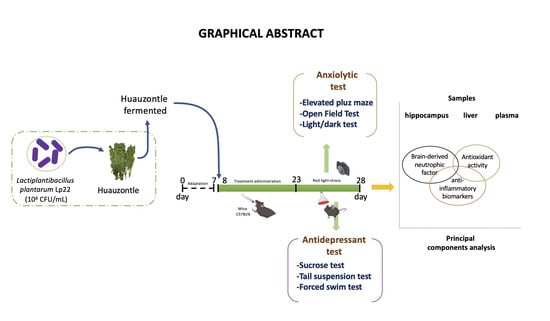
Antidepressant and Anxiolytic Effects of Fermented Huauzontle, a Prehispanic Mexican Pseudocereal
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Culture and Preparation of Fermented Huauzontle
2.2. Experimental Animals
2.3. Induced Stress Mice and Behavioural Testing
2.3.1. Anxiolytic Evaluation
2.3.2. Antidepressant Evaluation
2.4. Sample Collection
Determination of Biomarkers Associated with Oxidative Stress and Inflammatory Response
2.5. Statistical Analysis
3. Results and Discussion
3.1. Locomotor Activity and Reduced the Anxiety-like Behaviour
3.2. Depressive-like Behaviour Mice
3.3. Biochemical Parameters
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Assad-Bustillos, M.; Ramírez-Gilly, M.; Tecante, A.; Chaires-Martínez, L. Physicochemical, functional, thermal and rheological characterization of starch from huauzontle seeds (Chenopodium berlandieri spp. nuttalliae). Agrociencia 2014, 48, 789–803. [Google Scholar]
- Sánchez-Villa, C.E.; Zepeda-Bautista, R.; Ramírez-Ortiz, M.E.; Corzo-Ríos, L.J. Nixtamalized tortillas supplemented with proteins isolated from Phaseolus coccineus and huauzontle (Chenopodium berlandieri subsp. Nuttalliae) flour: Rheological, textural, and sensorial properties. Int. J. Gastron. Food Sci. 2020, 22, 100274. [Google Scholar] [CrossRef]
- Garcia, H.S.; Santiago-López, L.; González-Córdova, A.F.; Vallejo-Cordoba, B.; Hernández-Mendoza, A. Evaluation of a pseudocereal suitability to prepare a functional fermented beverage with epiphytic lactic acid bacteria of Huauzontle (Chenopodium berlandieri spp. nuttalliae). LWT 2022, 155, 112913. [Google Scholar] [CrossRef]
- Rodríguez-Yoldi, M.J. Anti-inflammatory and antioxidant properties of plant extracts. Antioxidants 2021, 10, 921. [Google Scholar] [CrossRef]
- Rawdin, B.J.; Mellon, S.H.; Dhabhar, F.S.; Epel, E.S.; Puterman, E.; Su, Y.; Burke, H.M.; Reus, V.I.; Rosser, R.; Hamilton, S.P.; et al. Dysregulated relationship of inflammation and oxidative stress in major depression. Brain. Behav. Immun. 2013, 31, 143–152. [Google Scholar] [CrossRef] [Green Version]
- Rahman, T.; Hosen, I.; Islam, M.T.; Shekhar, H.U. Oxidative stress and human health. Adv. Biosci. Biotechnol. 2012, 3, 997–1019. [Google Scholar] [CrossRef] [Green Version]
- Daly, M.; Robinson, E. Depression and anxiety during COVID-19. Lancet 2022, 399, 518. [Google Scholar] [CrossRef]
- Santomauro, D.F.; Herrera, A.M.M.; Shadid, J.; Zheng, P.; Ashbaugh, C.; Pigott, D.M.; Ferrari, A.J. Global prevalence and burden of depressive and anxiety disorders in 204 countries and territories in 2020 due to the COVID-19 pandemic. Lancet 2021, 398, 1700–1712. [Google Scholar] [CrossRef]
- Guevara, C.A.; Del Valle, P.; Mercedes, C.R. Microglia and reactive oxygen species are required for behavioral susceptibility to chronic social defeat stress. J. Neurosci. 2020, 40, 1370–1372. [Google Scholar] [CrossRef]
- Dang, R.; Wang, M.; Li, X.; Wang, H.; Liu, L.; Wu, Q.; Zhao, J.; Ji, P.; Zhong, L.; Licinio, J.; et al. Edaravone ameliorates depressive and anxiety-like behaviors via Sirt1/Nrf2/HO-1/Gpx4 pathway. J. Neuroinflammation 2022, 19, 1–29. [Google Scholar] [CrossRef] [PubMed]
- Gartlehner, G.; Wagner, G.; Matyas, N.; Titscher, V.; Greimel, J.; Lux, L.; Gaynes, B.N.; Viswnathan, M.; Patel, S.; Lohr, K.N. Pharmacological and non-pharmacological treatments for major depressive disorder: Review of systematic reviews. BMJ Open 2017, 7, e014912. [Google Scholar] [CrossRef] [PubMed]
- Rajaram, S.; Jones, J.; Lee, G.J. Plant-based dietary patterns, plant foods, and age-related cognitive decline. Adv. Nutr. 2019, 10, S422–S436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kenda, M.; Kočevar Glavač, N.; Nagy, M.; Sollner Dolenc, M. Medicinal Plants Used for Anxiety, Depression, or Stress Treatment: An Update. Molecules 2022, 27, 6021. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.S.; Eweys, A.S.; Zhang, J.Y.; Zhu, Y.; Bai, J.; Darwesh, O.M.; Zhang, H.-B.; Xiao, X. Fermentation affects the antioxidant activity of Plant-Based food material through the release and production of bioactive components. Antioxidants 2021, 10, 2004. [Google Scholar] [CrossRef]
- Tortitas de Huauzontle. Nutrición Con Sabor; Seguridad Alimentaria Mexicana: Zacatecas, Zacatecas, Mexico, 2022; No. 11. [Google Scholar]
- National Research Council (US). Committee for the Update for the Care and Use of Laboratory Animals, 8th ed.; National Academies Press: Washington, DA, USA, 2011. [Google Scholar] [CrossRef]
- Arifin, W.N.; Zahiruddin, W.M. Sample Size Calculation in Animal Studies Using Resource Equation Approach. Malays. J. Med. Sci. 2017, 24, 101–105. [Google Scholar]
- Janhavi, P.; Divyashree, S.; Sanjailal, K.P.; Muthukumar, S.P. DoseCal: A virtual calculator for dosage conversion between human and different animal species. Arch. Physiol. Biochem. 2022, 128, 426–430. [Google Scholar] [CrossRef] [PubMed]
- He, D.; Wang, X.; Zhang, P.; Luo, X.; Li, X.; Wang, L.; Li, S.; Xu, Y. Evaluation of the anxiolytic and antidepressant activities of the aqueous extract from Camellia euphlebia Merr. ex Sealy in mice. Evid. Based Complementary Altern. Med. 2015, 2015, 618409. [Google Scholar] [CrossRef] [Green Version]
- Torrisi, S.A.; Geraci, F.; Tropea, M.R.; Grasso, M.; Caruso, G.; Fidilio, A.; Musso, N.; Sanfilippo, G.; Tascedda, F.; Palmeri, A.; et al. Fluoxetine and vortioxetine reverse depressive-like phenotype and memory deficits induced by Aβ1-42 oligomers in mice: A key role of transforming growth factor-β1. Front. Pharmacol. 2019, 10, 693. [Google Scholar] [CrossRef]
- Stenman, L.K.; Patterson, E.; Meunier, J.; Roman, F.J.; Lehtinen, M.J. Strain specific stress-modulating effects of candidate probiotics: A systematic screening in a mouse model of chronic restraint stress. Behav. Brain Res. 2020, 379, 112376. [Google Scholar] [CrossRef]
- Xie, B.; Zhang, Y.; Qi, H.; Yao, H.; Shang, Y.; Yuan, S.; Zhang, J. Red light exaggerated sepsis-induced learning impairments and anxiety-like behaviors. Aging 2020, 12, 23739. [Google Scholar] [CrossRef]
- Rodriguez, A.; Zhang, H.; Klaminder, J.; Brodin, T.; Andersson, P.L.; Andersson, M. ToxTrac: A fast and robust software for tracking organisms. Methods Ecol. Evol. 2018, 9, 460–464. [Google Scholar] [CrossRef]
- Komada, M.; Takao, K.; Miyakawa, T. Elevated plus maze for mice. JoVE 2008, 22, e1088. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramalho, J.B.; Soares, M.B.; Spiazzi, C.C.; Bicca, D.F.; Soares, V.M.; Pereira, J.G.; da Silva, W.P.; Sehn, C.P.; Cibin, F.W. In vitro probiotic and antioxidant potential of Lactococcus lactis subsp. cremoris LL95 and its effect in mice behaviour. Nutrients 2019, 11, 901. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seibenhener, M.L.; Wooten, M.C. Use of the open field maze to measure locomotor and anxiety-like behavior in mice. JoVE 2015, 96, e52434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takao, K.; Miyakawa, T. Light/dark transition test for mice. JoVE 2006, 1, e104. [Google Scholar] [CrossRef] [PubMed]
- Serchov, T.; van Calker, D.; Biber, K. Light/dark transition test to assess anxiety-like behavior in mice. Bio-Protocol 2016, 6, e1957. [Google Scholar] [CrossRef]
- Liu, M.Y.; Yin, C.Y.; Zhu, L.J.; Zhu, X.H.; Xu, C.; Luo, C.X.; Chen, H.; Zhu, D.-Y.; Zhou, Q.G. Sucrose preference test for measurement of stress-induced anhedonia in mice. Nat. Protoc. 2018, 13, 1686–1698. [Google Scholar] [CrossRef]
- Yankelevitch-Yahav, R.; Franko, M.; Huly, A.; Doron, R. The forced swim test as a model of depressive-like behavior. JoVE 2015, 97, e52587. [Google Scholar] [CrossRef] [Green Version]
- Guerrero-Encinas, I.; González-González, J.N.; Santiago-López, L.; Muhlia-Almazán, A.; Garcia, H.S.; Mazorra-Manzano, M.A.; Vallejo-Córdoba, B.; González-Córdova, A.F.; Hernández-Mendoza, A. Protective effect of Lacticaseibacillus casei CRL 431 postbiotics on mitochondrial function and oxidative status in rats with aflatoxin b1–induced oxidative stress. Probiotics Antimicrob. Proteins 2021, 13, 1033–1043. [Google Scholar] [CrossRef]
- Cuevas-González, P.F.; Aguilar-Toalá, J.E.; García, H.S.; González-Córdova, A.F.; Vallejo-Cordoba, B.; Hernández-Mendoza, A. Protective Effect of the Intracellular Content from Potential Probiotic Bacteria against Oxidative Damage Induced by Acrylamide in Human Erythrocytes. Probiotics Antimicrob. Proteins 2020, 12, 1459–1470. [Google Scholar] [CrossRef]
- Flohé, L.; Günzler, W.A. Assays of glutathione peroxidase. Methods Enzymol. 1984, 105, 114–120. [Google Scholar] [PubMed]
- Aebi, H. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar] [PubMed]
- Sinha, M.; Manna, P.; Sil, P.C. A 43 kDa protein from the herb, Cajanus indicus L., protects against fluoride induced oxidative stress in mice erythrocytes. Pathophysiology 2007, 14, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Caetano, A.C.; da Veiga, L.F.; Capaldi, F.R.; de Alencar, S.M.; Azevedo, R.A.; Bezerra, R.M. The antioxidant response of the liver of male Swiss mice raised on a AIN 93 or commercial diet. BMC Physiol. 2013, 13, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Motor, S.; Ozturk, S.; Ozcan, O.; Gurpina, A.; Can, Y.; Yuksel, R.; Yenin, J.Z.; Seraslan, G.; Ozturk, O.H. Evaluation of total antioxidant status, total oxidant status and oxidative stress index in patients with alopecia areata. Int. J. Clin. Exp. Med. 2014, 7, 1089–1093. [Google Scholar] [PubMed]
- Walf, A.A.; Frye, C.A. The use of the elevated plus maze as an assay of anxiety-related behavior in rodents. Nat. Protoc. 2007, 2, 322–328. [Google Scholar] [CrossRef] [Green Version]
- Lezak, K.R.; Missig, G.; Carlezon, W.A., Jr. Behavioral methods to study anxiety in rodents. Dialogues Clin. Neurosci. 2022, 19, 181–191. [Google Scholar] [CrossRef]
- Lebow, M.A.; Chen, A. Overshadowed by the amygdala: The bed nucleus of the stria terminalis emerges as key to psychiatric disorders. Mol. Psychiatry 2016, 21, 450–463. [Google Scholar] [CrossRef] [Green Version]
- Cornish, S.; Mehl-Madrona, L. The role of vitamins and minerals in psychiatry. Integr. Med. Insights. 2008, 3, 33–42. [Google Scholar] [CrossRef]
- Castagné, V.; Moser, P.; Roux, S.; Porsolt, R.D. Rodent models of depression: Forced swim and tail suspension behavioral despair tests in rats and mice. Curr. Protocols Pharmacol. 2010, 49, 5–8. [Google Scholar] [CrossRef] [PubMed]
- Planchez, B.; Surget, A.; Belzung, C. Animal models of major depression: Drawbacks and challenges. J. Neural Transm. 2019, 126, 1383–1408. [Google Scholar] [CrossRef]
- Chao, L.; Liu, C.; Sutthawongwadee, S.; Li, Y.; Lv, W.; Chen, W.; Yu, L.; Zhou, J.; Guo, A.; Li, S.; et al. Effects of probiotics on depressive or anxiety variables in healthy participants under stress conditions or with a depressive or anxiety diagnosis: A meta-analysis of randomized controlled trials. Front. Neurol. 2020, 11, 421. [Google Scholar] [CrossRef]
- Lee, G.; Bae, H. Therapeutic effects of phytochemicals and medicinal herbs on depression. BioMed Res. Int. 2017, 2017, 6596241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, A.; Kukreti, R.; Saso, L.; Kukreti, S. Oxidative stress: A key modulator in neurodegenerative diseases. Molecules 2019, 24, 1583. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, I.B.; Lee, J.H.; Park, S.C. The relationship between stress, inflammation, and depression. Biomedicines 2022, 10, 1929. [Google Scholar] [CrossRef] [PubMed]
- Himmerich, H.; Patsalos, O.; Lichtblau, N.; Ibrahim, M.A.; Dalton, B. Cytokine research in depression: Principles, challenges, and open questions. Front. Psychiatry 2019, 10, 30. [Google Scholar] [CrossRef] [Green Version]
- Meyers, A.K.; Zhu, X. The NLRP3 inflammasome: Metabolic regulation and contribution to inflammaging. Cells 2020, 9, 1808. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Lu, K.; Lin, P.; Che, H.; Li, H.; Song, L.; Yang, X.; Xie, W. Saccharina japonica ethanol extract ameliorates depression/anxiety-like behavior by inhibiting inflammation, oxidative stress, and apoptosis in dextran sodium sulfate induced ulcerative colitis mice. Front. Nutr. 2021, 8, 784532. [Google Scholar] [CrossRef]
- Trojan, E.; Slusarczyk, J.; Chamera, K.; Kotarska, K.; Glombik, K.; Kubera, M.; Basta-Kaim, A. The Modulatory Properties of Chronic Antidepressant Drugs Treatment on the Brain Chemokine—Chemokine Receptor Network: A Molecular Study in an Animal Model of Depression. Front. Pharmacol. 2017, 8, 779. [Google Scholar] [CrossRef] [Green Version]
- Milenkovic, V.M.; Stanton, E.H.; Nothdurfter, C.; Rupprecht, R.; Wetzel, C.H. The role of chemokines in the pathophysiology of major depressive disorder. Int. J. Mol. Sci. 2019, 20, 2283. [Google Scholar] [CrossRef] [Green Version]
- Grassi-Oliveira, R.; Brieztke, E.; Teixeira, A.; Pezzi, J.C.; Zanini, M.; Lopes, R.P.; Bauer, M.E. Peripheral chemokine levels in women with recurrent major depression with suicidal ideation. Rev. Bras. Psiquiatr. 2012, 34, 71–75. [Google Scholar] [CrossRef]
- Lehto, S.M.; Niskanen, L.; Herzig, K.H.; Tolmunen, T.; Huotari, A.; Viinamäki, H.; Koivumaa-Honkanen, H.; Honkalampi, K.; Ruotsalainen, H.; Hintikka, J. Serum chemokine levels in major depressive disorder. Psychoneuroendocrinology 2010, 35, 226–232. [Google Scholar] [CrossRef] [PubMed]
- Proma, M.A.; Daria, S.; Nahar, Z.; Islam, S.M.A.; Bhuiyan, M.A.; Islam, M.R. Monocyte chemoattractant protein-1 levels are associated with major depressive disorder. J. Basic Clin. Physiol. Pharmacol. 2022, 533, 735–741. [Google Scholar] [CrossRef] [PubMed]
- Myung, W.; Lim, S.W.; Woo, H.I.; Park, J.H.; Shim, S.; Lee, S.Y.; Kim, D.K. Serum Cytokine Levels in Major Depressive Disorder and Its Role in Antidepressant Response. Psychiatry Investig. 2016, 13, 644–651. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manev, R.; Mrazovac, D.; Manev, H. Possible role for interactions between 5-lipoxygenase (5-LOX) and AMPA GluR1 receptors in depression and in antidepressant therapy. Med. Hypotheses 2007, 69, 1076–1079. [Google Scholar] [CrossRef] [Green Version]
- Furuyashiki, T.; Akiyama, S.; Kitaoka, S. Roles of multiple lipid mediators in stress and depression. Int. Immunol. 2019, 31, 579–587. [Google Scholar] [CrossRef] [Green Version]
- Kotlega, D.; Zembron-Lacny, A.; Golab-Janowska, M.; Nowacki, P.; Szczuko, M. The association of free fatty acids and eicosanoids with the severity of depressive symptoms in stroke patients. Int. J. Mol. Sci. 2020, 21, 5220. [Google Scholar] [CrossRef]
- Uz, T.; Dimitrijevic, N.; Imbesi, M.; Manev, H.; Manev, R. Effects of MK-886, a 5-lipoxygenase activating protein (FLAP) inhibitor, and 5-lipoxygenase deficiency on the forced swimming behavior of mice. Neurosci. Lett. 2008, 436, 269–272. [Google Scholar] [CrossRef] [Green Version]
- Härtig, W.; Michalski, D.; Seeger, G.; Voigt, C.; Donat, C.K.; Dulin, J.; Schuhmann, M.U. Impact of 5-lipoxygenase inhibitors on the spatiotemporal distribution of inflammatory cells and neuronal COX-2 expression following experimental traumatic brain injury in rats. Brain Res. 2013, 1498, 69–84. [Google Scholar] [CrossRef]
- Phillips, C. Brain-derived neurotrophic factor, depression, and physical activity: Making the neuroplastic connection. Neural Plast. 2017, 2017, 7260130. [Google Scholar] [CrossRef]
- Riveros, M.E.; Ávila, A.; Schruers, K.; Ezquer, F. Antioxidant Biomolecules and Their Potential for the Treatment of Difficult-to-Treat Depression and Conventional Treatment-Resistant Depression. Antioxidants 2022, 11, 540. [Google Scholar] [CrossRef] [PubMed]
- Salim, S. Oxidative stress and psychological disorders. Curr. Neuropharmacol. 2014, 12, 140–147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kessas, K.; Chouari, Z.; Ghzaiel, I.; Zarrouk, A.; Ksila, M.; Ghrairi, T.; Midaoui, A.E.; Lizard, G.; Kharoubi, O. Role of Bioactive Compounds in the Regulation of Mitochondrial Dysfunctions in Brain and Age-Related Neurodegenerative Diseases. Cells 2022, 11, 257. [Google Scholar] [CrossRef]
- Giacobbo, B.L.; Doorduin, J.; Klein, C.H.; Dierckx, R.A.J.O.; Bromberg, E.; de Vries, E.F.J. Brain-Derived Neurotrophic Factor in Brain Disorders: Focus on Neuroinflammation. Mol. Neurobiol. 2019, 56, 3295–3312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- González-Sarrías, A.; Núñez-Sánchez, M.Á.; Tomás-Barberán, F.A.; Espín, J.C. Neuroprotective effects of bioavailable polyphenol-derived metabolites against oxidative stress-induced cytotoxicity in human neuroblastoma sh-sy5y cells. J. Agric. Food Chem. 2017, 65, 752–758. [Google Scholar] [CrossRef] [PubMed]





| Liver | ||||||
| Treatments | ABTS | ORAC | CAT | GPx | OSi | MDA |
| Non-stressed | 1512. 75 ± 76.37 a | 5102.76 ± 1068.48 a | 7.32 ± 2.00 a | 35.92 ± 13.60 a | 0.02 ± 0.01 b | 0.39 ± 0.23 a |
| Red light | 1435.64 ±134.66 a | 5189.70 ± 1786.08 a | 10.74 ± 2.86 a | 19.50 ± 2.12 a | 0.29 ± 0.11 a | 0.63 ± 0.14 b |
| Unfermented huauzontle | 1772.44 ± 77.42 b | 4411.40 ± 1313.12 a | 11.76 ± 4.47 a | 16.74 ± 6.28 a | 0.22 ± 0.14 a | 0.68 ± 0.12 b |
| Fermented huauzontle | 1707.27 ± 80.11 b | 4840.90 ± 182.04 a | 9.12 ± 0.93 a | 13.33 ± 2.32 a | 0.05 ± 0.18 b | 0.52 ± 0.19 b |
| Hippocampus | ||||||
| Non-stressed | 235.75 ± 71.54 a | 195.12 ± 85.27 ac | 1.16 ± 0.52 a | 2.61 ± 0.38 b | 0.26 ± 0.05 a | 0.75 ± 0.07 a |
| Red light | 210.60 ± 72.15 a | 157.3 ± 72.40 a | 1.09 ± 0.30 a | 2.29 ± 0.45 b | 0.27 ± 0.09 a | 0.69 ± 0.06 a |
| Unfermented huauzontle | 259.05 ± 85.12 a | 404.8 ± 53.09 b | 1.00 ± 0.39 ab | 1.57 ± 0.37 ab | 0.21 ± 0.11 a | 0.62 ± 0.15 a |
| Fermented huauzontle | 259.12 ± 63.08 a | 297.7 ± 65.76 c | 0.66 ± 0.13 b | 1.91 ± 0.77 a | 0.22 ± 0.08 a | 0.73 ± 0.04 a |
| Plasma | ||||
| Cytokines (pg/mL) | ||||
| Treatments | IL-10 | MCP1 | TNFα | BDNF (pg/mL) |
| Non-stressed | 49.05 ± 3.96 a | 16.42 ± 5.33 a | 9.10 ± 2.29 a | 171.87 ± 63.13 a |
| Red light | 13.87 ± 1.80 b | 18.76 ± 1.17 a | 6.64 ± 1.45 a | 179.62 ± 37.71 a |
| Unfermented huauzontle | 10.69 ± 2.78 b | 24.56 ± 3.78 b | 6.34 ± 1.44 a | 191.11 ± 23.14 a |
| Fermented huauzontle | 24.97 ± 7.30 c | 27.02 ± 5.34 b | 5.92 ± 0.64 a | 232.20 ± 23.59 b |
| Hippocampus | ||||
| Non-stressed | 10.16 ± 2.94 a | 16.33 ± 1.79 a | <7.3 | 75.00 ± 35.31 * |
| Red light | 11.44 ± 3.14 a | 16.96 ± 2.38 a | 10.47 ± 0.86 a | <18.75 |
| Huauzontle unfermented | 13.35 ± 2.64 a | 17.18 ± 1.54 a | 15.87 ± 4.40 a | <18.75 |
| Huauzontle fermented | 10.62 ± 1.66 a | 16.43 ± 1.23 a | 10.81 ± 0.84 a | 77.80 ± 5.24 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santiago-López, L.; Almada-Corral, A.; García, H.S.; Mata-Haro, V.; González-Córdova, A.F.; Vallejo-Cordoba, B.; Hernández-Mendoza, A. Antidepressant and Anxiolytic Effects of Fermented Huauzontle, a Prehispanic Mexican Pseudocereal. Foods 2023, 12, 53. https://doi.org/10.3390/foods12010053
Santiago-López L, Almada-Corral A, García HS, Mata-Haro V, González-Córdova AF, Vallejo-Cordoba B, Hernández-Mendoza A. Antidepressant and Anxiolytic Effects of Fermented Huauzontle, a Prehispanic Mexican Pseudocereal. Foods. 2023; 12(1):53. https://doi.org/10.3390/foods12010053
Chicago/Turabian StyleSantiago-López, Lourdes, Arantxa Almada-Corral, Hugo S. García, Verónica Mata-Haro, Aarón F. González-Córdova, Belinda Vallejo-Cordoba, and Adrián Hernández-Mendoza. 2023. "Antidepressant and Anxiolytic Effects of Fermented Huauzontle, a Prehispanic Mexican Pseudocereal" Foods 12, no. 1: 53. https://doi.org/10.3390/foods12010053