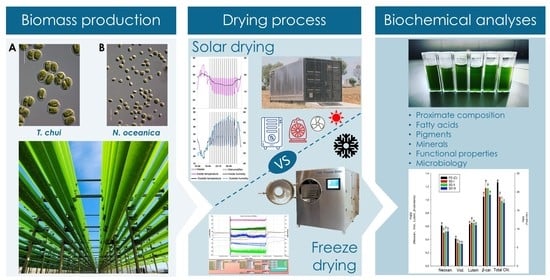
Drying Microalgae Using an Industrial Solar Dryer: A Biomass Quality Assessment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microalgal Biomass Cultivation and Harvesting
2.2. Drying Procedures and Moisture Analyses
2.2.1. Freeze-Drying Trials
2.2.2. Solar Drying
Hybrid Solar Dryer Characteristics and Operation
Experimental Setup
2.2.3. Moisture Analyses
2.3. Biochemical Analyses and Physical Properties
2.3.1. Proximate Composition
Protein Content
Lipid Content
Ash Content
Carbohydrate Content
2.3.2. Pigments’ Analyses
2.3.3. Fatty Acid Profile
2.3.4. Mineral Element Content
2.3.5. Microbial Safety Analyses
2.3.6. Functional Properties
Water- and Oil-Holding Capacities
Foaming Properties
Water Solubility Index
Emulsion Capacity
2.4. Statistical Analyses
3. Results
3.1. Drying Process
3.1.1. Solar-Drying Conditions
3.1.2. Moisture Analyses
3.2. Biomass Analyses
3.2.1. Proximate Composition
3.2.2. Pigment Analyses
3.2.3. Fatty Acid Profile
3.2.4. Mineral Contents
3.2.5. Microbial Safety Analyses
3.2.6. Functional Properties
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO. The State of Food Security and Nutrition in the World. Available online: www.fao.org.http://www.fao.org/state-of-food-security-nutrition/en/ (accessed on 13 February 2022).
- Crist, E.; Mora, C.; Engelman, R. The Interaction of Human Population, Food Production, and Biodiversity Protection. Science 2017, 356, 260–264. [Google Scholar] [CrossRef] [PubMed]
- Lynch, H.; Johnston, C.; Wharton, C. Plant-Based Diets: Considerations for Environmental Impact, Protein Quality, and Exercise Performance. Nutrients 2018, 10, 1841. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joyce, A.; Dixon, S.; Comfort, J.; Hallett, J. Reducing the Environmental Impact of Dietary Choice: Perspectives from a Behavioural and Social Change Approach. J. Environ. Public Health 2012, 2012, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Rzymski, P.; Kulus, M.; Jankowski, M.; Dompe, C.; Bryl, R.; Petitte, J.N.; Kempisty, B.; Mozdziak, P. COVID-19 Pandemic Is a Call to Search for Alternative Protein Sources as Food and Feed: A Review of Possibilities. Nutrients 2021, 13, 150. [Google Scholar] [CrossRef] [PubMed]
- Sexton, A.E.; Garnett, T.; Lorimer, J. Framing the Future of Food: The Contested Promises of Alternative Proteins. Environ. Plann. E 2019, 2, 47–72. [Google Scholar] [CrossRef] [Green Version]
- Dragone, G.; Fernandes, B.; Vicente, A.A.; Teixeira, J.A. Third Generation Biofuels from Microalgae. In Current Research, Technology and Education Topics in Applied Microbiology and Microbial Biotechnology; Mendez-Vilas, A., Ed.; Formatex: Badajos, Spain, 2010; pp. 1355–1366. [Google Scholar]
- Guo, Z.; Liu, Y.; Guo, H.; Yan, S.; Mu, J. Microalgae Cultivation Using an Aquaculture Wastewater as Growth Medium for Biomass and Biofuel Production. J. Environ. Sci. 2013, 25, S85–S88. [Google Scholar] [CrossRef]
- Lam, M.K.; Lee, K.T. Microalgae Biofuels: A Critical Review of Issues, Problems and the Way Forward. Biotechnol. Adv. 2012, 30, 673–690. [Google Scholar] [CrossRef] [PubMed]
- Coêlho, D.d.F.; Tundisi, L.L.; Cerqueira, K.S.; da Silva Rodrigues, J.R.; Mazzola, P.G.; Tambourgi, E.B.; de Souza, R.R. Microalgae: Cultivation Aspects and Bioactive Compounds. Braz. Arch. Biol. Technol. 2019, 62, e19180343. [Google Scholar] [CrossRef]
- Yadav, G.; Dubey, B.K.; Sen, R. A Comparative Life Cycle Assessment of Microalgae Production by CO2 Sequestration from Flue Gas in Outdoor Raceway Ponds under Batch and Semi-Continuous Regime. J. Clean. Prod. 2020, 258, 120703. [Google Scholar] [CrossRef]
- Vrenna, M.; Peruccio, P.P.; Liu, X.; Zhong, F.; Sun, Y. Microalgae as Future Superfoods: Fostering Adoption through Practice-Based Design Research. Sustainability 2021, 13, 2848. [Google Scholar] [CrossRef]
- Yadav, D.K.; Singh, A.; Agrawal, V.; Yadav, N. Algal Biomass: A Natural Resource of High-Value Biomolecules. In Bioprospecting of Plant Biodiversity for Industrial Molecules; Upadhyay, S.K., Singh, S.P., Eds.; Wiley: Hoboken, NJ, USA, 2021; pp. 303–334. [Google Scholar]
- Barkia, I.; Saari, N.; Manning, S.R. Microalgae for High-Value Products Towards Human Health and Nutrition. Mar. Drugs 2019, 17, 304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torres-Tiji, Y.; Fields, F.J.; Mayfield, S.P. Microalgae as a Future Food Source. Biotechnol. Adv. 2020, 41, 107536. [Google Scholar] [CrossRef] [PubMed]
- Lafarga, T. Effect of Microalgal Biomass Incorporation into Foods: Nutritional and Sensorial Attributes of the End Products. Algal Res. 2019, 41, 101566. [Google Scholar] [CrossRef]
- Pereira, H.; Sardinha, M.; Santos, T.; Gouveia, L.; Barreira, L.; Dias, J.; Varela, J. Incorporation of Defatted Microalgal Biomass (Tetraselmis sp. CTP4) at the Expense of Soybean Meal as a Feed Ingredient for Juvenile Gilthead Seabream (Sparus Aurata). Algal Res. 2020, 47, 101869. [Google Scholar] [CrossRef]
- Cavonius, L.R.; Albers, E.; Undeland, I. In Vitro Bioaccessibility of Proteins and Lipids of PH-Shift Processed Nannochloropsis Oculata Microalga. Food Funct. 2016, 7, 2016–2024. [Google Scholar] [CrossRef] [Green Version]
- Ye, C.; Mu, D.; Horowitz, N.; Xue, Z.; Chen, J.; Xue, M.; Zhou, Y.; Klutts, M.; Zhou, W. Life Cycle Assessment of Industrial Scale Production of Spirulina Tablets. Algal Res. 2018, 34, 154–163. [Google Scholar] [CrossRef]
- Pienkos, P.T.; Darzins, A. The Promise and Challenges of Microalgal-Derived Biofuels. Biofuel. Bioprod. Bior. 2009, 3, 431–440. [Google Scholar] [CrossRef]
- Porcelli, R.; Dotto, F.; Pezzolesi, L.; Marazza, D.; Greggio, N.; Righi, S. Comparative Life Cycle Assessment of Microalgae Cultivation for Non-Energy Purposes Using Different Carbon Dioxide Sources. Sci. Total Environ. 2020, 721, 137714. [Google Scholar] [CrossRef] [PubMed]
- Fasaei, F.; Bitter, J.H.; Slegers, P.M.; van Boxtel, A.J.B. Techno-Economic Evaluation of Microalgae Harvesting and Dewatering Systems. Algal Res. 2018, 31, 347–362. [Google Scholar] [CrossRef]
- Chen, C.-L.; Chang, J.-S.; Lee, D.-J. Dewatering and Drying Methods for Microalgae. Dry. Technol. 2015, 33, 443–454. [Google Scholar] [CrossRef]
- Ryckebosch, E.; Muylaert, K.; Eeckhout, M.; Ruyssen, T.; Foubert, I. Influence of Drying and Storage on Lipid and Carotenoid Stability of the Microalga Phaeodactylum tricornutum. J. Agric. Food Chem. 2011, 59, 11063–11069. [Google Scholar] [CrossRef] [PubMed]
- Medipally, S.R.; Yusoff, F.M.; Banerjee, S.; Shariff, M. Microalgae as Sustainable Renewable Energy Feedstock for Biofuel Production. Biomed. Res. Int. 2015, 2015, 519513. [Google Scholar] [CrossRef] [PubMed]
- Nail, S.L.; Jiang, S.; Chongprasert, S.; Knopp, S.A. Fundamentals of Freeze-Drying. In Development and Manufacture of Protein Pharmaceuticals; Nail, S.L., Akers, M.J., Eds.; Springer: Boston, MA, USA, 2002; Volume 14, pp. 281–360. [Google Scholar] [CrossRef]
- Nelson, J.A. Postharvest Degradation of Microalgae: Effect of Temperature and Water Activity. Masters’ Thesis, Utah State University, Logan, UT, USA, 2015. [Google Scholar] [CrossRef]
- Hosseinizand, H.; Lim, C.J.; Webb, E.; Sokhansanj, S. Economic Analysis of Drying Microalgae Chlorella in a Conveyor Belt Dryer with Recycled Heat from a Power Plant. Appl. Therm. Eng. 2017, 124, 525–532. [Google Scholar] [CrossRef]
- Uduman, N.; Qi, Y.; Danquah, M.K.; Forde, G.M.; Hoadley, A. Dewatering of Microalgal Cultures: A Major Bottleneck to Algae-Based Fuels. J. Renew. Sustain. Energy 2010, 2, 012701. [Google Scholar] [CrossRef]
- Silva, J.P.S.; Veloso, C.R.R.; de Souza Barrozo, M.A.; Vieira, L.G.M. Indirect Solar Drying of Spirulina Platensis and the Effect of Operating Conditions on Product Quality. Algal Res. 2021, 60, 102521. [Google Scholar] [CrossRef]
- Lingayat, A.B.; Chandramohan, V.P.; Raju, V.R.K.; Meda, V. A Review on Indirect Type Solar Dryers for Agricultural Crops—Dryer Setup, Its Performance, Energy Storage and Important Highlights. Appl. Energy. 2020, 258, 114005. [Google Scholar] [CrossRef]
- Mohammed, S.; Edna, M.; Siraj, K. The Effect of Traditional and Improved Solar Drying Methods on the Sensory Quality and Nutritional Composition of Fruits: A Case of Mangoes and Pineapples. Heliyon 2020, 6, e04163. [Google Scholar] [CrossRef] [PubMed]
- Foegeding, E.A.; Davis, J.P. Food Protein Functionality: A Comprehensive Approach. Food Hydrocoll. 2011, 25, 1853–1864. [Google Scholar] [CrossRef]
- dos Reis, M.G.; Ribeiro, A. Conversion Factors and General Equations Applied in Agricultural and Forest Meteorology. Agrom. 2020, 27, 227–258. [Google Scholar] [CrossRef] [Green Version]
- Barreira, L.; Resek, E.; Rodrigues, M.J.; Rocha, M.I.; Pereira, H.; Bandarra, N.; da Silva, M.M.; Varela, J.; Custódio, L. Halophytes: Gourmet Food with Nutritional Health Benefits? J. Food Compos. Anal. 2017, 59, 35–42. [Google Scholar] [CrossRef]
- Uslu, L.; Durmaz, Y.; Duyar, H.A.; Bandarra, N.M. Fatty Acids, α-Tocopherol and Proximate Composition of Four Red Macroalgae in the Sinop Bay (Turkey). J. Anim. Vet. Adv. 2013, 12, 29–33. [Google Scholar]
- AOAC. Official Methods of Analysis of the Association of Analytical, 21st ed.; AOAC: Washington, DC, USA, 2019. [Google Scholar]
- Bligh, E.G.G.; Dyer, W.J.J. A Rapid Method of Total Lipid Extraction and Purification. Can. J. Biochem. Phys. 1959, 37, 911–917. [Google Scholar] [CrossRef]
- Pereira, H.; Barreira, L.; Mozes, A.; Florindo, C.; Polo, C.; Duarte, C.V.; Custódio, L.; Varela, J. Microplate-Based High Throughput Screening Procedure for the Isolation of Lipid-Rich Marine Microalgae. Biotechnol. Biofuels. 2011, 4, 61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schüler, L.M.; Bombo, G.; Duarte, P.; Santos, T.F.; Maia, I.B.; Pinheiro, F.; Marques, J.; Jacinto, R.; Schulze, P.S.C.; Pereira, H.; et al. Carotenoid Biosynthetic Gene Expression, Pigment and n-3 Fatty Acid Contents in Carotenoid-Rich Tetraselmis striata CTP4 Strains under Heat Stress Combined with High Light. Bioresource Technol. 2021, 337, 125385. [Google Scholar] [CrossRef] [PubMed]
- Lichtenthaler, H.K.; Wellburn, A.R. Determinations of Total Carotenoids and Chlorophylls a and b of Leaf Extracts in Different Solvents. Biochem. Soc. Trans. 1983, 11, 591–592. [Google Scholar] [CrossRef] [Green Version]
- Vollenweider, R.A. Photosynthetic pigments. In A Manual on Methods for Measuring Primary Production in Aquatic Environments; Vollenweider, R.A., Ed.; Blackwell Scientific Pub.: Oxford, UK, 1974; pp. 21–26. [Google Scholar]
- Lepage, G.; Roy, C.C. Improved Recovery of Fatty Acid through Direct Transesterification without Prior Extraction or Purification. J. Lipid Res. 1984, 25, 1391–1396. [Google Scholar] [CrossRef]
- Pereira, H.; Barreira, L.; Figueiredo, F.; Custódio, L.; Vizetto-Duarte, C.; Polo, C.; Rešek, E.; Engelen, A.; Varela, J. Polyunsaturated Fatty Acids of Marine Macroalgae: Potential for Nutritional and Pharmaceutical Applications. Mar. Drugs 2012, 10, 1920–1935. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bundesamt für Verbraucherschutz und Lebensmittelsicherheit (BVL). Amtliche Sammlung von Untersuchungsverfahren Nach § 64 LFGB; Bundesamt für Verbraucherschutz und Lebensmittelsicherheit: Braunschweig, Germany, 2014. [Google Scholar]
- EN 15510:2007; Animal Feeding Stuffs—Determination of Calcium, Sodium, Phosphorus, Magnesium, Potassium, Iron, Zinc, Copper, Manganese, Cobalt, Molybdenum, Arsenic, Lead and Cadmium by ICP-AES. Beuth Verlag: Berlin, Germany, 2007. (In German)
- ISO 4833-2:2013; Microbiology of the Food Chain—Horizontal Method for the Enumeration of Microorganisms—Part 2: Colony Count at 30 °C by the Surface Plating Technique. ISO: Geneva, Switzerland, 2013. Available online: Https://Www.Iso.Org/Standard/59509.Html (accessed on 17 January 2022).
- ISO 7954:1987; Microbiology—General Guidance for Enumeration of Yeasts and Moulds—Colony Count Technique at 25 °C. ISO: Geneva, Switzerland, 1987. Available online: Https://Www.Iso.Org/Standard/14931.Html (accessed on 17 January 2022).
- ISO 21527-2:2008; Microbiology of Food and Animal Feeding Stuffs—Horizontal Method for the Enumeration of Yeasts and Moulds—Part 2: Colony Count Technique in Products with Water Activity Less than or Equal to 0.95. ISO: Geneva, Switzerland, 2008. Available online: Https://Www.Iso.Org/Standard/38276.Html (accessed on 17 January 2022).
- ISO 16649-2:2001; Microbiology of Food and Animal Feeding Stuffs—Horizontal Method for the Enumeration of Beta-Glucuronidase-Positive Escherichia Coli—Part 2: Colony-Count Technique at 44 °C Using 5-Bromo-4-Chloro-3-Indolyl Beta-D-Glucuronide. ISO: Geneva, Switzerland, 2001. Available online: Https://Www.Iso.Org/Standard/29824.Html (accessed on 17 January 2022).
- Stone, A.K.; Avarmenko, N.A.; Warkentin, T.D.; Nickerson, M.T. Functional Properties of Protein Isolates from Different Pea Cultivars. Food Sci. Biotechnol. 2015, 24, 827–833. [Google Scholar] [CrossRef]
- Xiong, T.; Xiong, W.; Ge, M.; Xia, J.; Li, B.; Chen, Y. Effect of High Intensity Ultrasound on Structure and Foaming Properties of Pea Protein Isolate. Food Res. Int. 2018, 109, 260–267. [Google Scholar] [CrossRef] [PubMed]
- Calderón-Castro, A.; Vega-García, M.O.; de Jesús Zazueta-Morales, J.; Fitch-Vargas, P.R.; Carrillo-López, A.; Gutiérrez-Dorado, R.; Limón-Valenzuela, V.; Aguilar-Palazuelos, E. Effect of Extrusion Process on the Functional Properties of High Amylose Corn Starch Edible Films and Its Application in Mango (Mangifera indica L.) Cv. Tommy Atkins. J. Food Sci. Technol. 2018, 55, 905–914. [Google Scholar] [CrossRef] [PubMed]
- Benelhadj, S.; Gharsallaoui, A.; Degraeve, P.; Attia, H.; Ghorbel, D. Effect of P.H. on the Functional Properties of Arthrospira (Spirulina) Platensis Protein Isolate. Food Chem. 2016, 194, 1056–1063. [Google Scholar] [CrossRef]
- Gouveia, L.; Graça, S.; Sousa, C.; Ambrosano, L.; Ribeiro, B.; Botrel, E.P.; Neto, P.C.; Ferreira, A.F.; Silva, C.M. Microalgae Biomass Production Using Wastewater: Treatment and Costs. Algal Res. 2016, 16, 167–176. [Google Scholar] [CrossRef]
- Balasubramanian, R.K.; Yen Doan, T.T.; Obbard, J.P. Factors Affecting Cellular Lipid Extraction from Marine Microalgae. Chem. Eng. J. 2013, 215–216, 929–936. [Google Scholar] [CrossRef]
- Lin, L.P. Microstructure of Spray-Dried and Freeze-Dried Microalgal Powders. Food Struct. 1985, 4, 17. [Google Scholar]
- Agbede, O.O.; Oke, E.O.; Akinfenwa, S.I.; Wahab, K.T.; Ogundipe, S.; Aworanti, O.A.; Arinkoola, A.O.; Agarry, S.E.; Ogunleye, O.O.; Osuolale, F.N.; et al. Thin Layer Drying of Green Microalgae (Chlorella sp.) Paste Biomass: Drying Characteristics, Energy Requirement and Mathematical Modeling. Bioresource Technol. Rep. 2020, 11, 100467. [Google Scholar] [CrossRef]
- Viswanathan, T.; Mani, S.; Das, K.C.; Chinnasamy, S.; Bhatnagar, A. Drying Characteristics of a Microalgae Consortium Developed for Biofuels Production. Trans. ASABE 2011, 54, 2245–2252. [Google Scholar] [CrossRef]
- Stramarkou, M.; Papadaki, S.; Kyriakopoulou, K.; Krokida, M. Effect of Drying and Extraction Conditions on the Recovery of Bioactive Compounds from Chlorella vulgaris. J. Appl. Phycol. 2017, 29, 2947–2960. [Google Scholar] [CrossRef]
- Desmorieux, H.; Hernandez, F. Biochemical and Physical Criteria of Spirulina after Different Drying Processes. In Proceedings of the 14th International Drying Symposium (IDS 2004), Sào Paulo, Brazil, 22–25 August 2004; pp. 900–907. [Google Scholar]
- Takaichi, S. Carotenoids in Algae: Distributions, Biosyntheses and Functions. Mar. Drugs 2011, 9, 1101–1118. [Google Scholar] [CrossRef]
- Neves, F.d.F.; Demarco, M.; Tribuzi, G. Drying and Quality of Microalgal Powders for Human Alimentation. In Microalgae—From Physiology to Application, 1st ed.; Vítová, M., Ed.; IntechOpen: London, UK, 2019. [Google Scholar]
- Ljubic, A.; Safafar, H.; Jacobsen, C. Recovery of Microalgal Biomass and Metabolites from Homogenized, Swirl Flash-Dried Microalgae. J. Appl. Phycol. 2019, 31, 2355–2363. [Google Scholar] [CrossRef] [Green Version]
- Hosseini Shekarabi, S.P. Biochemical composition and fatty acid profile of the marine microalga Isochrysis galbana dried with different methods. J. Microbiol. Biotechnol. Food Sci. 2019, 9, 521–524. [Google Scholar] [CrossRef]
- European Parliament and Council of the European Union. Regulation (E.U.) No 1169/2011 of the European Parliament and of the Council of 25 October 2011 on the provision of food information to consumers. Off. J. Eur. Union 2011, 50, 18–63. [Google Scholar]
- U.S. Department of Agriculture. Agricultural Research Service. “FoodData Central”. 2019. Available online: https://fdc.nal.usda.gov/ (accessed on 28 February 2022).
- Schiraldi, C.; De Rosa, M. Mesophilic Organisms. In Encyclopedia of Membranes; Drioli, E., Giorno, L., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 1–2. ISBN 978-3-642-40872-4. [Google Scholar]
- Doğan-Halkman, H.B.; Çakır, İ.; Keven, F.; Worobo, R.W.; Halkman, A.K. Relationship among Fecal Coliforms and Escherichia Coli in Various Foods. Eur. Food Res. Technol. 2003, 216, 331–334. [Google Scholar] [CrossRef]
- Uyttendaele, M.; De Loy-Hendrickx, A.; Vermeulen, A.; Jacxsens, L.; Debevere, J.; Devlieghere, F. Microbiological Guidelines—Support for Interpretation of Microbiological Test Results of Foods; Uyttendaele, M., Ed.; Die Keure Publishing: Bruge, Belgium, 2018; pp. 1–95. [Google Scholar]
- Gaiani, C.; Morand, M.; Sanchez, C.; Tehrany, E.A.; Jacquot, M.; Schuck, P.; Jeantet, R.; Scher, J. How Surface Composition of High Milk Proteins Powders Is Influenced by Spray-Drying Temperature. Colloid. Surface. B 2010, 75, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Köhler, K.; Schuchmann, H.P. Emulgiertechnik: Grundlagen, Verfahren und Anwendungen, 3rd ed.; Behr’s Verlag: Hamburg, Germany, 2012; pp. 1–527. [Google Scholar]
- Walstra, P. Physical Chemistry of Foods; Food Science and Technology; Marcel Dekker: New York, NY, USA, 2003; pp. 41–52. ISBN 978-0-8247-9355-5. [Google Scholar]
- Lam, R.S.H.; Nickerson, M.T. Food Proteins: A Review on Their Emulsifying Properties Using a Structure–Function Approach. Food Chem. 2013, 141, 975–984. [Google Scholar] [CrossRef]
- Nakai, S.; Ho, L.; Helbig, N.; Kato, A.; Tung, M.A. Relationship Between Hydrophobicity and Emulsifying Properties of Some Plant Proteins. Can. Inst. Food Sci. Technol. J. 1980, 13, 23–27. [Google Scholar] [CrossRef]
- Liang, H.-N.; Tang, C.-H. PH-Dependent Emulsifying Properties of Pea Pisum sativum (L.) Proteins. Food Hydrocoll. 2013, 33, 309–319. [Google Scholar] [CrossRef]
- McClements, D.J. Food Emulsions: Principles, Practices, and Techniques, 3rd ed.; CRC Press: Boca Raton, FL, USA, 1999; pp. 1–714. [Google Scholar]
- Guil-Guerrero, J.L.; Navarro-Juárez, R.; López-Martínez, J.C.; Campra-Madrid, P.; Rebolloso-Fuentes, M. Functional Properties of the Biomass of Three Microalgal Species. J. Food Eng. 2004, 65, 511–517. [Google Scholar] [CrossRef]
- Kamath, S.; Huppertz, T.; Houlihan, A.V.; Deeth, H.C. The Influence of Temperature on the Foaming of Milk. Int. Dairy J. 2008, 18, 994–1002. [Google Scholar] [CrossRef]
- Raikos, V. Effect of Heat Treatment on Milk Protein Functionality at Emulsion Interfaces. A Review. Food Hydrocoll. 2010, 24, 259–265. [Google Scholar] [CrossRef]






| Parameters | SD I | SD II | SD III |
|---|---|---|---|
| Indoor temperature min.–max. (°C) | 23.9–49.3 | 24.3–39.0 | 27.4–46.0 |
| Average indoor temperature (°C) | 35.4 | 33.5 | 35.3 |
| Indoor humidity min.–max. (%) | 15.3–41.9 | 23.4–47.6 | 18.7–46.9 |
| Average indoor humidity (%) | 28.4 | 32.1 | 32.2 |
| Outdoor temperature min.–max. (°C) | 10.9–21.8 | 16.2–20.1 | 16.7–20.5 |
| Average outdoor temperature (°C) | 15.63 | 18.20 | 18.21 |
| Outdoor humidity min.–max. (%) | 51.1–91.7 | 46.1–84.0 | 51.9–91.6 |
| Average outdoor humidity (%) | 76.2 | 65.8 | 76.6 |
| Average solar radiation (Rs; W.m−2) | 275.3 | 114.1 | 209.9 |
| Proximate Composition (%) | Tetraselmis chui | Nannochloropsis oceanica | ||||||
|---|---|---|---|---|---|---|---|---|
| SD I | SD II | SD III | FDc | SD I | SD II | SD III | FDc | |
| Proteins | 38.53 ± 0.78 a | 37.80 ± 0.64 a | 38.01 ± 0.88 a | 37.93 ± 0.11 a | 46.72 ± 0.31 a | 46.01 ± 0.94 a | 48.09 ± 0.41 a | 47.39 ± 2.18 a |
| Total lipids | 18.61 ± 1.46 a | 18.40 ± 1.62 a | 18.24 ± 0.50 a | 16.75 ± 1.62 a | 21.46 ± 2.78 a | 24.69 ± 1.95 a | 23.25 ± 1.97 a | 24.25 ± 3.37 a |
| Carbohydrates | 16.73 ± 1.27 a | 16.06 ± 1.05 a | 16.27 ± 1.18 a | 15.49 ± 1.78 a | 17.11 ± 2.54 a | 14.69 ± 2.61 a | 14.24 ± 1.62 a | 15.28 ± 4.25 a |
| Ashes | 28.46 ± 0.06 a | 30.44 ± 0.06 a | 30.24 ± 0.05 a | 30.86 ± 0.10 a | 14.71 ± 0.28 b | 14.62 ± 0.45 b | 14.42 ± 0.15 b | 13.08 ± 0.16 a |
| Fatty Acid (%) | Tetraselmis chui | Nannochloropsis oceanica | ||||||
|---|---|---|---|---|---|---|---|---|
| SD I | SD II | SD III | FDc | SD I | SD II | SD III | FDc | |
| C14:0 | 1.12 ± 0.22 | 1.07 ± 0.15 | 1.05 ± 0.17 | 1.16 ± 0.18 | 4.85 ± 1.67 | 4.18 ± 0.74 | 4.20 ± 0.77 | 4.22 ± 0.61 |
| C16:0 | 20.48 ± 1.88 | 20.58 ± 1.87 | 20.67 ± 1.68 | 22.41 ± 1.91 | 19.95 ± 5.60 | 18.09 ± 3.52 | 17.79 ± 3.29 | 18.11 ± 2.89 |
| ∑ SFA | 21.59 ± 2.10 | 21.65 ± 2.03 | 21.72 ± 1.84 | 23.57 ± 2.09 | 24.80 ± 7.26 | 22.28 ± 4.26 | 21.99 ± 4.06 | 22.32 ± 3.50 |
| C16:1n-9 | 3.96 ± 1.92 | 3.84 ± 1.20 | 3.02 ± 1.97 | 4.12 ± 1.22 | 25.30 ± 7.68 | 22.03 ± 3.33 | 21.80 ± 3.43 | 21.94 ± 2.85 |
| C18:1n-9 | 17.29 ± 0.73 | 17.78 ± 0.49 | 17.96 ± 0.60 | 16.09 ± 4.42 | 3.61 ± 0.63 | 2.70 ± 0.80 | 2.95 ± 0.71 | 3.37±0.29 |
| C20:1 | 1.14 ± 0.68 | 1.20 ± 0.70 | 1.42 ± 0.34 | 1.48 ± 0.33 | n.d. | n.d. | n.d. | n.d. |
| ∑ MUFA | 22.39 ± 3.34 | 22.82 ± 2.40 | 22.40 ± 2.91 | 21.69 ± 5.97 | 28.9 ± 8.31 | 24.74 ± 4.13 | 24.75 ± 4.14 | 25.31 ± 3.14 |
| C16:3n-3 | 1.56 ± 0.20 | 1.57 ± 0.16 | 1.58 ± 0.20 | 1.58 ± 0.17 | n.d. | n.d. | n.d. | n.d. |
| C16:4n-3 | 19.32 ± 1.01 | 19.43 ± 0.52 | 19.87 ± 0.96 | 19.49 ± 1.10 | n.d. | n.d. | n.d. | n.d. |
| C18:2n-6 | 5.09 ± 0.18 | 5.17 ± 0.11 | 5.24 ± 0.20 | 5.36 ± 0.40 | 4.61 ± 0.89 | 3.89 ± 0.34 | 3.92 ± 0.39 | 4.22 ± 0.21 |
| C18:3n-6 | 5.08 ± 0.39 | 5.06 ± 0.29 | 5.17 ± 0.34 | 5.14 ± 0.58 | n.d. | n.d. | n.d. | n.d. |
| C18:4n-3 | 9.03 ± 0.67 | 9.01 ± 0.38 | 9.19 ± 0.57 | 8.89 ± 0.92 | n.d. | n.d. | n.d. | n.d. |
| C20:4n-6 | 1.84 ± 0.26 | 1.70 ± 0.23 | 1.62 ± 0.24 | 1.62 ± 0.29 | 10.31 ± 0.66 | 8.69 ± 1.24 | 9.20 ± 0.60 | 9.31 ± 0.34 |
| C20:5n-3 | 12.12 ± 3.50 | 11.47 ± 3.15 | 11.15 ± 3.31 | 10.62 ± 3.58 | 28.72 ± 9.22 | 37.95 ± 6.70 | 37.29 ± 7.25 | 36.37 ± 7.27 |
| ∑ PUFA | 54.04 ± 6.11 | 53.42 ± 4.85 | 53.82 ± 5.83 | 52.69 ± 7.05 | 43.64 ± 10.76 | 50.53 ± 8.28 | 50.41 ± 8.24 | 49.89 ± 7.82 |
| Minerals (mg/g) | Tetraselmis chui | Nannochloropsis oceanica | ||||||
|---|---|---|---|---|---|---|---|---|
| SD I | SD II | SD III | FDc | SD I | SD II | SD III | FDc | |
| Phosphorus | 10.73 ± 0.06 b | 10.67 ± 0.02 ab | 10.75 ± 0.08 b | 10.53 ± 0.07 a | 11.44 ± 0.07 a | 11.51 ± 0.06 a | 11.48 ± 0.08 a | 11.26 ± 0.34 a |
| Sodium | 56.67 ± 2.28 d | 59.29 ± 1.57 c | 60.28 ± 0.04 b | 68.99 ± 0.45 a | 30.22 ± 0.44 b | 28.84 ± 0.16 a | 27.14 ± 0.80 a | 37.58 ± 0.24 a |
| Potassium | 17.28 ± 0.16 a | 17.73 ± 0.04 a | 17.64 ± 0.39 a | 18.54 ± 0.03 b | 5.07 ± 0.03 ab | 5.09 ± 0.09 b | 4.92 ± 0.06 a | 5.42 ± 0.03 c |
| Magnesium | 10.98 ± 0.07 a | 10.91 ± 0.12 a | 11.04 ± 0.10 a | 11.46 ± 0.07 b | 7.92 ± 0.08 a | 7.93 ± 0.07 a | 7.96 ± 0.12 a | 8.10 ± 0.03 a |
| Calcium | 16.46 ± 0.07 b | 16.37 ± 0.16 a | 16.02 ± 0.23 a | 16.33 ± 0.06 ab | 4.10 ± 0.03 b | 4.06 ± 0.03 ab | 4.05 ± 0.04 ab | 4.01 ± 0.04 a |
| Iron | 2.75 ± 1.55 b | 2.75 ± 0.31 b | 2.78 ± 0.78 b | 2.68 ± 1.92 a | 0.72 ± 2.71 a | 0.70 ± 0.91 a | 0.72 ± 0.06 a | 0.70 ± 0.48 a |
| Copper | 0.01 ± 0.00 ab | 0.01 ± 0.00 ab | 0.01 ± 0.00 b | 0.01 ± 0.00 a | 0.02 ± 0.00 a | 0.02 ± 0.00 a | 0.02 ± 0.00 b | 0.02 ± 0.00 a |
| Manganese | 0.11 ± 0.00 c | 0.10 ± 0.00 b | 0.11 ± 0.00 c | 0.10 ± 0.00 a | 0.04 ± 0.00 a | 0.04 ± 0.00 a | 0.05 ± 0.00 b | 0.04 ± 0.00 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schmid, B.; Navalho, S.; Schulze, P.S.C.; Van De Walle, S.; Van Royen, G.; Schüler, L.M.; Maia, I.B.; Bastos, C.R.V.; Baune, M.-C.; Januschewski, E.; et al. Drying Microalgae Using an Industrial Solar Dryer: A Biomass Quality Assessment. Foods 2022, 11, 1873. https://doi.org/10.3390/foods11131873
Schmid B, Navalho S, Schulze PSC, Van De Walle S, Van Royen G, Schüler LM, Maia IB, Bastos CRV, Baune M-C, Januschewski E, et al. Drying Microalgae Using an Industrial Solar Dryer: A Biomass Quality Assessment. Foods. 2022; 11(13):1873. https://doi.org/10.3390/foods11131873
Chicago/Turabian StyleSchmid, Benjamin, Sofia Navalho, Peter S. C. Schulze, Simon Van De Walle, Geert Van Royen, Lisa M. Schüler, Inês B. Maia, Carolina R. V. Bastos, Marie-Christin Baune, Edwin Januschewski, and et al. 2022. "Drying Microalgae Using an Industrial Solar Dryer: A Biomass Quality Assessment" Foods 11, no. 13: 1873. https://doi.org/10.3390/foods11131873