Amanitins in Wild Mushrooms: The Development of HPLC-UV-EC and HPLC-DAD-MS Methods for Food Safety Purposes
Abstract
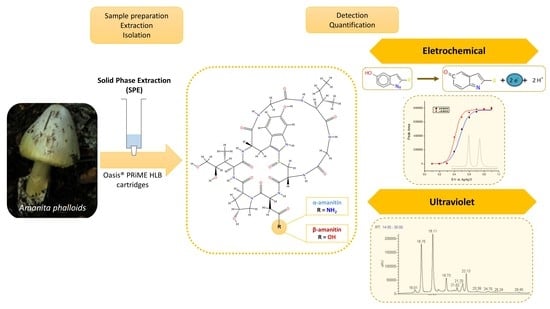
:1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
2.2. Working Solutions, Calibration Curve, and Quality Control Samples
2.3. Mushroom Collection
2.4. Sample Preparation and Extraction Procedure
2.5. Chromatographic System and Conditions
2.6. Validation of the Method
2.6.1. Selectivity
2.6.2. Linearity
2.6.3. Precision
2.6.4. Limit of Detection (LOD) and Limit of Quantification (LOQ)
2.6.5. Recovery and Matrix Effect
2.7. Method Application
3. Results and Discussion
3.1. Optimization of Electrochemical Detection
3.2. Optimization of Sample Extraction Procedures
3.3. Optimization of the Chromatographic Conditions
3.4. Validation of the Method
3.5. Method Application
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kalač, P. A review of chemical composition and nutritional value of wild-growing and cultivated mushrooms. J. Sci. Food Agric. 2013, 93, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Wasser, S.P. Medicinal mushrooms as a source of antitumor and immunomodulating polysaccharides. Appl. Microbiol. Biotechnol. 2002, 60, 258–274. [Google Scholar] [PubMed]
- Gao, Y.; Chan, E.; Zhou, S. Immunomodulating Activities of Ganoderma, a Mushroom with Medicinal Properties. Food Rev. Int. 2004, 20, 123–161. [Google Scholar] [CrossRef]
- Jayachandran, M.; Xiao, J.; Xu, B. A Critical Review on Health Promoting Benefits of Edible Mushrooms through Gut Microbiota. Int. J. Mol. Sci. 2017, 18, 1934. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muszynska, K.; Ostrowska, D.; Bartnicki, F.; Kowalska, E.; Bodaszewska-Lubas, M.; Hermanowicz, P.; Faulstich, H.; Strzalka, W. Selection and analysis of a DNA aptamer binding alpha-amanitin from Amanita phalloides. Acta Biochim. Pol. 2017, 64, 401–406. [Google Scholar] [CrossRef]
- Valverde, M.E.; Hernandez-Pérez, T.; Paredes-López, O. Edible Mushrooms: Improving Human Health and Promoting Quality Life. Int. J. Microbiol. 2015, 2015, 376387. [Google Scholar] [CrossRef] [Green Version]
- Kulshreshtha, S.; Mathur, N.; Bhatnagar, P. Mushroom as a product and their role in mycoremediation. AMB Express 2014, 4, 29. [Google Scholar] [CrossRef] [Green Version]
- Enjalbert, F.; Rapior, S.; Nouguier-Soule, J.; Guillon, S.; Amouroux, N. Treatment of amatoxin poisoning: 20-year retrospective analysis. J. Toxicol.-Clin. Toxicol. 2002, 40, 715–757. [Google Scholar] [CrossRef]
- Karlson-Stiber, C.; Persson, H. Cytotoxic fungi—An overview. Toxicon 2003, 42, 339–349. [Google Scholar] [CrossRef]
- Erden, A.; Esmeray, K.; Karagoz, H.; Karahan, S.; Gumuscu, H.H.; Basak, M.; Cetinkaya, A.; Avci, D.; Poyrazoglu, O.K. Acute liver failure caused by mushroom poisoning: A case report and review of the literature. Int. Med. Case Rep. J. 2013, 6, 85–90. [Google Scholar]
- Berger, K.J.; Guss, D.A. Mycotoxins revisited: Part I. J. Emerg. Med. 2005, 28, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Vetter, J.; Vetter, S. Death cap poisoning and the animals. Literature review. Magy. Allatorv. Lapja 2014, 136, 750–758. [Google Scholar]
- Garcia, J.; Costa, V.M.; Baptista, P.; Bastos, M.D.; Carvalho, F. Quantification of alpha-amanitin in biological samples by HPLC using simultaneous UV-diode array and electrochemical detection. J. Chromatogr. B-Anal. Technol. Biomed. Life Sci. 2015, 997, 85–95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vargas, N.; Bernal, A.; Sarria, V.; Franco-Molano, A.; Restrepo, S. Amatoxin and phallotoxin composition in species of the genus Amanita in Colombia: A taxonomic perspective. Toxicon 2011, 58, 583–590. [Google Scholar] [CrossRef] [PubMed]
- Durand, F.; Valla, D. Mushroom Poisoning: A Clinical Model of Toxin-Induced Centrilobular Necrosis. In Drug-Induced Liver Disease, 3rd ed.; Academic Press: Cambridge, MA, USA, 2013; pp. 621–629. [Google Scholar]
- Wieland, T.; Faulstich, H. 50 Years of Amanitin. Experientia 1991, 47, 1186–1193. [Google Scholar] [CrossRef] [PubMed]
- Vetter, J. Toxins of Amanita phalloides. Toxicon 1998, 36, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Robinson-Fuentes, V.A.; Jaime-Sanchez, J.L.; Garcia-Aguilar, L.; Gomez-Peralta, M.; Vazquez-Garciduenas, M.S.; Vazquez-Marrufo, G. Determination of alpha- and beta-amanitin in clinical urine samples by Capillary Zone Electrophoresis. J. Pharmaceut. Biomed. 2008, 47, 913–917. [Google Scholar] [CrossRef]
- Lawton, L.D.; Bhraonain, S.N. Accidental poisoning by death cap mushrooms: Be careful what you eat. Wilderness Environ. Med. 2013, 24, 168–170. [Google Scholar] [CrossRef] [Green Version]
- Garcia, J.; Costa, V.M.; Bovolini, A.; Duarte, J.A.; Rodrigues, D.F.; Bastos, M.D.; Carvalho, F. An effective antidotal combination of polymyxin B and methylprednisolone for alpha-amanitin intoxication. Arch. Toxicol. 2019, 93, 1449–1463. [Google Scholar] [CrossRef]
- Garcia, J.; Carvalho, A.T.P.; Dourado, D.F.A.R.; Baptista, P.; Bastos, M.D.; Carvalho, F. New in silico insights into the inhibition of RNAP II by alpha-amanitin and the protective effect mediated by effective antidotes. J. Mol. Graph. Model. 2014, 51, 120–127. [Google Scholar] [CrossRef] [Green Version]
- Arici, M.A.; Sahin, A.; Cavdar, Z.; Ergur, B.U.; Ural, C.; Akokay, P.; Kalkan, S.; Tuncok, Y. Effects of resveratrol on alpha-amanitin-induced nephrotoxicity in BALB/c mice. Hum. Exp. Toxicol. 2020, 39, 328–337. [Google Scholar] [CrossRef] [PubMed]
- Enjalbert, F.; Gallion, C.; Jehl, F.; Monteil, H.; Faulstich, H. Simultaneous Assay for Amatoxins and Phallotoxins in Amanita-Phalloides Fr by High-Performance Liquid-Chromatography. J. Chromatogr. 1992, 598, 227–236. [Google Scholar] [CrossRef] [PubMed]
- Enjalbert, F.; Cassanas, G.; Guinchard, C.; Chaumont, J.P. Toxin composition of Amanita phalloides tissues in relation to the collection site. Mycologia 1996, 88, 909–921. [Google Scholar] [CrossRef]
- Kaya, E.; Yilmaz, I.; Sinirlioglu, Z.A.; Karahan, S.; Bayram, R.; Yaykasli, K.O.; Colakoglu, S.; Saritas, A.; Severoglu, Z. Amanitin and phallotoxin concentration in Amanita phalloides var. alba mushroom. Toxicon 2013, 76, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Kaya, E.; Karahan, S.; Hanci, M. The Measurement Of Alpha Amanitin Levels Using Hplc Method In Amanita Phalloides From Düzce Province. Duzce Med. J. 2012, 14, 15–17. [Google Scholar]
- Yoshioka, N.; Akamatsu, S.; Mitsuhashi, T.; Todo, C.; Asano, M.; Ueno, Y. A simple method for the simultaneous determination of mushroom toxins by liquid chromatography-time-of-flight mass spectrometry. Forensic Toxicol. 2014, 32, 89–96. [Google Scholar] [CrossRef]
- Sgambelluri, R.M.; Epis, S.; Sassera, D.; Luo, H.; Angelos, E.R.; Walton, J.D. Profiling of Amatoxins and Phallotoxins in the Genus Lepiota by Liquid Chromatography Combined with UV Absorbance and Mass Spectrometry. Toxins 2014, 6, 2336–2347. [Google Scholar] [CrossRef] [Green Version]
- Garcia, J.; Oliveira, A.; de Pinho, P.G.; Freitas, V.; Carvalho, A.; Baptista, P.; Pereira, E.; Bastos, M.D.L.; Carvalho, F. Determination of amatoxins and phallotoxins in Amanita phalloides mushrooms from northeastern Portugal by HPLC-DAD-MS. Mycologia 2015, 107, 679–687. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, W.H.A.; Gonmori, K.; Suzuki, M.; Watanabe, K.; Suzuki, O. Simultaneous analysis of alpha-amanitin, beta-amanitin, and phalloidin in toxic mushrooms by liquid chromatography coupled to time-of-flight mass spectrometry. Forensic Toxicol. 2010, 28, 69–76. [Google Scholar] [CrossRef]
- Maio, M.; Di Fiore, C.; Iannone, A.; Carriera, F.; Notardonato, I.; Avino, P. Review of the Analytical Methods Based on HPLC-Electrochemical Detection Coupling for the Evaluation of Organic Compounds of Nutritional and Environmental Interest. Analytica 2022, 3, 54–78. [Google Scholar] [CrossRef]
- Sontag, G.; Pinto, M.I.; Noronha, J.P.; Burrows, H.D. Analysis of Food by High Performance Liquid Chromatography Coupled with Coulometric Detection and Related Techniques: A Review. J. Agric. Food Chem. 2019, 67, 4113–4144. [Google Scholar] [CrossRef] [PubMed]
- Silah, H.; Erkmen, C.; Demir, E.; Uslu, B. Modified indium tin oxide electrodes: Electrochemical applications in pharmaceutical, biological, environmental and food analysis. TrAC Trends Anal. Chem. 2021, 141, 116289. [Google Scholar] [CrossRef]
- FDA. Guidance for Industry: Bioanalytical Method Validation; Food and Drug Administration (FDA): Silver Spring, MD, USA, 2018. [Google Scholar]
- EMA. ICH Guideline M10 on Bioanalytical Method Validation; EMA: Amsterdam, The Netherlands, 2019. [Google Scholar]
- ICH. Validation of Analytical Procedures: Text and Methodology Q2(R1); The International Conference on Harmonisation of Thecnical Requirements for Registartion of Pharmaceuticals for Human Use: Geneva, Switzerland, 2005. [Google Scholar]
- Defendenti, C.; Bonacina, E.; Mauroni, M.; Gelosa, L. Validation of a high performance liquid chromatographic method for alpha amanitin determination in urine. Forensic Sci. Int. 1998, 92, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Tagliaro, F.; Schiavon, G.; Bontempelli, G.; Carli, G.; Marigo, M. Improved High-Performance Liquid-Chromatographic Determination with Amperometric Detection of Alpha-Amanitin in Human Plasma Based on Its Voltammetric Study. J. Chromatogr.-Biomed. Appl. 1991, 563, 299–311. [Google Scholar] [CrossRef] [PubMed]
- Tagliaro, F.; Chiminazzo, S.; Maschio, S.S.; Alberton, F.; Marigo, M. Improved High Performance Liquid Chromatographic Dtermination of amanitins with Electrochemical Detection. Chromatographia 1987, 24, 482–486. [Google Scholar] [CrossRef]
- Nováková, L.; Vlcková, H. A review of current trends and advances in modern bio-analytical methods: Chromatography and sample preparation. Anal. Chim. Acta 2009, 656, 8–35. [Google Scholar] [CrossRef]
- Clarke, D.B.; Lloyd, A.S.; Robb, P. Application of liquid chromatography coupled to time-of-flight mass spectrometry separation for rapid assessment of toxins in Amanita mushrooms. Anal. Methods 2012, 4, 1298–1309. [Google Scholar] [CrossRef]
- Chung, W.C.; Tso, S.C.; Sze, S.T. Separation of polar mushroom toxins by mixed-mode hydrophilic and ionic interaction liquid chromatography-electrospray ionization-mass spectrometry. J. Chromatogr. Sci. 2007, 45, 104–111. [Google Scholar] [CrossRef] [Green Version]
- Rittgen, J.; Putz, M.; Pyell, U. Identification of toxic oligopeptides in Amanita fungi employing capillary electrophoresis-electrospray ionization-mass spectrometry with positive and negative ion detection. Electrophoresis 2008, 29, 2094–2100. [Google Scholar] [CrossRef]
- Zhang, P.; Chen, Z.H.; Hu, J.S.; Wei, B.Y.; Zhang, Z.G.; Hu, W.Q. Production and characterization of Amanitin toxins from a pure culture of Amanita exitialis. Fems Microbiol. Lett. 2005, 252, 223–228. [Google Scholar] [CrossRef] [Green Version]
- Tang, S.S.; Zhou, Q.; He, Z.M.; Luo, T.; Zhang, P.; Cai, Q.; Yang, Z.L.; Chen, J.; Chen, Z.H. Cyclopeptide toxins of lethal amanitas: Compositions, distribution and phylogenetic implication. Toxicon 2016, 120, 78–88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Enjalbert, F.; Cassanas, G.; Salhi, S.L.; Guinchard, C.; Chaumont, J.P. Distribution of the amatoxins and phallotoxins in Amanita phalloides. Influence of the tissues and the collection site. Comptes Rendus Acad. Sci. III 1999, 322, 855–862. [Google Scholar] [CrossRef]
- Hallen, H.E.; Watling, R.; Adams, G.C. Taxonomy and toxicity of Conocybe lactea and related species. Mycol. Res. 2003, 107, 969–979. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zhao, Y.F.; Li, H.J.; Zhou, S.; Chen, D.W.; Zhang, Y.Z.; Yao, Q.M.; Sun, C.Y. A Simple and High-Throughput Analysis of Amatoxins and Phallotoxins in Human Plasma, Serum and Urine Using UPLC-MS/MS Combined with PRiME HLB Elution Platform. Toxins 2016, 8, 128. [Google Scholar] [CrossRef] [Green Version]
- Gouveia, F.; Bicker, J.; Santos, J. Development, validation and application of a new HPLC-DAD method for simultaneous quantification of apixaban, dabigatran, edoxaban and rivaroxaban in human plasma. J. Pharm. Biomed. Anal. 2020, 181, 113109. [Google Scholar] [CrossRef] [PubMed]
- Wieland, T. Peptides of Poisonous Amanita Mushrooms; Springer: Berlin/Heidelberg, Germany, 1986. [Google Scholar]
- Leite, M.; Freitas, A.; Azul, A.M.; Barbosa, J.; Costa, S.; Ramos, F. Development, optimization and application of an analytical methodology by ultra performance liquid chromatography-tandem mass spectrometry for determination of amanitins in urine and liver samples. Anal. Chim. Acta 2013, 799, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Kaya, E.; Karahan, S.; Bayram, R.; Yaykasli, K.O.; Colakoglu, S.; Saritas, A. Amatoxin and phallotoxin concentration in Amanita phalloides spores and tissues. Toxicol. Ind. Health 2015, 31, 1172–1177. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.C.; Kalb, S.R.; Barr, J.R. Toxin Analysis Using Mass Spectrometry. In Microbial Forensics, 2nd ed.; Academic Press: Cambridge, MA, USA, 2011; pp. 405–420. [Google Scholar]
- Filigenzi, M.S.; Poppenga, R.H.; Tiwary, A.K.; Puschner, B. Determination of alpha-amanitin in serum and liver by multistage linear ion trap mass spectrometry. J. Agric. Food Chem. 2007, 55, 2784–2790. [Google Scholar] [CrossRef]
- Li, C.L.; Wei, F.; Muhammad, S.; Yang, G.D.; Wang, S.C.; Liu, X.S. A cost-effective LC-MS/MS method for identification and quantification of a-amanitin in rat plasma: Application to toxicokinetic study. J. Chromatogr. B-Anal. Technol. Biomed. Life Sci. 2017, 1064, 36–39. [Google Scholar] [CrossRef]






| Compound | Detection | Range (µg mL−1) | Linearity (R2) | Linear Regression Equation |
|---|---|---|---|---|
| α-amanitin | EC | 0.5–20.0 | 0.9998 | y = 621,020x + 10,701 |
| UV | 0.5–20.0 | 0.9993 | y = 36,624x + 5896.6 | |
| β-amanitin | EC | 0.5–20.0 | 0.9997 | y = 346,265x + 31,251 |
| UV | 0.5–20.0 | 0.9996 | y = 27,942x + 1373.6 |
| Matrix | Mushroom Toxin | LOD (ng g−1) | LOQ (ng g−1) | Method | Reference |
|---|---|---|---|---|---|
| Mushroom tissue | α-amanitin β-amanitin | EC-62 UV-55 EC-24 UV-64 | EC-189 UV-168 EC-72.6 UV-193 | HPLC-UV-EC | Present Work |
| Mushroom tissue | α-amanitin β-amanitin | 20 20 | NS | HPLC-ESI-MS | [42] |
| Mushroom tissue | α-amanitin β-amanitin | 30 30 | NS | HPLC-TOF-MS | [30] |
| Mushroom tissue | α-amanitin β-amanitin | 230 190 | 26.8 33.3 | HPLC-TOF-MS | [27] |
| Mushroom tissue | α-amanitin β-amanitin | 2 2 | NS | HPLC-TOF-MS | [52] |
| Compound | Detection | Concentration (µg mL−1) | Recovery (%) (RSD) | Matrix Effect (%) (RSD) |
|---|---|---|---|---|
| α-amanitin | EC | 0.5 | 117 (4.78) | 96.9 (8.01) |
| 2.0 | 99.4 (1.37) | |||
| 10.0 | 96.1 (1.48) | |||
| UV | 0.5 | 104 (3.72) | 95.8 (8.87) | |
| 2.0 | 92.5 (1.64) | |||
| 10.0 | 96.8 (0.34) | |||
| β-amanitin | EC | 0.5 | 114 (3.25) | 95.8 (11.4) |
| 2.0 | 103 (2.94) | |||
| 10.0 | 93.6 (0.48) | |||
| UV | 0.5 | 92.0 (3.9) | 97.6 (10.9) | |
| 2.0 | 89.0 (2.7) | |||
| 10.0 | 93.3 (0.6) |
| Compound | Detection | Concentration (μg mL−1) | Precision (%RSD) | |
|---|---|---|---|---|
| Intra-day | Inter-day | |||
| α-amanitin | EC | 0.5 | 5.35 | 12.95 |
| 2.0 | 1.05 | 7.64 | ||
| 10.0 | 0.41 | 9.95 | ||
| UV | 0.5 | 10.23 | 3.73 | |
| 2.0 | 1.13 | 2.46 | ||
| 10.0 | 0.62 | 11.75 | ||
| β-amanitin | EC | 0.5 | 4.20 | 10.38 |
| 2.0 | 3.34 | 5.45 | ||
| 10.0 | 1.22 | 3.81 | ||
| UV | 0.5 | 5.33 | 5.80 | |
| 2.0 | 4.55 | 3.69 | ||
| 10.0 | 0.69 | 3.51 | ||
| Mushroom Species | HPLC-UV-EC |
|---|---|
| Amanita citrine; | |
| Amanita pantherine; | |
| Amanita boudieri; | |
| Amanita gemmate; | |
| Amanita muscaria; | |
| Amanita rubescens; | |
| Amanita vaginatae; | ND |
| Amanita var alba; | |
| Boletus edulis; | |
| Cantharellus cibarius; | |
| Tricholoma equestre; | |
| Tricholoma joachimii; | |
| Tricholoma portentosum | |
| Amanita phalloides | α-amanitin: (EC) < > 290 µg g−1 β-amanitin: (EC) < > 280 µg g−1 α-amanitin: (UV) < > 280 µg g−1 β-amanitin: (UV) < > 288 µg g−1 |
| Compounds (Formula) | Molecular Weight | Retention Time (min) | Protonated Molecular Ions (MS1, m/z) | Major Fragment Ions (MS2, m/z) |
|---|---|---|---|---|
| α-amanitin (C39H54N10O14S) | 918.97 | 18.1 | [M+H]+ 919.00 | 901.14 (100%) 661.43 (44.8%) 546.71 (60%) |
| β-amanitin (C39H53N9O15S) | 919.95 | 16.6 | [M+H]+ 920.35 | 902.09 (100%) 661.77 (45%) 546.64 (64.8%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barbosa, I.; Domingues, C.; Barbosa, R.M.; Ramos, F. Amanitins in Wild Mushrooms: The Development of HPLC-UV-EC and HPLC-DAD-MS Methods for Food Safety Purposes. Foods 2022, 11, 3929. https://doi.org/10.3390/foods11233929
Barbosa I, Domingues C, Barbosa RM, Ramos F. Amanitins in Wild Mushrooms: The Development of HPLC-UV-EC and HPLC-DAD-MS Methods for Food Safety Purposes. Foods. 2022; 11(23):3929. https://doi.org/10.3390/foods11233929
Chicago/Turabian StyleBarbosa, Isabel, Cátia Domingues, Rui M. Barbosa, and Fernando Ramos. 2022. "Amanitins in Wild Mushrooms: The Development of HPLC-UV-EC and HPLC-DAD-MS Methods for Food Safety Purposes" Foods 11, no. 23: 3929. https://doi.org/10.3390/foods11233929