata, Y.; Kamihira, S.; Kohno, S.; Nakashima, M.; Sasaki, H. Antimicrobial activity of saturated fatty acids and fatty amines against methicillin-resistant Staphylococcus aureus. Biol. Pharm. Bull. 2004, 27, 1321–1326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Altieri, C.; Bevilacqua, A.; Cardillo, D.; Sinigaglia, M. Antifungal activity of fatty acids and their monoglycerides against Fusarium spp. in a laboratory medium. Int. J. Food Sci. Technol. 2009, 44, 242–245. [Google Scholar] [CrossRef]



| Fatty Acids (%) | Crude Shea Butter | Refined Shea Stearin | Refined Shea Olein | Mixture |
|---|---|---|---|---|
| C16:0 | 3.17 ± 0.02 b | 2.43 ± 0.01 d | 3.89 ± 0.01 a | 3.06 ± 0.02 c |
| C18:0 | 43.53 ± 0.07 c | 60.37 ± 0.16 a | 30.25 ± 0.00 d | 46.37 ± 0.08 b |
| C18:1 | 45.77 ± 0.09 b | 32.85 ± 0.08 d | 56.04 ± 0.01 a | 43.79 ± 0.07 c |
| C18:2 n-6 | 5.85 ± 0.02 b | 2.86 ± 0.00 d | 8.06 ± 0.01 a | 5.13 ± 0.02 c |
| C18:3 n-3 | 0.13 ± 0.00 b | ND b | ND b | ND b |
| C20:0 | 1.47 ± 0.02 ab | 1.50 ± 0.06 a | 1.26 ± 0.01 c | 1.42 ± 0.01 b |
| C20:1 | 0.28 ± 0.02 b | ND c | 0.52 ± 0.01 a | 0.25 ± 0.02 b |
| SFA | 48.17 ± 0.02 c | 64.30 ± 0.14 a | 35.40 ± 0.07 d | 50.84 ± 0.01 b |
| MUFA | 46.05 ± 0.02 b | 32.85 ± 0.01 d | 56.55 ± 0.01 a | 44.05 ± 0.01 c |
| PUFA | 5.98 ± 0.01 b | 2.85 ± 0.01 d | 8.06 ± 0.01 a | 5.13 ± 0.01 c |
| ECN | Triacylglycerols (%) | Crude Shea Butter | Refined Shea Stearin | Refined Shea Olein | Mixture |
|---|---|---|---|---|---|
| 46 | OLO | 0.42 ± 0.12 b | ND c | 1.06 ± 0.37 a | 0.45 ± 0.09 b |
| 46 | SLL | 0.39 ± 0.13 b | ND c | 0.96 ± 0.19 a | 0.39 ± 0.11 b |
| 48 | OOO | 2.89 ± 0.30 b | ND d | 7.24 ± 0.51 a | 2.28 ± 0.03 c |
| 48 | SLO | 4.23 ± 0.19 b | ND d | 8.45 ± 0.26 a | 2.49 ± 0.15 c |
| 48 | POO | 0.72 ± 0.08 c | ND d | 2.59 ± 0.11 a | 1.11 ± 0.15 b |
| 50 | SOO | 42.12 ± 0.42 b | 4.75 ± 0.61 d | 72.66 ± 3.03 a | 32.39 ± 0.80 c |
| 50 | AOO | 0.32 ± 0.04 b | ND c | 0.68 ± 0.10 a | 0.31 ± 0.12 b |
| 50 | PPS | ND b | 1.59 ± 0.05 a | ND b | ND b |
| 50 | POS | 2.15 ± 0.26 b | 2.94 ± 0.45 a | 2.01 ± 0.25 b | 2.82 ± 0.07 a |
| 50 | SOS | 46.77 ± 0.69 c | 90.72 ± 1.12 a | 2.82 ± 0.07 a | 57.77 ± 1.02 b |
| Parameters | Crude Shea Butter | Refined Shea Stearin | Refined Shea Olein | Mixture |
|---|---|---|---|---|
| FFAs (%) | 12.24 ± 0.30 a | 0.00 ± 0.00 b | 0.00 ± 0.00 b | 0.11 ± 0.00 b |
| USM (%) | 5.06 ± 0.11 b | 0.22 ± 0.04 d | 6.38 ± 0.57 a | 4.06 ± 0.11 c |
| TPC (mg/g) | 0.21 ± 0.01 a | 0.19 ± 0.01 b | 0.19 ± 0.01 b | 0.21 ± 0.02 a |
| TFC (mg catechin/100 g) | 40.14 ± 0.20 a | 26.46 ± 0.46 b | 27.61 ± 0.70 c | 29.20 ± 0.44 d |
| PV (meq O2/kg) | 5.91 ± 0.40 a | 0.24 ± 0.08 b | 0.63 ± 0.01 c | 0.30 ± 0.04 b |
| RSA (%) | 28.33 ± 0.12 b | 6.79 ± 0.20 d | 28.61 ± 0.13 a | 17.68 ± 0.15 c |
| EC50 (mg/mL) | 189.04 c | 835.83 a | 183.31 c | 315.86 b |
| Melting point (°C) | 32 ± 0 b | 36 ± 0 a | 25 ± 0 d | 31 ± 1 c |
| Tocopherols (mg/100 g) | Crude Shea Butter | Refined Shea Stearin | Refined Shea Olein | Mixture |
|---|---|---|---|---|
| α-T | 8.52 ± 0.03 a | 1.06 ± 0.03 d | 8.28 ± 0.03 b | 4.58 ± 0.08 c |
| β-T | 1.04 ± 0.02 a | 0.21 ± 0.04 d | 0.97 ± 0.05 b | 0.36 ± 0.03 c |
| γ-T | 0.36 ± 0.02 a | ND b | ND b | ND b |
| δ-T | 0.15 ± 0.03 a | ND b | ND b | ND b |
| Total | 10.06 ± 0.06 a | 1.27 ± 0.07 d | 9.25 ± 0.02 b | 4.94 ± 0.11 c |
| Sterols (mg/g) | Crude Shea Butter | Refined Shea Stearin | Refined Shea Olein | Mixture |
|---|---|---|---|---|
| Squalene | 0.20 ± 0.01 a | 0.03 ± 0.00 d | 0.05 ± 0.00 b | 0.04 ± 0.00 c |
| Lanosterol | 0.25 ± 0.01 a | 0.04 ± 0.00 c | 0.24 ± 0.00 a | 0.18 ± 0.01 b |
| α-Amyrin | 13.09 ± 1.33 a | 2.32 ± 0.03 c | 12.43 ± 0.59 a | 9.31 ± 0.23 b |
| β-Amyrin | 7.32 ± 0.75 a | 1.31 ± 0.01 c | 7.12 ± 0.39 a | 5.28 ± 0.14 b |
| Total | 20.83 ± 0.62 c | 3.69± 0.03 a | 19.85 ± 0.51 c | 14.81 ± 0.21 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdel-Razek, A.G.; Abo-Elwafa, G.A.; Al-Amrousi, E.F.; Badr, A.N.; Hassanein, M.M.M.; Qian, Y.; Siger, A.; Grygier, A.; Radziejewska-Kubzdela, E.; Rudzińska, M. Effect of Refining and Fractionation Processes on Minor Components, Fatty Acids, Antioxidant and Antimicrobial Activities of Shea Butter. Foods 2023, 12, 1626. https://doi.org/10.3390/foods12081626
Abdel-Razek AG, Abo-Elwafa GA, Al-Amrousi EF, Badr AN, Hassanein MMM, Qian Y, Siger A, Grygier A, Radziejewska-Kubzdela E, Rudzińska M. Effect of Refining and Fractionation Processes on Minor Components, Fatty Acids, Antioxidant and Antimicrobial Activities of Shea Butter. Foods. 2023; 12(8):1626. https://doi.org/10.3390/foods12081626
Chicago/Turabian StyleAbdel-Razek, Adel G., Ghada A. Abo-Elwafa, Eman F. Al-Amrousi, Ahmed N. Badr, Minar Mahmoud M. Hassanein, Ying Qian, Aleksander Siger, Anna Grygier, Elżbieta Radziejewska-Kubzdela, and Magdalena Rudzińska. 2023. "Effect of Refining and Fractionation Processes on Minor Components, Fatty Acids, Antioxidant and Antimicrobial Activities of Shea Butter" Foods 12, no. 8: 1626. https://doi.org/10.3390/foods12081626