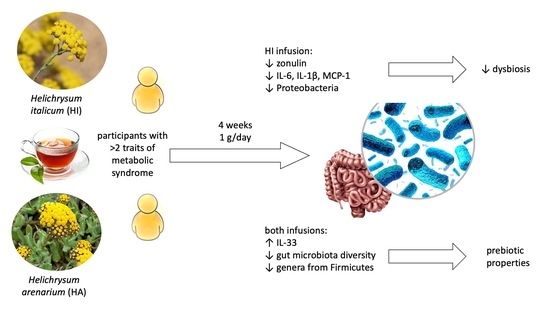
Helichrysum italicum (Roth) G. Don and Helichrysum arenarium (L.) Moench Infusion Consumption Affects the Inflammatory Status and the Composition of Human Gut Microbiota in Patients with Traits of Metabolic Syndrome: A Randomized Comparative Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. Study Protocol
2.3. Test Beverages and Dosages
2.4. Inflammatory Parameters
2.5. Gastrointestinal Symptoms
2.6. Stool Sample Analysis
2.7. Microbiota Data Analysis
2.8. Statistics
3. Results
3.1. Baseline Characteristics of Subjects
3.2. Effects of HI and HA Infusions on Inflammatory Markers and Zonulin
3.3. Effects of HI and HA Infusions on Gut Microbiota
3.4. Effects of HI and HA Infusions on Gastrointestinal Symptoms
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kahn, B.B.; Flier, J.S. Obesity and Insulin Resistance. J. Clin. Investig. 2021, 106, 473–481. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Odegaard, J.I.; Chawla, A. The Immune System as a Sensor of the Metabolic State. Immunity 2013, 38, 644–654. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kopelman, P.G. Obesity as a Medical Problem. Nature 2000, 404, 635–643. [Google Scholar] [CrossRef] [PubMed]
- Moeller, A.H.; Suzuki, T.A.; Phifer-Rixey, M.; Nachman, M.W. Transmission Modes of the Mammalian Gut Microbiota. Science 2018, 362, 453–457. [Google Scholar] [CrossRef] [Green Version]
- Salvucci, E. Microbiome, Holobiont and the Net of Life. Crit. Rev. Microbiol. 2016, 42, 485–494. [Google Scholar] [CrossRef]
- Salehi, B.; Dimitrijević, M.; Aleksić, A.; Neffe-Skocińska, K.; Zielińska, D.; Kołożyn-Krajewska, D.; Sharifi-Rad, J.; Stojanović-Radić, Z.; Prabu, S.M.; Célia, F. Human Microbiome and Homeostasis: Insights into the Key Role of Prebiotics, Probiotics, and Symbiotics. Crit. Rev. Food Sci. Nutr. 2021, 61, 1415–1428. [Google Scholar]
- Rinninella, E.; Cintoni, M.; Raoul, P.; Lopetuso, L.R.; Scaldaferri, F.; Pulcini, G.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C. Food Components and Dietary Habits: Keys for a Healthy Gut Microbiota Composition. Nutrients 2019, 11, 2393. [Google Scholar] [CrossRef] [Green Version]
- Delzenne, N.M.; Rodriguez, J.; Olivares, M.; Neyrinck, A.M. Microbiome Response to Diet: Focus on Obesity and Related Diseases. Rev. Endocr. Metab. Disord. 2020, 21, 369–380. [Google Scholar] [CrossRef]
- Ma, J.; Li, H. The Role of Gut Microbiota in Atherosclerosis and Hypertension. Front. Pharmacol. 2018, 9, 1082. [Google Scholar] [CrossRef]
- Wang, Z.; Zhao, Y. Gut Microbiota Derived Metabolites in Cardiovascular Health and Disease. Protein Cell 2018, 9, 416–431. [Google Scholar] [CrossRef] [Green Version]
- Thingholm, L.B.; Rühlemann, M.C.; Koch, M.; Fuqua, B.; Laucke, G.; Boehm, R.; Bang, C.; Franzosa, E.A.; Hübenthal, M.; Rahnavard, A.; et al. Obese Individuals with and without Type 2 Diabetes Show Different Gut Microbial Functional Capacity and Composition. Cell Host Microbe 2019, 26, 252–264.e10. [Google Scholar] [CrossRef]
- Bezek, K.; Petelin, A.; Pražnikar, J.; Nova, E.; Redondo, N.; Marcos, A.; Jenko Pražnikar, Z. Obesity Measures and Dietary Parameters as Predictors of Gut Microbiota Phyla in Healthy Individuals. Nutrients 2020, 12, 2695. [Google Scholar] [CrossRef] [PubMed]
- Zmora, N.; Suez, J.; Elinav, E. You Are What You Eat: Diet, Health and the Gut Microbiota. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 35–56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, G.D.; Chen, J.; Hoffmann, C.; Bittinger, K.; Chen, Y.-Y.; Keilbaugh, S.A.; Bewtra, M.; Knights, D.; Walters, W.A.; Knight, R.; et al. Linking Long-Term Dietary Patterns with Gut Microbial Enterotypes. Science 2011, 334, 105–108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sung, M.M.; Kim, T.T.; Denou, E.; Soltys, C.-L.M.; Hamza, S.M.; Byrne, N.J.; Masson, G.; Park, H.; Wishart, D.S.; Madsen, K.L.; et al. Improved Glucose Homeostasis in Obese Mice Treated with Resveratrol Is Associated with Alterations in the Gut Microbiome. Diabetes 2017, 66, 418–425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kramberger, K.; Barlič-Maganja, D.; Bandelj, D.; Baruca Arbeiter, A.; Peeters, K.; Miklavčič Višnjevec, A.; Jenko Pražnikar, Z. HPLC-DAD-ESI-QTOF-MS Determination of Bioactive Compounds and Antioxidant Activity Comparison of the Hydroalcoholic and Water Extracts from Two Helichrysum italicum Species. Metabolites 2020, 10, 403. [Google Scholar] [CrossRef] [PubMed]
- Kramberger, K.; Jenko Pražnikar, Z.; Baruca Arbeiter, A.; Petelin, A.; Bandelj, D.; Kenig, S. A Comparative Study of the Antioxidative Effects of Helichrysum italicum and Helichrysum arenarium Infusions. Antioxidants 2021, 10, 380. [Google Scholar] [CrossRef]
- Kenig, S.; Kramberger, K.; Petelin, A.; Bandelj, D.; Baruca Arbeiter, A.; Miklavčič Višnjevec, A.; Peeters, K.; Mohorko, N.; Šik Novak, K.; Jenko Pražnikar, Z. Helichrysum italicum ssp. italicum Infusion Promotes Fat Oxidation in Hepatocytes and Stimulates Energy Expenditure and Fat Oxidation after Acute Ingestion in Humans: A Pilot Study. Plants 2021, 10, 1516. [Google Scholar] [CrossRef]
- Kenig, S.; Kramberger, K.; Šik Novak, K.; Karnjuš, I.; Bandelj, D.; Petelin, A.; Jenko Pražnikar, Z. Helichrysum italicum (Roth) G. Don and Helichrysum arenarium (L.) Moench Infusions in Reversing the Traits of Metabolic Syndrome: A Double-Blind Randomized Comparative Trial. Food Funct. 2022, 13, 7697–7706. [Google Scholar] [CrossRef]
- Kramberger, K.; Barlič-Maganja, D.; Pražnikar, Z.J.; Režen, T.; Rozman, D.; Pražnikar, J.; Kenig, S. Whole Transcriptome Expression Array Analysis of Human Colon Fibroblasts Culture Treated with Helichrysum italicum Supports Its Use in Traditional Medicine. J. Ethnopharmacol. 2022, 296, 115505. [Google Scholar] [CrossRef]
- Plamada, D.; Vodnar, D.C. Polyphenols-Gut Microbiota Interrelationship: A Transition to a New Generation of Prebiotics. Nutrients 2021, 14, 137. [Google Scholar] [CrossRef]
- Fraga, C.G.; Croft, K.D.; Kennedy, D.O.; Tomás-Barberán, F.A. The Effects of Polyphenols and Other Bioactives on Human Health. Food Funct. 2019, 10, 514–528. [Google Scholar] [CrossRef] [Green Version]
- Parada, A.E.; Needham, D.M.; Fuhrman, J.A. Every Base Matters: Assessing Small Subunit RRNA Primers for Marine Microbiomes with Mock Communities, Time Series and Global Field Samples. Environ. Microbiol. 2016, 18, 1403–1414. [Google Scholar] [CrossRef]
- Apprill, A.; McNally, S.; Parsons, R.; Weber, L. Minor Revision to V4 Region SSU RRNA 806R Gene Primer Greatly Increases Detection of SAR11 Bacterioplankton. Aquat. Microb. Ecol. 2015, 75, 129–137. [Google Scholar] [CrossRef] [Green Version]
- Thompson, L.R.; Sanders, J.G.; McDonald, D.; Amir, A.; Ladau, J.; Locey, K.J.; Prill, R.J.; Tripathi, A.; Gibbons, S.M.; Ackermann, G.; et al. A Communal Catalogue Reveals Earth’s Multiscale Microbial Diversity. Nature 2017, 551, 457–463. [Google Scholar] [CrossRef] [Green Version]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, Interactive, Scalable and Extensible Microbiome Data Science Using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef]
- Martin, M. Cutadapt Removes Adapter Sequences from High-Throughput Sequencing Reads. EMBnet. J. 2011, 17, 10–12. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-Resolution Sample Inference from Illumina Amplicon Data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef] [Green Version]
- Bokulich, N.A.; Kaehler, B.D.; Rideout, J.R.; Dillon, M.; Bolyen, E.; Knight, R.; Huttley, G.A.; Gregory Caporaso, J. Optimizing Taxonomic Classification of Marker-Gene Amplicon Sequences with QIIME 2’s Q2-Feature-Classifier Plugin. Microbiome 2018, 6, 90. [Google Scholar] [CrossRef]
- Pedregosa, F.; Varoquaux, G.; Gramfort, A.; Michel, V.; Thirion, B.; Grisel, O.; Blondel, M.; Prettenhofer, P.; Weiss, R.; Dubourg, V.; et al. Scikit-Learn: Machine Learning in Python. J. Mach. Learn. Res. 2011, 12, 2825–2830. [Google Scholar]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA Ribosomal RNA Gene Database Project: Improved Data Processing and Web-Based Tools. Nucleic Acids Res. 2013, 41, D590–D596. [Google Scholar] [CrossRef] [PubMed]
- Robeson, M.S.; O’Rourke, D.R.; Kaehler, B.D.; Ziemski, M.; Dillon, M.R.; Foster, J.T.; Bokulich, N.A. RESCRIPt: Reproducible Sequence Taxonomy Reference Database Management. PLoS Comput. Biol. 2021, 17, e1009581. [Google Scholar] [CrossRef] [PubMed]
- Willis, A.D.; Martin, B.D. DivNet: An R Package to Estimate Diversity When Taxa in the Community Cooccur via a Ecological Network. Biostatistics 2020, 23, 207–2022. [Google Scholar] [CrossRef] [PubMed]
- Willis, A.; Bunge, J. Estimating Diversity via Frequency Ratios. Biometrics 2015, 71, 1042–1049. [Google Scholar] [CrossRef] [Green Version]
- Willis, A.; Bunge, J.; Whitman, T. Improved Detection of Changes in Species Richness in High-Diversity Microbial Communities. J. R. Stat. Soc. Ser. C 2017, 66, 963–977. [Google Scholar] [CrossRef] [Green Version]
- Lahti, L.; Shetty, S. Microbiome R package. Bioconductor 2017. [Google Scholar] [CrossRef]
- McMurdie, P.J.; Holmes, S. Phyloseq: An R Package for Reproducible Interactive Analysis and Graphics of Microbiome Census Data. PLoS ONE 2013, 8, e61217. [Google Scholar] [CrossRef] [Green Version]
- Mallick, H.; Rahnavard, A.; McIver, L.J.; Ma, S.; Zhang, Y.; Nguyen, L.H.; Tickle, T.L.; Weingart, G.; Ren, B.; Schwager, E.H.; et al. Multivariable Association Discovery in Population-Scale Meta-Omics Studies. PLoS Comput. Biol. 2021, 17, e1009442. [Google Scholar] [CrossRef]
- Gérard, P. Gut Microbiota and Obesity. Cell. Mol. Life Sci. 2016, 73, 147–162. [Google Scholar] [CrossRef]
- Cardona, F.; Andrés-Lacueva, C.; Tulipani, S.; Tinahones, F.J.; Queipo-Ortuño, M.I. Benefits of Polyphenols on Gut Microbiota and Implications in Human Health. J. Nutr. Biochem. 2013, 24, 1415–1422. [Google Scholar] [CrossRef] [Green Version]
- Kothavade, P.S.; Nagmoti, D.M.; Bulani, V.D.; Juvekar, A.R. Arzanol, a Potent MPGES-1 Inhibitor: Novel Anti-Inflammatory Agent. Sci. World J. 2013, 2013, 986429. [Google Scholar] [CrossRef] [Green Version]
- Loader, T.B.; Taylor, C.G.; Zahradka, P.; Jones, P.J.H. Chlorogenic Acid from Coffee Beans: Evaluating the Evidence for a Blood Pressure-Regulating Health Claim. Nutr. Rev. 2017, 75, 114–133. [Google Scholar] [CrossRef]
- Tajik, N.; Tajik, M.; Mack, I.; Enck, P. The Potential Effects of Chlorogenic Acid, the Main Phenolic Components in Coffee, on Health: A Comprehensive Review of the Literature. Eur. J. Nutr. 2017, 56, 2215–2244. [Google Scholar] [CrossRef]
- Monteiro, M.; Farah, A.; Perrone, D.; Trugo, L.C.; Donangelo, C. Chlorogenic Acid Compounds from Coffee Are Differentially Absorbed and Metabolized in Humans. J. Nutr. 2007, 137, 2196–2201. [Google Scholar] [CrossRef] [Green Version]
- Miller, A.M.; Asquith, D.L.; Hueber, A.J.; Anderson, L.A.; Holmes, W.M.; McKenzie, A.N.; Xu, D.; Sattar, N.; McInnes, I.B.; Liew, F.Y. Interleukin-33 Induces Protective Effects in Adipose Tissue Inflammation during Obesity in Mice. Circ. Res. 2010, 107, 650–658. [Google Scholar] [CrossRef]
- Nani, A.; Murtaza, B.; Sayed Khan, A.; Khan, N.A.; Hichami, A. Antioxidant and Anti-Inflammatory Potential of Polyphenols Contained in Mediterranean Diet in Obesity: Molecular Mechanisms. Molecules 2021, 26, 985. [Google Scholar] [CrossRef]
- Corrêa, T.A.F.; Rogero, M.M.; Hassimotto, N.M.A.; Lajolo, F.M. The Two-Way Polyphenols-Microbiota Interactions and Their Effects on Obesity and Related Metabolic Diseases. Front. Nutr. 2019, 6, 188. [Google Scholar] [CrossRef] [Green Version]
- Klimenko, N.S.; Tyakht, A.V.; Popenko, A.S.; Vasiliev, A.S.; Altukhov, I.A.; Ischenko, D.S.; Shashkova, T.I.; Efimova, D.A.; Nikogosov, D.A.; Osipenko, D.A.; et al. Microbiome Responses to an Uncontrolled Short-Term Diet Intervention in the Frame of the Citizen Science Project. Nutrients 2018, 10, 576. [Google Scholar] [CrossRef] [Green Version]
- Le Chatelier, E.; Nielsen, T.; Qin, J.; Prifti, E.; Hildebrand, F.; Falony, G.; Almeida, M.; Arumugam, M.; Batto, J.-M.; Kennedy, S.; et al. Richness of Human Gut Microbiome Correlates with Metabolic Markers. Nature 2013, 500, 541–546. [Google Scholar] [CrossRef]
- Rizzatti, G.; Lopetuso, L.R.; Gibiino, G.; Binda, C.; Gasbarrini, A. Proteobacteria: A Common Factor in Human Diseases. BioMed Res. Int. 2017, 2017, 9351507. [Google Scholar] [CrossRef] [Green Version]
- Hiippala, K.; Kainulainen, V.; Suutarinen, M.; Heini, T.; Bowers, J.R.; Jasso-Selles, D.; Lemmer, D.; Valentine, M.; Barnes, R.; Engelthaler, D.M.; et al. Isolation of Anti-Inflammatory and Epithelium Reinforcing Bacteroides and Parabacteroides spp. from A Healthy Fecal Donor. Nutrients 2020, 12, 935. [Google Scholar] [CrossRef] [Green Version]
- Candela, M.; Maccaferri, S.; Turroni, S.; Carnevali, P.; Brigidi, P. Functional Intestinal Microbiome, New Frontiers in Prebiotic Design. Int. J. Food Microbiol. 2010, 140, 93–101. [Google Scholar] [CrossRef]
- Clemente, J.C.; Ursell, L.K.; Parfrey, L.W.; Knight, R. The Impact of the Gut Microbiota on Human Health: An Integrative View. Cell 2012, 148, 1258–1270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lazar, V.; Ditu, L.-M.; Pircalabioru, G.G.; Gheorghe, I.; Curutiu, C.; Holban, A.M.; Picu, A.; Petcu, L.; Chifiriuc, M.C. Aspects of Gut Microbiota and Immune System Interactions in Infectious Diseases, Immunopathology, and Cancer. Front. Immunol. 2018, 9, 1830. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rastmanesh, R. High Polyphenol, Low Probiotic Diet for Weight Loss Because of Intestinal Microbiota Interaction. Chem. Biol. Interact. 2011, 189, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Koliada, A.; Syzenko, G.; Moseiko, V.; Budovska, L.; Puchkov, K.; Perederiy, V.; Gavalko, Y.; Dorofeyev, A.; Romanenko, M.; Tkach, S.; et al. Association between Body Mass Index and Firmicutes/Bacteroidetes Ratio in an Adult Ukrainian Population. BMC Microbiol. 2017, 17, 120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lyu, M.; Wang, Y.-F.; Fan, G.-W.; Wang, X.-Y.; Xu, S.-Y.; Zhu, Y. Balancing Herbal Medicine and Functional Food for Prevention and Treatment of Cardiometabolic Diseases through Modulating Gut Microbiota. Front. Microbiol. 2017, 8, 2146. [Google Scholar] [CrossRef] [Green Version]
- Pataky, Z.; Genton, L.; Spahr, L.; Lazarevic, V.; Terraz, S.; Gaïa, N.; Rubbia-Brandt, L.; Golay, A.; Schrenzel, J.; Pichard, C. Impact of Hypocaloric Hyperproteic Diet on Gut Microbiota in Overweight or Obese Patients with Nonalcoholic Fatty Liver Disease: A Pilot Study. Dig. Dis. Sci. 2016, 61, 2721–2731. [Google Scholar] [CrossRef]
- Gomez-Arango, L.F.; Barrett, H.L.; McIntyre, H.D.; Callaway, L.K.; Morrison, M.; Dekker Nitert, M.; SPRING Trial Group. Connections Between the Gut Microbiome and Metabolic Hormones in Early Pregnancy in Overweight and Obese Women. Diabetes 2016, 65, 2214–2223. [Google Scholar] [CrossRef] [Green Version]
- Harrison, C.A.; Laubitz, D.; Ohland, C.L.; Midura-Kiela, M.T.; Patil, K.; Besselsen, D.G.; Jamwal, D.R.; Jobin, C.; Ghishan, F.K.; Kiela, P.R. Microbial Dysbiosis Associated with Impaired Intestinal Na+/H+ Exchange Accelerates and Exacerbates Colitis in Ex-Germ Free Mice. Mucosal Immunol. 2018, 11, 1329–1341. [Google Scholar] [CrossRef] [Green Version]
- Gao, Z.; Yin, J.; Zhang, J.; Ward, R.E.; Martin, R.J.; Lefevre, M.; Cefalu, W.T.; Ye, J. Butyrate Improves Insulin Sensitivity and Increases Energy Expenditure in Mice. Diabetes 2009, 58, 1509–1517. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, B.; Zhong, M.; Shen, Q.; Wu, Y.; Cao, M.; Ju, S.; Chen, L. Gut Microbiota in Early Pregnancy among Women with Hyperglycaemia vs. Normal Blood Glucose. BMC Pregnancy Childbirth 2020, 20, 284. [Google Scholar] [CrossRef]
- Tuovinen, E.; Keto, J.; Nikkilä, J.; Mättö, J.; Lähteenmäki, K. Cytokine Response of Human Mononuclear Cells Induced by Intestinal Clostridium Species. Anaerobe 2013, 19, 70–76. [Google Scholar] [CrossRef]
- Kostic, A.D.; Gevers, D.; Siljander, H.; Vatanen, T.; Hyötyläinen, T.; Hämäläinen, A.-M.; Peet, A.; Tillmann, V.; Pöhö, P.; Mattila, I.; et al. The Dynamics of the Human Infant Gut Microbiome in Development and in Progression toward Type 1 Diabetes. Cell Host Microbe 2015, 17, 260–273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kemperman, R.A.; Gross, G.; Mondot, S.; Possemiers, S.; Marzorati, M.; de Wiele, T.V.; Doré, J.; Vaughan, E.E. Impact of Polyphenols from Black Tea and Red Wine/Grape Juice on a Gut Model Microbiome. Food Res. Int. 2013, 2, 659–669. [Google Scholar] [CrossRef]
- Costabile, A.; Bergillos-Meca, T.; Rasinkangas, P.; Korpela, K.; de Vos, W.M.; Gibson, G.R. Effects of Soluble Corn Fiber Alone or in Synbiotic Combination with Lactobacillus Rhamnosus GG and the Pilus-Deficient Derivative GG-PB12 on Fecal Microbiota, Metabolism, and Markers of Immune Function: A Randomized, Double-Blind, Placebo-Controlled, Crossover Study in Healthy Elderly (Saimes Study). Front. Immunol. 2017, 8, 1443. [Google Scholar] [CrossRef] [PubMed]
- Koh, A.; De Vadder, F.; Kovatcheva-Datchary, P.; Bäckhed, F. From Dietary Fiber to Host Physiology: Short-Chain Fatty Acids as Key Bacterial Metabolites. Cell 2016, 165, 1332–1345. [Google Scholar] [CrossRef] [Green Version]
- Lopez-Siles, M.; Duncan, S.H.; Garcia-Gil, L.J.; Martinez-Medina, M. Faecalibacterium Prausnitzii: From Microbiology to Diagnostics and Prognostics. ISME J. 2017, 11, 841–852. [Google Scholar] [CrossRef]
- Quévrain, E.; Maubert, M.A.; Michon, C.; Chain, F.; Marquant, R.; Tailhades, J.; Miquel, S.; Carlier, L.; Bermúdez-Humarán, L.G.; Pigneur, B.; et al. Identification of an Anti-Inflammatory Protein from Faecalibacterium Prausnitzii, a Commensal Bacterium Deficient in Crohn’s Disease. Gut 2016, 65, 415–425. [Google Scholar] [CrossRef] [Green Version]
- Walters, W.A.; Xu, Z.; Knight, R. Meta-Analyses of Human Gut Microbes Associated with Obesity and IBD. FEBS Lett. 2014, 588, 4223–4233. [Google Scholar] [CrossRef] [Green Version]
- Lozano, C.P.; Wilkens, L.R.; Shvetsov, Y.B.; Maskarinec, G.; Park, S.-Y.; Shepherd, J.A.; Boushey, C.J.; Hebert, J.R.; Wirth, M.D.; Ernst, T.; et al. Associations of the Dietary Inflammatory Index with Total Adiposity and Ectopic Fat through the Gut Microbiota, LPS, and C-Reactive Protein in the Multiethnic Cohort-Adiposity Phenotype Study. Am. J. Clin. Nutr. 2022, 115, 1344–1356. [Google Scholar] [CrossRef]
- Chambers, E.S.; Viardot, A.; Psichas, A.; Morrison, D.J.; Murphy, K.G.; Zac-Varghese, S.E.K.; MacDougall, K.; Preston, T.; Tedford, C.; Finlayson, G.S.; et al. Effects of Targeted Delivery of Propionate to the Human Colon on Appetite Regulation, Body Weight Maintenance and Adiposity in Overweight Adults. Gut 2015, 64, 1744–1754. [Google Scholar] [CrossRef]
- Albenberg, L.; Esipova, T.V.; Judge, C.P.; Bittinger, K.; Chen, J.; Laughlin, A.; Grunberg, S.; Baldassano, R.N.; Lewis, J.D.; Li, H.; et al. Correlation between Intraluminal Oxygen Gradient and Radial Partitioning of Intestinal Microbiota. Gastroenterology 2014, 147, 1055–1063.e8. [Google Scholar] [CrossRef] [Green Version]
- Takakura, W.; Pimentel, M. Small Intestinal Bacterial Overgrowth and Irritable Bowel Syndrome—An Update. Front. Psychiatry 2020, 11, 664. [Google Scholar] [CrossRef]
- Rao, S.S.C.; Bhagatwala, J. Small Intestinal Bacterial Overgrowth: Clinical Features and Therapeutic Management. Clin. Transl. Gastroenterol. 2019, 10, e00078. [Google Scholar] [CrossRef]
- Antunes Viegas, D.; Palmeira-de-Oliveira, A.; Salgueiro, L.; Martinez-de-Oliveira, J.; Palmeira-de-Oliveira, R. Helichrysum Italicum: From Traditional Use to Scientific Data. J. Ethnopharmacol. 2014, 151, 54–65. [Google Scholar] [CrossRef]
- Suzuki, A.; Ito, M.; Hamaguchi, T.; Mori, H.; Takeda, Y.; Baba, R.; Watanabe, T.; Kurokawa, K.; Asakawa, S.; Hirayama, M.; et al. Quantification of Hydrogen Production by Intestinal Bacteria That Are Specifically Dysregulated in Parkinson’s Disease. PLoS ONE 2018, 13, e0208313. [Google Scholar] [CrossRef] [Green Version]
- Srivastava, D.; Ghoshal, U.; Mittal, R.D.; Ghoshal, U.C. Associations between IL-1RA Polymorphisms and Small Intestinal Bacterial Overgrowth among Patients with Irritable Bowel Syndrome from India. Neurogastroenterol. Motil. 2014, 26, 1408–1416. [Google Scholar] [CrossRef]
- Khairudin, M.A.S.; Mhd Jalil, A.M.; Hussin, N. Effects of Polyphenols in Tea (Camellia sinensis sp.) on the Modulation of Gut Microbiota in Human Trials and Animal Studies. Gastroenterol. Insights 2021, 12, 202–216. [Google Scholar] [CrossRef]




| Helichrysum arenarium Group (N = 13) | Helichrysum italicum Group (N = 14) | |||||
|---|---|---|---|---|---|---|
| Baseline | Week 4 | Follow-Up | Baseline | Week 4 | Follow-Up | |
| IL-6 (pg/mL) | 2.06 ± 2.60 | 1.74 ± 2.43 | 1.67 ± 1.86 | 4.13 ± 5.34 | 2.74 ± 3.14 a,c | 4.12 ± 4.48 |
| IL-1ß (pg/mL) | 1.06 ± 0.59 | 0.61 ± 0.47 a | 0.59 ± 0.56 c | 2.02 ± 1.97 | 1.81 ± 2.56 c | 1.58 ± 2.99 |
| MCP-1 (pg/mL) | 70.66 ± 28.89 | 90.91 ± 41.38 | 92.53 ± 39.44 c | 101.53 ± 46.52 b | 97.32 ± 45.99 c | 96.90 ± 64.24 |
| IL-33 (pg/mL) | 25.06 ± 9.81 | 32.65 ± 30.99 | 36.74 ± 11.93 a | 26.02 ± 14.37 | 40.86 ± 33.50 a | 28.94 ± 14.61 |
| Zonulin (ng/mL) | 6.06 ± 3.04 | 6.93 ± 3.34 | 6.95 ± 3.86 | 6.19 ± 1.91 | 5.65 ± 1.53 c | 6.68 ± 3.28 |
| Helichrysum arenarium Group (N = 13); Median/Mean ± SD | Helichrysum italicum Group (N = 14); Median/Mean ± SD | |||
|---|---|---|---|---|
| Baseline | Week 4 | Baseline | Week 4 | |
| Phylum Bacteroidota | 50.41/49.39 ± 18.12 | 62.59/55.82 ± 21.3 | 49.18/52.05 ± 19.13 | 64.86/59.15 ± 19.12 |
| Genera | ||||
| Bacteroides | 36.17/33.56 ± 22.11 | 25.98/38.8 ± 27.01 | 27.85/35.82 ± 23.98 | 46.13/43 ± 24.96 |
| Alistipes | 4.39/4.41 ± 2.67 | 2.76/3.72 ± 2.78 | 4.71/4.88 ± 3.92 | 4.60/4.74 ± 3.22 |
| Parabacteroides | 0.93/1.11 ± 0.51 | 1.86/1.80 ± 1.07 | 0.98/1.47 ± 1.06 | 1.28/1.32 ± 0.68 |
| Barnesiella | 0.43/1.09 ± 2.09 | 0.49/0.55 ± 0.53 | 0.35/0.96 ± 1.25 | 0.32/0.74 ± 0.85 |
| Odoribacter | 0.45/0.47 ± 0.35 | 0.53/0.47 ± 0.3 | 0.40/0.64 ± 0.66 | 0.63/0.68 ± 0.44 |
| Phylum Firmicutes | 41.47/41.67 ± 14.82 | 33.43/35.57 ± 19.18 | 35.68/37.06 ± 18.78 | 29.65/33.66 ± 18 |
| Genera | ||||
| Faecalibacterium | 5.52/6.58 ± 3.73 | 3.58/5.17 ± 4.15 | 9.35/9.74 ± 6.86 | 6.39/8.28 ± 6.27 |
| Lachnospiraceae NK4A136 | 1.61/2.30 ± 2.84 | 2.46/2.32 ± 2.27 | 1.34/1.57 ± 1.32 | 1.39/1.99 ± 2.22 |
| Clostridia UCG-014 | 1.48/2.74 ± 3.49 | 1.38/1.8 ± 1.57 | 0.70/1.12 ± 1.4 | 0.56/1.62 ± 3.43 |
| Roseburia | 1.48/1.96 ± 1.77 | 1.07/1.9 ± 2.48 | 0.82/1.48 ± 1.53 | 1.64/1.7 ± 1.39 |
| Agathobacter | 2.99/2.96 ± 2.43 | 1.66/2.08 ± 2.48 | 0.82/0.95 ± 0.74 | 0.71/0.96 ± 1.03 |
| Subdoligranulum | 1.17/1.21 ± 0.76 | 1.62/1.47 ± 0.88 | 0.95/1.33 ± 1.07 | 0.85/1.39 ± 2.21 |
| Lachnospiraceae unidentified | 1.13/1.41 ± 1.38 | 0.67/0.86 ± 0.55 | 1.19/1.48 ± 1.17 | 1.25/1.62 ± 1.59 |
| Oscillospiraceae UCG-002 | 0.99/1.62 ± 1.76 | 1.14/1.11 ± 0.83 | 0.94/1.25 ± 1.09 | 0.85/1.09 ± 1.05 |
| Christensenellaceae R-7 | 0.36/1.17 ± 2.21 | 0.19/1.39 ± 3.65 | 0.57/1.06 ± 1.36 | 0.28/0.67 ± 0.94 |
| Coprococcus | 0.82/0.74 ± 0.33 | 0.79/0.81 ± 0.53 | 0.67/1.10 ± 1.29 | 0.69/0.91 ± 0.74 |
| Ruminococcus | 0.53/0.75 ± 0.85 | 0.32/0.53 ± 0.66 | 0.42/1.12 ± 1.68 | 0.52/1.08 ± 1.43 |
| Blautia | 0.99/1.05 ± 0.54 | 0.52/0.64 ± 0.58 a | 0.79/0.86 ± 0.56 | 0.53/0.6 ± 0.38 |
| Lachnosphira | 0.43/0.63 ± 0.96 | 0.38/1.03 ± 2.2 | 0.50/0.52 ± 0.4 | 0.15/0.26 ± 0.29 |
| [Eubacterium] coprost. group | 0.26/0.53 ± 0.84 | 0.14/0.38 ± 0.55 | 0.42/0.67 ± 0.66 | 0.22/0.65 ± 1.07 |
| Ruminococcaceae CAG-352 | 0.22/0.47 ± 0.68 | 0.17/0.24 ± 0.27 | 0.42/0.94 ± 1.98 | 0.39/0.46 ± 0.47 |
| Clostridia vanidin BB60 | 0.50/0.81 ± 1.11 | 0.29/0.52 ± 0.6 | 0.38/0.53 ± 0.62 | 0.06/0.27 ± 0.43 a |
| Lachnospiraceae UCG-004 | 0.47/0.45 ± 0.33 | 0.26/0.35 ± 0.28 | 0.47/0.48 ± 0.39 | 0.48/0.73 ± 0.82 |
| Phylum Proteobacteria | 6.05/6.74 ± 5.67 | 5.40/5.68 ± 3.21 | 5.43/8.79 ± 8.14 | 3.53/4.62 ± 3.56 |
| Genera | ||||
| Sutterella | 3.06/2.75 ± 1.81 | 2.57/2.92 ± 1.59 | 1.97/2.7 ± 2.5 | 1.06/1.74 ± 1.44 |
| Helichrysum arenarium Group (N = 13) | Helichrysum italicum Group (N = 14) | |||||
|---|---|---|---|---|---|---|
| Scores | Baseline | Week 4 | Follow-up | Baseline | Week 4 | Follow-up |
| Nausea | 1.15 ± 0.38 | 1.00 ± 0.00 | 1.00 ± 0.00 | 1.14 ± 0.54 | 1.50 ± 0.94 | 1.29 ± 0.61 |
| Bloating | 2.62 ± 1.04 | 1.69 ± 0.86 a | 1.54 ± 0.52 a | 2.36 ± 1.08 | 1.71 ± 0.83 a | 1.93 ± 0.92 |
| Borborygmi | 2.00 ± 0.91 | 1.38 ± 0.65 a | 1.38 ± 0.51 a | 2.07 ± 0.62 | 1.86 ± 0.95 | 1.79 ± 0.80 |
| Abdominal pain | 1.69 ± 0.95 | 1.08 ± 0.28 a | 1.00 ± 0.00 a | 1.36 ± 0.63 | 1.43 ± 0.85 | 1.43 ± 0.76 |
| Flatulence | 2.61 ± 1.12 | 1.85 ± 0.90 a | 1.85 ± 0.38 a | 2.36 ± 0.93 | 2.00 ± 0.78 a | 2.00 ± 0.68 |
| Burning or epigastric pain | 1.69 ± 1.03 | 1.15 ± 0.56 a | 1.31 ± 0.48 | 1.93 ± 0.92 | 1.43 ± 0.76 a | 1.50 ± 0.76 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petelin, A.; Šik Novak, K.; Hladnik, M.; Bandelj, D.; Baruca Arbeiter, A.; Kramberger, K.; Kenig, S.; Jenko Pražnikar, Z. Helichrysum italicum (Roth) G. Don and Helichrysum arenarium (L.) Moench Infusion Consumption Affects the Inflammatory Status and the Composition of Human Gut Microbiota in Patients with Traits of Metabolic Syndrome: A Randomized Comparative Study. Foods 2022, 11, 3277. https://doi.org/10.3390/foods11203277
Petelin A, Šik Novak K, Hladnik M, Bandelj D, Baruca Arbeiter A, Kramberger K, Kenig S, Jenko Pražnikar Z. Helichrysum italicum (Roth) G. Don and Helichrysum arenarium (L.) Moench Infusion Consumption Affects the Inflammatory Status and the Composition of Human Gut Microbiota in Patients with Traits of Metabolic Syndrome: A Randomized Comparative Study. Foods. 2022; 11(20):3277. https://doi.org/10.3390/foods11203277
Chicago/Turabian StylePetelin, Ana, Karin Šik Novak, Matjaž Hladnik, Dunja Bandelj, Alenka Baruca Arbeiter, Katja Kramberger, Saša Kenig, and Zala Jenko Pražnikar. 2022. "Helichrysum italicum (Roth) G. Don and Helichrysum arenarium (L.) Moench Infusion Consumption Affects the Inflammatory Status and the Composition of Human Gut Microbiota in Patients with Traits of Metabolic Syndrome: A Randomized Comparative Study" Foods 11, no. 20: 3277. https://doi.org/10.3390/foods11203277