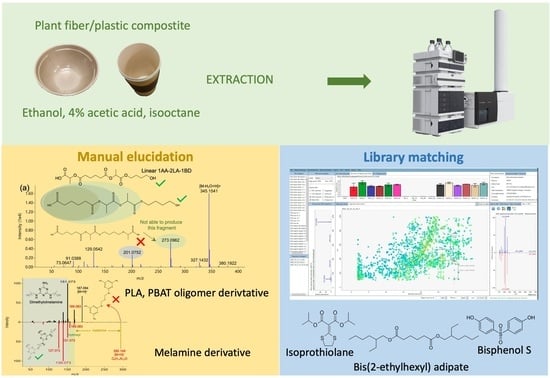
Elucidation of Non-Intentionally Added Substances from Plant Fiber/Plastic Composites by UPLC-QTOF/MS
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Samples
2.2. Compound Extraction
2.3. Analysis of Non-Volatile Substances by UPLC-QTOF/MS
2.4. Data Processing and Compound Identification
3. Results and Discussions
3.1. Identification by Matching against MS/MS Libraries
3.2. Elucidation of Melamine Derivatives
3.3. Elucidation of PLA Oligomers and Their Derivatives
3.4. Elucidation of PBAT Oligomers and Their Derivatives
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, H.; Weng, Y. Safety Risks of Plant Fiber/Plastic Composites (Ppcs) Intended for Food Contact: A Review of Potential Hazards and Risk Management Measures. Toxics 2021, 9, 343. [Google Scholar] [CrossRef] [PubMed]
- Saber, D.; Abdelnaby, A.H. Recent Developments in Natural Fiber as Reinforcement in Polymeric Composites: A Review. Asian J. Appl. Sci. Technol. 2022, 6, 56–75. [Google Scholar] [CrossRef]
- Ramesh, M.; Palanikumar, K.; Reddy, K.H. Plant Fibre Based Bio-Composites: Sustainable and Renewable Green Materials. Renew. Sustain. Energy Rev. 2017, 79, 558–584. [Google Scholar] [CrossRef]
- Väisänen, T.; Haapala, A.; Lappalainen, R.; Tomppo, L. Utilization of Agricultural and Forest Industry Waste and Residues in Natural Fiber-Polymer Composites: A Review. Waste Manag. 2016, 54, 62–73. [Google Scholar] [CrossRef] [PubMed]
- Mohanty, A.K.; Vivekanandhan, S.; Pin, J.-M.; Misra, M. Composites from Renewable and Sustainable Resources: Challenges and Innovations. Science 2018, 362, 536–542. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, L.; Dombrowski, A.; Völker, C.; Wagner, M. Are Bioplastics and Plant-Based Materials Safer than Conventional Plastics? In Vitro Toxicity and Chemical Composition. Environ. Int. 2020, 145, 106066. [Google Scholar] [CrossRef]
- Naziruddin, M.A.; Jawaid, M.; Yusof, N.L.; Abdul-Mutalib, N.A.; Ahmad, M.F.; Sanny, M.; Alzahari, A. Assessment and Detection of the Potential Contaminants from Oil Palm Empty Fruit Bunch Fiber-Based Biodegradable Tray. Food Packag. Shelf Life 2021, 29, 100685. [Google Scholar] [CrossRef]
- Welch, K. Plant Toxins. Toxicon 2019, 168, 140. [Google Scholar] [CrossRef]
- Jones, D.; Ormondroyd, G.; Curling, S.; Popescu, C.-M.; Popescu, M.-C. Chemical Compositions of Natural Fibres. In Advanced High Strength Natural Fibre Composites in Construction; Elsevier: Amsterdam, The Netherlands, 2017; pp. 23–58. [Google Scholar]
- Su, Q.Z.; Vera, P.; van de Wiele, C.; Nerín, C.; Lin, Q.B.; Zhong, H.N. Non-Target Screening of (Semi-)Volatiles in Food-Grade Polymers by Comparison of Atmospheric Pressure Gas Chromatography Quadrupole Time-of-Flight and Electron Ionization Mass Spectrometry. Talanta 2019, 202, 285–296. [Google Scholar] [CrossRef]
- Vera, P.; Canellas, E.; Nerín, C. Identification of Non Volatile Migrant Compounds and NIAS in Polypropylene Films Used as Food Packaging Characterized by UPLC-MS/QTOF. Talanta 2018, 188, 750–762. [Google Scholar] [CrossRef]
- Maiza, M.; Benaniba, M.T.; Massardier-Nageotte, V. Plasticizing Effects of Citrate Esters on Properties of Poly(Lactic Acid). J. Polym. Eng. 2016, 36, 371–380. [Google Scholar] [CrossRef]
- Nerín, C.; Alfaro, P.; Aznar, M.; Domeño, C. The Challenge of Identifying Non-Intentionally Added Substances from Food Packaging Materials: A Review. Anal. Chim. Acta 2013, 775, 14–24. [Google Scholar] [CrossRef]
- Geueke, B. Dossier—Non-Intentionally Added Substances (NIAS), 2nd ed.; CERN: Meyrin, Switzerland, 2018; pp. 1–10. [Google Scholar] [CrossRef]
- Leeman, W.; Krul, L. Non-Intentionally Added Substances in Food Contact Materials: How to Ensure Consumer Safety. Curr. Opin. Food Sci. 2015, 6, 33–37. [Google Scholar] [CrossRef]
- Federal Office of Consumer Protection and Food Safety. Report on the National Monitoring; Federal Office of Consumer Protection and Food Safety: Braunschweig, Germany, 2020. [Google Scholar]
- Petrova, S.P.; Christova-Bagdassarian, V. Migration of Melamine and Formaldehyde from Kids’ Bamboo Dinner Sets. Food Environ. Saf. J. 2021, 20. [Google Scholar] [CrossRef]
- Ubeda, S.; Aznar, M.; Nerín, C. Determination of Oligomers in Virgin and Recycled Polyethylene Terephthalate (PET) Samples by UPLC-MS-QTOF. Anal. Bioanal. Chem. 2018, 410, 2377–2384. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Lee, Y.J.; Koo, Y.J.; Pack, E.C.; Lim, K.M.; Choi, D.W. Migration of Monomers, Plastic Additives, and Non-Intentionally Added Substances from Food Utensils Made of Melamine–Formaldehyde Resin Following Ultraviolet Sterilization. Food Control 2021, 125, 107981. [Google Scholar] [CrossRef]
- Osorio, J.; Aznar, M.; Nerín, C.; Birse, N.; Elliott, C.; Chevallier, O. Ambient Mass Spectrometry as a Tool for a Rapid and Simultaneous Determination of Migrants Coming from a Bamboo-Based Biopolymer Packaging. J. Hazard. Mater. 2020, 398, 122891. [Google Scholar] [CrossRef]
- Wrona, M.; Nerín, C. Analytical Approaches for Analysis of Safety of Modern Food Packaging: A Review. Molecules 2020, 25, 752. [Google Scholar] [CrossRef]
- Peters, R.J.B.; Groeneveld, I.; Sanchez, P.L.; Gebbink, W.; Gersen, A.; de Nijs, M.; van Leeuwen, S.P.J. Review of Analytical Approaches for the Identification of Non-Intentionally Added Substances in Paper and Board Food Contact Materials. Trends Food Sci. Technol. 2019, 85, 44–54. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.; Carver, J.J.; Phelan, V.V.; Sanchez, L.M.; Garg, N.; Peng, Y.; Nguyen, D.D.; Watrous, J.; Kapono, C.A.; Luzzatto-Knaan, T.; et al. Sharing and Community Curation of Mass Spectrometry Data with Global Natural Products Social Molecular Networking. Nat. Biotechnol. 2016, 34, 828–837. [Google Scholar] [CrossRef]
- Patlewicz, G.; Jeliazkova, N.; Safford, R.J.; Worth, A.P.; Aleksiev, B. An Evaluation of the Implementation of the Cramer Classification Scheme in the Toxtree Software. SAR QSAR Environ. Res. 2008, 19, 495–524. [Google Scholar] [CrossRef] [PubMed]
- Tsochatzis, E.D.; Alberto Lopes, J.; Hoekstra, E.; Emons, H. Development and Validation of a Multi-Analyte GC-MS Method for the Determination of 84 Substances from Plastic Food Contact Materials. Anal. Bioanal. Chem. 2020, 412, 5419–5434. [Google Scholar] [CrossRef] [PubMed]
- Deshwal, G.K.; Panjagari, N.R.; Alam, T. An Overview of Paper and Paper Based Food Packaging Materials: Health Safety and Environmental Concerns. J. Food Sci. Technol. 2019, 56, 4391–4403. [Google Scholar] [CrossRef] [PubMed]
- European Union. European Commission Regulation (EC) No 10/2011 on Plastic Materials and Articles Intened to Come into Contact with Food. Off. J. Eur. Union 2011, 45, 42–130. [Google Scholar]
- Qiu, W.; Zhan, H.; Hu, J.; Zhang, T.; Xu, H.; Wong, M.; Xu, B.; Zheng, C. The Occurrence, Potential Toxicity, and Toxicity Mechanism of Bisphenol S, a Substitute of Bisphenol A: A Critical Review of Recent Progress. Ecotoxicol. Environ. Saf. 2019, 173, 192–202. [Google Scholar] [CrossRef]
- Viñas, P.; Campillo, N.; Martínez-Castillo, N.; Hernández-Córdoba, M. Comparison of Two Derivatization-Based Methods for Solid-Phase Microextraction-Gas Chromatography-Mass Spectrometric Determination of Bisphenol A, Bisphenol S and Biphenol Migrated from Food Cans. Anal. Bioanal. Chem. 2010, 397, 115–125. [Google Scholar] [CrossRef]
- Liao, C.; Liu, F.; Kannan, K. Bisphenol S, a New Bisphenol Analogue, in Paper Products and Currency Bills and Its Association with Bisphenol a Residues. Environ. Sci. Technol. 2012, 46, 6515–6522. [Google Scholar] [CrossRef]
- Pivnenko, K.; Pedersen, G.A.; Eriksson, E.; Astrup, T.F. Bisphenol A and Its Structural Analogues in Household Waste Paper. Waste Manag. 2015, 44, 39–47. [Google Scholar] [CrossRef]
- Kruve, A.; Kaupmees, K.; Liigand, J.; Oss, M.; Leito, I. Sodium Adduct Formation Efficiency in ESI Source. J. Mass Spectrom. 2013, 48, 695–702. [Google Scholar] [CrossRef]
- Tsugawa, H.; Kind, T.; Nakabayashi, R.; Yukihira, D.; Tanaka, W.; Cajka, T.; Saito, K.; Fiehn, O.; Arita, M. Hydrogen Rearrangement Rules: Computational MS/MS Fragmentation and Structure Elucidation Using MS-FINDER Software. Anal. Chem. 2016, 88, 7946–7958. [Google Scholar] [CrossRef]
- Ebner, I.; Haberer, S.; Sander, S.; Kappenstein, O.; Luch, A.; Bruhn, T. Release of Melamine and Formaldehyde from Melamine-Formaldehyde Plastic Kitchenware. Molecules 2020, 25, 3629. [Google Scholar] [CrossRef] [PubMed]
- Ubeda, S.; Aznar, M.; Alfaro, P.; Nerín, C. Migration of Oligomers from a Food Contact Biopolymer Based on Polylactic Acid (PLA) and Polyester. Anal. Bioanal. Chem. 2019, 411, 3521–3532. [Google Scholar] [CrossRef] [PubMed]
- Kruve, A.; Kaupmees, K.; Liigand, J.; Leito, I. Negative Electrospray Ionization via Deprotonation: Predicting the Ionization Efficiency. Anal. Chem. 2014, 86, 4822–4830. [Google Scholar] [CrossRef] [PubMed]
- Aznar, M.; Ubeda, S.; Dreolin, N.; Nerín, C. Determination of Non-Volatile Components of a Biodegradable Food Packaging Material Based on Polyester and Polylactic Acid (PLA) and Its Migration to Food Simulants. J. Chromatogr. A 2019, 1583, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, D.; Wu, F.; Mohanty, A.K.; Hirai, S.; Misra, M. Biodegradable Composites Developed from PBAT/PLA Binary Blends and Silk Powder: Compatibilization and Performance Evaluation. ACS Omega 2018, 3, 12412–12421. [Google Scholar] [CrossRef] [Green Version]





| Number | Plant Fiber | Plastic | Type | Weight (g) | Area (dm2) |
|---|---|---|---|---|---|
| 1# | Bamboo | MF | Bowl | 86 | 1.9 |
| 2# | Bamboo | MF | Bowl | 212 | 3.2 |
| 3# | Bamboo | PP | Cup | 125 | 3.4 |
| 4# | Rice husk | PP | Bowl | 238 | 3.4 |
| 5# | Rice husk | PP | Bowl | 128 | 1.9 |
| 6# | Rice husk | PLA | Cup | 78 | 1.7 |
| Nº | RT (min) | Precursor Ion | Adduct | Name | CAS | Formula | SML or CR | Score | Sample |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2.12 | 150.1127 | [M + H]+ | Triethanolamine | 102-71-6 | C6H15NO3 | 0.05 | 97 | E; #1, #2, #3, #4, #5, #6 |
| 2 | 2.33 | 127.0732 | [M + H]+ | Melamine | 108-78-1 | C3H6N6 | 2.5 | 93 | E, H; #1, #2 |
| 3 | 2.36 | 365.1048 | [M + Na]+ | Sucrose | 57-50-1 | C12H22O11 | III | 86 | E; #1, #2, #3, #4, #5, #6 |
| 4 * | 2.62 | 157.0835 | [M + H]+ | Methylolmelamine | 937-35-9 | C4H8N6O | III | - | H; #1, #2 |
| 5 | 3.02 | 144.0653 | [M + H − H2O]+ | N-Methyl-L-glutamic acid | 35989-16-3 | C6H11NO4 | III | 81 | E; #5 |
| 6 * | 3.29 | 187.0937 | [M + H]+ | Dimethylol melamine | 5001-80-9 | C5H10N6O2 | III | - | H; #1, #2 |
| 7 * | 4.45 | 295.1487 | [M + H]+ | ((4-amino-6-((((4,6-diamino-1,3,5-triazin-2-yl)amino)methyl)amino)-1,3,5-triazin-2-yl)amino)methanol | - | C8H14N12O | III | - | H; #1, #2 |
| 8 * | 5.68 | 325.1593 | [M + H]+ | (((methylenebis(azanediyl))bis(6-amino-1,3,5-triazine-4,2-diyl))bis(azanediyl))dimethanol | - | C9H16N12O2 | III | - | H; #1, #2 |
| 9º | 8.12 | 161.0463 | [M − H]− | Linear 2LA | - | C6H10O5 | I | - | E; #6 |
| 10 | 8.63 | 250.1439 | [M + H]+ | 4-Coumaroylcholine | - | C14H20NO3 | III | 87 | E; #1, #2, #3, #6 |
| 11 | 8.84 | 171.0124 | [M − H]− | p-Toluenesulfonic acid | 104-15-4 | C7H8O3S | I | 81 | E, M; #6 |
| 12 | 8.90 | 359.0979 | [M − H]− | 1-O-(3-Hydroxy-4,5-dimethoxybenzoyl)hexopyranose | - | C15H20O10 | III | 76 | H; #1 |
| 13 | 9.00 | 145.0510 | [M − H]− | adipic acid | 124-04-9 | C6H10O4 | I | 94 | E; #6 |
| 14 | 9.46 | 137.0245 | [M − H]− | 2,5-Dihydroxybenzaldehyde | 1194-98-5 | C7H6O3 | I | 89 | E; #4, #6 |
| 15 | 9.91 | 327.1081 | [M − H]− | 3-(4-Hydroxyphenyl)-3-oxopropyl.beta.-D-glucopyranoside | - | C15H20O8 | III | 81 | H; #1 |
| 16 | 10.27 | 137.0247 | [M − H]− | 4-Hydroxybenzoic acid | 99-96-7 | C7H6O3 | I | 88 | E; #2, #3, #4, #6 |
| 17 | 10.34 | 165.0556 | [M − H]− | 3,4’-Dihydroxypropiophenone | 53170-93-7 | C9H10O3 | I | 78 | E; #1, #2, #3, #4, #5, #6 |
| 18 | 10.35 | 367.1028 | [M − H]− | 5-O-Feruloylquinic acid | 62929-69-5 | C17H20O9 | II | 78 | E; #2, #3 |
| 19 | 10.48 | 496.2020 | [M + NH4]+ | Kelampayoside A | 87562-76-3 | C20H30O13 | III | 84 | E; #2 |
| 20 | 11.34 | 121.0296 | [M − H]− | 4-Hydroxybenzaldehyde | 123-08-0 | C7H6O2 | I | 89 | E, H; #1, #2, #3, #4, #5, #6 |
| 21º | 12.06 | 252.1078 | [M + NH4]+ | Linear 3LA | - | C9H14O7 | I | - | E; #6 |
| 22 | 12.33 | 367.1026 | [M − H]− | 1,3,5-Trihydroxy-4-(((2E)-3-(3-hydroxy-4-methoxyphenyl)prop-2-enoyl)oxy)cyclohexanecarboxylic acid | - | C16H20O10 | III | 77 | E; #2, #3, #6 |
| 23 | 12.65 | 183.0651 | [M + H]+ | Syringaldehyde | 134-96-3 | C9H10O4 | I | 87 | E, M; #1, #2, #3, #4, #5, #6 |
| 24º | 12.76 | 252.1442 | [M + NH4]+ | Linear 2LA-1BD | - | C10H18O6 | I | - | E; #6 |
| 25 | 12.76 | 371.0971 | [M − H]− | 3-(Benzoyloxy)-2-hydroxypropyl.beta.-D-glucopyranosiduronic acid | - | C16H20O10 | III | 77 | E; #2, #3, #6 |
| 26 | 12.87 | 760.3018 | [M + NH4]+ | Syringaresinol diglucoside | 96038-87-8 | C34H46O18 | III | 82 | H; #1, #2 |
| 27 | 12.89 | 787.2673 | [M + FA − H]− | Eleutheroside E | 39432-56-9 | C34H46O18 | III | 75 | H; #1 |
| 28 | 13.24 | 163.0405 | [M − H]− | trans-4-Coumaric acid | 501-98-4 | C9H8O3 | I | 94 | E; #1, #2, #3, #4, #5, #6 |
| 29º | 13.64 | 236.1488 | [M + NH4]+ | Linear 1AA-1BD | - | C10H18O5 | I | - | E; #6 |
| 30 | 13.86 | 147.0456 | [M − H − H2O]− | 3-(2-Hydroxyphenyl)propionic acid | 495-78-3 | C9H10O3 | I | 90 | E; #1, #2, #3, #4, #5, #6 |
| 31 | 13.99 | 251.0372 | [M + H]+ | Bisphenol S | 80-09-1 | C12H10O4S | 0.05 | 89 | E, H; #1, #2, #3, #4, #5, #6 |
| 32 | 14.19 | 207.0667 | [M − H]− | trans-3,5-Dimethoxy-4-hydroxycinnamaldehyde | 4206-58-0 | C11H12O4 | I | 84 | E; #1, #2, #3, #4, #5, #6 |
| 33 | 14.45 | 598.2492 | [M + NH4]+ | (-)-Syringaresinol-4-O-.beta.-D-glucopyranoside | 7374-79-0 | C28H36O13 | III | 79 | E; #1, #2, #3, #6 |
| 34º | 14.73 | 266.1231 | [M + NH4]+ | Linear 3LA-MeOH | - | C10H16O7 | I | - | M; #6 |
| 35º | 14.88 | 324.1297 | [M + NH4]+ | Linear 4LA | - | C12H18O9 | I | - | E; #6 |
| 36 | 15.88 | 183.0780 | [M + H]+ | Triethyl phosphate | 78-40-0 | C6H15O4P | III | 90 | E; #6 |
| 37º | 15.89 | 291.1438 | [M + H]+ | Linear 1AA-1LA-1BD | - | C13H22O7 | I | - | E; #6 |
| 38º | 16.28 | 291.1805 | [M + H]+ | Linear 1AA-2BD | - | C14H26O6 | I | - | E; #6 |
| 39º | 16.68 | 280.1391 | [M + NH4]+ | Linear 3LA-EtOH | - | C11H18O7 | I | - | E, M; #6 |
| 40 | 16.84 | 441.2018 | [M + H]+ | Diferuloyl putrescine | 42369-86-8 | C24H28N2O6 | III | 83 | E; #1, #2, #3, #6 |
| 41º | 16.95 | 396.1513 | [M + NH4]+ | Linear 5LA | - | C15H22O11 | I | - | E; #6 |
| 42º | 16.97 | 338.1443 | [M + NH4]+ | Linear 4LA-MeOH | - | C13H20O9 | I | - | M; #6 |
| 43º | 17.50 | 380.1917 | [M + NH4]+ | Linear 1AA-2LA-1BD | - | C16H26O9 | I | - | E; #6 |
| 44º | 17.81 | 380.2279 | [M + NH4]+ | Linear 1AA-1LA-2BD | - | C17H30O8 | I | - | E; #6 |
| 45º | 18.13 | 364.1971 | [M + NH4]+ | Linear 2AA-1BD | - | C16H26O8 | I | - | E; #6 |
| 46º | 18.39 | 468.1723 | [M + NH4]+ | Linear 6LA | - | C18H26O13 | III | - | E; #6 |
| 47º | 18.52 | 352.1606 | [M + NH4]+ | Linear 4LA-EtOH | - | C14H22O9 | I | - | E, M; #6 |
| 48º | 18.60 | 410.1658 | [M + NH4]+ | Linear 5LA-MeOH | - | C16H24O11 | III | - | M; #6 |
| 49º | 18.81 | 378.1398 | [M + NH4]+ | Cyclic 5LA | - | C15H20O10 | I | - | E; #6 |
| 50º | 18.96 | 452.2493 | [M + NH4]+ | Linear 1AA-2LA-2BD | - | C20H34O10 | I | - | E; #6 |
| 51º | 19.45 | 436.2546 | [M + NH4]+ | Linear 2AA-2BD | - | C20H34O9 | I | - | E; #6 |
| 52º | 19.54 | 540.1935 | [M + NH4]+ | Linear 7LA | - | C21H30O15 | III | - | E; #6 |
| 53º | 19.80 | 482.1865 | [M + NH4]+ | Linear 6LA-MeOH | - | C19H28O13 | III | - | M; #6 |
| 54º | 19.86 | 424.1816 | [M + NH4]+ | Linear 5LA-EtOH | - | C17H26O11 | III | - | E; #6 |
| 55º | 20.09 | 384.1652 | [M + NH4]+ | Linear 1TPA-1AA-1BD | - | C18H22O8 | I | - | E; #6 |
| 56º | 20.24 | 450.1609 | [M + NH4]+ | Cyclic 6LA | - | C18H24O12 | I | - | E, M; #6 |
| 57º | 20.45 | 612.2145 | [M + NH4]+ | Linear 8LA | - | C24H34O17 | III | - | E; #6 |
| 58º | 20.49 | 508.3116 | [M + NH4]+ | Linear 2AA-3BD | - | C24H42O10 | I | - | E; #6 |
| 59º | 20.74 | 554.2076 | [M + NH4]+ | Linear 7LA-MeOH | - | C22H32O15 | III | - | M; #6 |
| 60º | 20.84 | 496.2035 | [M + NH4]+ | Linear 6LA-EtOH | - | C20H30O13 | III | - | E; #6 |
| 61º | 20.97 | 580.2963 | [M + NH4]+ | Linear 2AA-2LA-2BD | - | C26H42O13 | III | - | E; #6 |
| 62º | 21.13 | 580.3326 | [M + NH4]+ | Linear 2AA-1LA-3BD | - | C27H46O12 | III | - | E; #6 |
| 63º | 21.18 | 689.1904 | [M + Na]+ | Linear 9LA | - | C27H38O19 | III | - | E; #6 |
| 64º | 21.22 | 522.1820 | [M + NH4]+ | Cyclic 7LA | - | C21H28O14 | I | - | E, M; #6 |
| 65º | 21.23 | 456.2225 | [M + NH4]+ | Linear 1TPA-1AA-2BD | - | C22H30O9 | I | - | E; #6 |
| 66º | 21.30 | 564.3017 | [M + NH4]+ | Linear 3AA-2BD | - | C26H42O12 | I | - | E; #6 |
| 67º | 21.48 | 626.2288 | [M + NH4]+ | Linear 8LA-MeOH | - | C25H36O17 | III | - | M; #6 |
| 68º | 21.53 | 568.2238 | [M + NH4]+ | Linear 7LA-EtOH | - | C23H34O15 | III | - | E; #6 |
| 69º | 21.61 | 418.2438 | [M + NH4]+ | Cyclic 2AA-2BD | - | C20H32O8 | I | - | E, M; #6 |
| 70º | 21.72 | 652.3535 | [M + NH4]+ | Linear 2AA-2LA-3BD | - | C30H50O14 | III | - | E; #6 |
| 71 | 21.72 | 291.0716 | [M + H]+ | Isoprothiolane | 50512-35-1 | C12H18O4S2 | III | 86 | E, M; #5 |
| 72º | 21.75 | 756.2562 | [M + NH4]+ | Linear 10LA | - | C30H42O21 | III | - | E, M; #6 |
| 73º | 21.80 | 594.2036 | [M + NH4]+ | Cyclic 8LA | - | C24H32O16 | I | - | E; #6 |
| 74º | 21.94 | 636.3594 | [M + NH4]+ | Linear 3AA-3BD | - | C30H50O13 | III | - | E; #6 |
| 75º | 22.01 | 528.2801 | [M + H]+ | Linear 1TPA-1AA-3BD | - | C26H38O10 | I | - | E; #6 |
| 76º | 22.23 | 640.2454 | [M + NH4]+ | Linear 8LA-EtOH | - | C26H38O17 | III | - | E; #6 |
| 77º | 22.27 | 828.2771 | [M + NH4]+ | Linear 11LA | - | C33H46O23 | III | - | E; #6 |
| 78º | 22.49 | 708.4163 | [M + NH4]+ | Linear 3AA-4BD | - | C34H58O14 | III | - | E; #6 |
| 79º | 22.55 | 666.2253 | [M + NH4]+ | Cyclic 9LA | - | C27H36O18 | I | - | E; #6 |
| 80º | 22.58 | 770.2703 | [M + NH4]+ | Linear 10LA-MeOH | - | C31H44O21 | III | - | M; #6 |
| 81º | 22.69 | 900.2977 | [M + H]+ | Linear 12LA | - | C36H50O25 | III | - | E; #6 |
| 82º | 22.73 | 712.2661 | [M + NH4]+ | Linear 9LA-EtOH | - | C29H42O19 | III | - | E; #6 |
| 83º | 22.75 | 584.2704 | [M + NH4]+ | Linear 1TPA-2AA-2BD | - | C28H38O12 | I | - | E; #6 |
| 84º | 22.96 | 764.4058 | [M + NH4]+ | Linear 4AA-3BD | - | C36H58O16 | III | - | E; #6 |
| 85º | 22.99 | 842.2909 | [M + NH4]+ | Linear 11LA-MeOH | - | C34H48O23 | III | - | M; #6 |
| 86º | 23.04 | 972.3190 | [M + NH4]+ | Linear 13LA | - | C39H54O27 | III | - | E; #6 |
| 87º | 23.16 | 784.2859 | [M + NH4]+ | Linear 10LA-EtOH | - | C32H46O21 | III | - | E; #6 |
| 88º | 23.20 | 738.2455 | [M + NH4]+ | Cyclic 10LA | - | C30H40O20 | I | - | E; #6 |
| 89º | 23.25 | 656.3275 | [M + NH4]+ | Linear 1TPA-2AA-3BD | - | C32H46O13 | I | - | E; #6 |
| 90º | 23.36 | 914.3131 | [M + NH4]+ | Linear 12LA-MeOH | - | C37H52O25 | III | - | M; #6 |
| 91º | 23.37 | 836.4641 | [M + NH4]+ | Linear 4AA-4BD | - | C40H66O17 | III | - | E; #6 |
| 92º | 23.40 | 438.2122 | [M + NH4]+ | Cyclic 1TPA-1AA-2BD | - | C22H30O9 | I | - | E, M; #6 |
| 93º | 23.51 | 856.3078 | [M + NH4]+ | Linear 11LA-EtOH | - | C35H50O23 | III | - | E; #6 |
| 94º | 23.54 | 728.3851 | [M + NH4]+ | Linear 1TPA-2AA-4BD | - | C36H54O14 | I | - | E; #6 |
| 95º | 23.65 | 810.2664 | [M + NH4]+ | Cyclic 11LA | - | C33H44O22 | I | - | E; #6 |
| 96º | 23.81 | 928.3292 | [M + NH4]+ | Linear 12LA-EtOH | - | C38H54O25 | III | - | E; #6 |
| 97º | 23.84 | 618.3482 | [M + NH4]+ | Cyclic 3AA-3BD | - | C30H48O12 | I | - | E, M; #6 |
| 98º | 24.01 | 784.3750 | [M + NH4]+ | Linear 1TPA-3AA-3BD | - | C38H54O16 | I | - | E; #6 |
| 99 | 24.08 | 318.2999 | [M + H]+ | Phytosphingosine | 554-62-1 | C18H39NO3 | II | 82 | E, M; #4, #5 |
| 100º | 24.10 | 882.2875 | [M + NH4]+ | Cyclic 12LA | - | C36H48O24 | I | - | E; #6 |
| 101º | 24.32 | 676.2963 | [M + NH4]+ | Linear 2TPA-1AA-3BD | - | C34H42O13 | I | - | E; #6 |
| 102º | 24.33 | 856.4323 | [M + NH4]+ | Linear 1TPA-3AA-4BD | - | C42H62O17 | I | - | E; #6 |
| 103º | 24.41 | 954.3080 | [M + NH4]+ | Cyclic 13LA | - | C39H52O26 | I | - | E; #6 |
| 104º | 24.51 | 928.4896 | [M + NH4]+ | Linear 1TPA-3AA-5BD | - | C46H70O18 | I | - | E; #6 |
| 105º | 24.65 | 748.3532 | [M + NH4]+ | Linear 2TPA-1AA-4BD | - | C38H50O14 | I | - | E; #6 |
| 106º | 24.83 | 984.4794 | [M + NH4]+ | Linear 1TPA-4AA-4BD | - | C48H70O20 | I | - | E; #6 |
| 107º | 25.07 | 638.3167 | [M + NH4]+ | Cyclic 1TPA-2AA-3BD | - | C32H44O12 | III | - | E, M; #6 |
| 108º | 25.18 | 876.4003 | [M + NH4]+ | Linear 2TPA-2AA-4BD | - | C44H58O17 | I | - | E; #6 |
| 109º | 25.28 | 948.4576 | [M + NH4]+ | Linear 2TPA-2AA-5BD | - | C48H66O18 | I | - | E; #6 |
| 110 | 25.74 | 403.2340 | [M + H]+ | Tributyl acetylcitrate (ATBC) | 77-90-7 | C20H34O8 | 60 | 89 | E, M; #6 |
| 111º | 26.33 | 658.2849 | [M + NH4]+ | Cyclic 2TPA-1AA-3BD | - | C34H40O12 | III | - | E, M; #6 |
| 112 | 26.52 | 343.2117 | [M + H]+ | Tributyl aconitate | 7568-58-3 | C18H30O6 | I | - | E, M; #6 |
| 113º | 26.79 | 858.3901 | [M + NH4]+ | Cyclic 2TPA-2AA-4BD | - | C44H56O16 | III | - | E, M; #6 |
| 114 | 27.27 | 496.3391 | [M + H]+ | 1-Palmitoyl-sn-glycero-3-phosphocholine | 17364-16-8 | C24H50NO7P | III | 88 | E; #2, #3, #5 |
| 115 | 29.60 | 371.3158 | [M + H]+ | Bis(2-ethylhexyl) adipate | 103-23-1 | C22H42O4 | 18 | 93 | E, M; #1, #2, #3, #4, #5, #6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Su, Q.-Z.; Shang, G.-Q.; Weng, Y.-X.; Zhu, L. Elucidation of Non-Intentionally Added Substances from Plant Fiber/Plastic Composites by UPLC-QTOF/MS. Foods 2023, 12, 678. https://doi.org/10.3390/foods12030678
Zhang H, Su Q-Z, Shang G-Q, Weng Y-X, Zhu L. Elucidation of Non-Intentionally Added Substances from Plant Fiber/Plastic Composites by UPLC-QTOF/MS. Foods. 2023; 12(3):678. https://doi.org/10.3390/foods12030678
Chicago/Turabian StyleZhang, Hong, Qi-Zhi Su, Gui-Qin Shang, Yun-Xuan Weng, and Lei Zhu. 2023. "Elucidation of Non-Intentionally Added Substances from Plant Fiber/Plastic Composites by UPLC-QTOF/MS" Foods 12, no. 3: 678. https://doi.org/10.3390/foods12030678