The Influence of Whey Protein Heating Parameters on Their Susceptibility to Digestive Enzymes and the Antidiabetic Activity of Hydrolysates
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
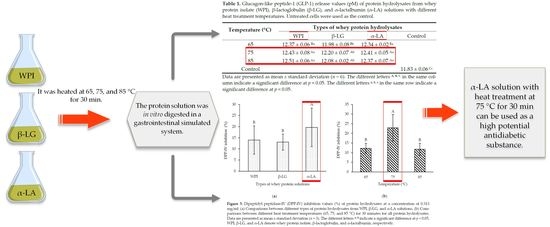
2.2. Preparation of WPI, β-LG, and α-LA Solution
2.3. In Vitro Digestion of Whey Proteins
2.4. Determination of Degree of Hydrolysis (DH)
2.5. Evaluation of Antidiabetic Properties
2.5.1. Glucagon-like Peptide-1 (GLP-1) Quantification Assay
- Cell culture
- Cell viability test
- GLP-1 secretion test
2.5.2. Dipeptidyl Peptidase-IV (DPP-IV) Inhibition Assay
- Measurement of DPP-IV inhibitory activity
- Measurement of DPP-IV half-maximal inhibitory concentration (IC50)
2.6. Statistical Analysis
3. Results
3.1. Degree of Hydrolysis of Protein Hydrolysate
3.2. Glucagon-like Peptide-1 Release Activity
3.3. Dipeptidyl Peptidase-IV Inhibitory Activity
3.4. Principal Component Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, P.-C.; Zhao, S.; Yang, B.-Y.; Wang, Q.-H.; Kuang, H.-X. Anti-diabetic polysaccharides from natural sources: A review. Carbohydr. Polym. 2016, 148, 86–97. Available online: http://www.sciencedirect.com/science/article/pii/S0144861716301254 (accessed on 20 August 2021). [CrossRef] [PubMed]
- Olesen, K.; Cleal, B.; Willaing, I. Discrimination and stigma among people with type 2 diabetes in the workplace: Prejudice against illness or obesity? Public Health 2020, 180, 100–101. Available online: http://www.sciencedirect.com/science/article/pii/S003335061930366X (accessed on 20 August 2021). [CrossRef] [PubMed]
- Omar, B.; Ahrén, B. Pleiotropic Mechanisms for the Glucose-Lowering Action of DPP-4 Inhibitors. Diabetes 2014, 63, 2196–2202. Available online: http://diabetes.diabetesjournals.org/content/63/7/2196.abstract (accessed on 20 August 2021). [CrossRef] [PubMed] [Green Version]
- Zhang, B.B.; Zhou, G.; Li, C. AMPK: An Emerging Drug Target for Diabetes and the Metabolic Syndrome. Cell Metab. 2009, 9, 407–416. Available online: https://www.sciencedirect.com/science/article/pii/S1550413109000904 (accessed on 20 August 2021). [CrossRef] [Green Version]
- Andrade, J.; Pereira, C.G.; Junior, J.C.D.A.; Viana, C.C.R.; Neves, L.N.D.O.; da Silva, P.H.F.; Bell, M.J.V.; Anjos, V.D.C.D. FTIR-ATR determination of protein content to evaluate whey protein concentrate adulteration. LWT 2019, 99, 166–172. Available online: http://www.sciencedirect.com/science/article/pii/S0023643818308107 (accessed on 23 August 2021). [CrossRef]
- Nongonierma, A.B.; FitzGerald, R.J. Strategies for the discovery, identification and validation of milk protein-derived bioactive peptides. Trends Food Sci. Technol. 2016, 50, 26–43. Available online: http://www.sciencedirect.com/science/article/pii/S092422441530114X (accessed on 23 August 2021). [CrossRef] [Green Version]
- Tulipano, G.; Faggi, L.; Nardone, A.; Cocchi, D.; Caroli, A.M. Characterisation of the potential of β-lactoglobulin and α-lactalbumin as sources of bioactive peptides affecting incretin function: In silico and in vitro comparative studies. Int. Dairy J. 2015, 48, 66–72. Available online: http://www.sciencedirect.com/science/article/pii/S0958694615000199 (accessed on 23 August 2021). [CrossRef]
- Flaim, C.; Kob, M.; Di Pierro, A.M.; Herrmann, M.; Lucchin, L. Effects of a whey protein supplementation on oxidative stress, body composition and glucose metabolism among overweight people affected by diabetes mellitus or impaired fasting glucose: A pilot study. J. Nutr. Biochem. 2017, 50, 95–102. Available online: http://www.sciencedirect.com/science/article/pii/S0955286316302790 (accessed on 23 August 2021). [CrossRef]
- Corrochano, A.R.; Arranz, E.; De Noni, I.; Stuknytė, M.; Ferraretto, A.; Kelly, P.M.; Buckin, V.; Giblin, L. Intestinal health benefits of bovine whey proteins after simulated gastrointestinal digestion. J. Funct. Foods 2018, 49, 526–535. Available online: http://www.sciencedirect.com/science/article/pii/S1756464618304754 (accessed on 23 August 2021). [CrossRef]
- Thoma, R.; Löffler, B.; Stihle, M.; Huber, W.; Ruf, A.; Hennig, M. Structural Basis of Proline-Specific Exopeptidase Activity as Observed in Human Dipeptidyl Peptidase-IV. Structure 2003, 11, 947–959. [Google Scholar] [CrossRef] [Green Version]
- White, J.R. Dipeptidyl Peptidase-IV Inhibitors: Pharmacological Profile and Clinical Use. Clin. Diabetes 2008, 26, 53–57. Available online: https://clinical.diabetesjournals.org/content/diaclin/26/2/53.full.pdf (accessed on 26 August 2021). [CrossRef] [Green Version]
- Müller, T.D.; Finan, B.; Bloom, S.R.; D’Alessio, D.; Drucker, D.J.; Flatt, P.R.; Fritsche, A.; Gribble, F.; Grill, H.J.; Habener, J.F.; et al. Glucagon-like peptide 1 (GLP-1). Mol. Metab. 2019, 30, 72–130. Available online: http://www.sciencedirect.com/science/article/pii/S2212877819309135 (accessed on 26 August 2021). [CrossRef] [PubMed]
- Nongonierma, A.B.; FitzGerald, R.J. Structure activity relationship modelling of milk protein-derived peptides with dipeptidyl peptidase IV (DPP-IV) inhibitory activity. Peptides 2016, 79, 1–7. Available online: http://www.sciencedirect.com/science/article/pii/S0196978116300298 (accessed on 26 August 2021). [CrossRef] [PubMed] [Green Version]
- Nongonierma, A.B.; FitzGerald, R.J. An in silico model to predict the potential of dietary proteins as sources of dipeptidyl peptidase IV (DPP-IV) inhibitory peptides. Food Chem. 2014, 165, 489–498. Available online: http://www.sciencedirect.com/science/article/pii/S0308814614008061 (accessed on 30 August 2021). [CrossRef] [PubMed] [Green Version]
- Corrochano, A.R.; Sariçay, Y.; Arranz, E.; Kelly, P.M.; Buckin, V.; Giblin, L. Comparison of antioxidant activities of bovine whey proteins before and after simulated gastrointestinal digestion. J. Dairy Sci. 2019, 102, 54–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Relkin, P.; Mulvihill, D.M. Thermal unfolding of β-lactoglobulin, α-lactalbumin, and bovine serum albumin. A thermodynamic approach. Crit. Rev. Food Sci. Nutr. 1996, 36, 565–601. [Google Scholar] [CrossRef]
- Nongonierma, A.B.; Paolella, S.; Mudgil, P.; Maqsood, S.; FitzGerald, R.J. Dipeptidyl peptidase IV (DPP-IV) inhibitory prop-erties of camel milk protein hydrolysates generated with trypsin. J. Funct. Foods 2017, 34, 49–58. Available online: http://www.sciencedirect.com/science/article/pii/S1756464617302050 (accessed on 30 August 2021). [CrossRef] [Green Version]
- Nongonierma, A.B.; Lalmahomed, M.; Paolella, S.; FitzGerald, R.J. Milk protein isolate (MPI) as a source of dipeptidyl pep-tidase IV (DPP-IV) inhibitory peptides. Food Chem. 2017, 231, 202–211. Available online: http://www.sciencedirect.com/science/article/pii/S0308814617305186 (accessed on 30 August 2021). [CrossRef] [Green Version]
- Zhang, L.; Zhou, R.; Zhang, J.; Zhou, P. Heat-induced denaturation and bioactivity changes of whey proteins. Int. Dairy J. 2021, 123, 105175. Available online: https://www.sciencedirect.com/science/article/pii/S095869462100203X (accessed on 5 January 2022). [CrossRef]
- Arrutia, F.; Puente, Á.; Riera, F.A.; Menéndez, C.; González, U.A. Influence of heat pre-treatment on BSA tryptic hydrolysis and peptide release. Food Chem. 2016, 202, 40–48. Available online: https://www.sciencedirect.com/science/article/pii/S030881461630108X (accessed on 30 August 2021). [CrossRef]
- AOAC International. Official Methods of Analysis of AOAC International, 19th ed.; AOAC International: Gaithersburg, MD, USA, 2012. [Google Scholar]
- Yi, D.; Lin, Q.; Johns, P.W. Estimation of Degree of Hydrolysis of Protein Hydrolysates by Size Exclusion Chromatography. Food Anal. Methods 2021, 14, 805–813. [Google Scholar] [CrossRef]
- Gruppi, A.; Dermiki, M.; Spigno, G.; FitzGerald, R.J. Impact of Enzymatic Hydrolysis and Heat Inactivation on the Physico-chemical Properties of Milk Protein Hydrolysates. Foods 2022, 11, 516. [Google Scholar] [CrossRef] [PubMed]
- Shao, C.; Furusawa, Y.; Aoki, M.; Matsumoto, H.; Ando, K. Nitric oxide-mediated bystander effect induced by heavy-ions in human salivary gland tumour cells. Int. J. Radiat. Biol. 2002, 78, 837–844. [Google Scholar] [CrossRef] [PubMed]
- Jeffers, L.K.; Madden, V.; Webster-Cyriaque, J. BK virus has tropism for human salivary gland cells in vitro: Implications for transmission. Virology 2009, 394, 183–193. Available online: http://www.sciencedirect.com/science/article/pii/S0042682209004267 (accessed on 3 September 2021). [CrossRef] [Green Version]
- Qiu, Y.; Qiu, L.; Cui, J.; Wei, Q. Bacterial cellulose and bacterial cellulose-vaccarin membranes for wound healing. Mater. Sci. Eng. C 2016, 59, 303–309. [Google Scholar] [CrossRef]
- Arteaga-Cardona, F.; Gutiérrez-García, E.; Hidalgo-Tobón, S.; López-Vasquez, C.; Brito-Barrera, Y.A.; Flores-Tochihuitl, J.; Angulo-Molina, A.; Reyes-Leyva, J.R.; González-Rodríguez, R.; Coffer, J.; et al. Cell viability and MRI performance of highly efficient polyol-coated magnetic nanoparticles. J. Nanoparticle Res. 2016, 18, 345. Available online: https://ui.adsabs.harvard.edu/abs/2016JNR....18..345A (accessed on 3 September 2021). [CrossRef]
- Komatsu, Y.; Wada, Y.; Izumi, H.; Shimizu, T.; Takeda, Y.; Hira, T.; Hara, H. Casein materials show different digestion patterns using an in vitro gastrointestinal model and different release of glucagon-like peptide-1 by enteroendocrine GLUTag cells. Food Chem. 2019, 277, 423–431. Available online: http://www.sciencedirect.com/science/article/pii/S030881461831906X (accessed on 3 September 2021). [CrossRef]
- Mousa, S.A.; Ayoub, B.M. Repositioning of dipeptidyl peptidase-4 inhibitors and glucagon like peptide-1 agonists as potential neuroprotective agents. Neural Regen. Res. 2019, 14, 745–748. Available online: http://www.nrronline.org/article.asp?issn=1673-5374;year=2019;volume=14;issue=5;spage=745;epage=748;aulast=Mousa (accessed on 8 September 2021). [CrossRef]
- Chakrabarti, R.; Bhavtaran, S.; Narendra, P.; Varghese, N.; Vanchhawng, L.; Hyder, M.S.S.H.; Thirumurugan, K. Dipeptidyl Peptidase- IV Inhibitory Activity of Berberis aristata. Journal of Natural Products 2011, 4, 158–163. [Google Scholar]
- Hair, J.F.; Black, W.C.; Babin, B.J.; Anderson, R.E. Multivariate Data Analysis, 8th ed.; Cengage Learning: Hampshire, UK, 2019. [Google Scholar]
- Lagace, M. Effect of α-Lactalbumin and β-Lactoglobulin Hydrolysates on Markers of Metabolic Syndrome; University of Manitoba: Winnipeg, MB, Canada, 2012. [Google Scholar]
- Creamer, L.; Parry, D.; Malcolm, G. Secondary structure of bovine β-lactoglobulin B. Arch. Biochem. Biophys. 1983, 227, 98–105. Available online: http://www.sciencedirect.com/science/article/pii/000398618390351X (accessed on 17 February 2022). [CrossRef]
- Permyakov, E.A. α-Lactalbumin. In Encyclopedia of Metalloproteins; Kretsinger, R.H., Uversky, V.N., Permyakov, E.A., Eds.; Springer: New York, NY, USA, 2013; pp. 1070–1076. [Google Scholar] [CrossRef]
- Baum, B.J.; Adriaansen, J.; Cotrim, A.P.; Goldsmith, C.M.; Perez, P.; Qi, S.; Rowzee, A.M.; Zheng, C. Gene therapy of salivary diseases. Methods Mol. Biol. 2010, 666, 3–20. [Google Scholar] [CrossRef] [PubMed]
- Rowzee, A.M.; Cawley, N.X.; Chiorini, J.A.; Di Pasquale, G. Glucagon-Like Peptide-1 Gene Therapy. Exp. Diabetes Res. 2011, 2011, 601047. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Samuni, Y.; Baum, B.J. Gene delivery in salivary glands: From the bench to the clinic. Biochim. Biophys. Acta Mol. Basis Dis. 2011, 1812, 1515–1521. Available online: http://www.sciencedirect.com/science/article/pii/S0925443911001402 (accessed on 20 October 2021). [CrossRef] [PubMed] [Green Version]
- Chevalier, F.; Chobert, J.-M.; Genot, C.; Haertlé, T. Scavenging of Free Radicals, Antimicrobial, and Cytotoxic Activities of the Maillard Reaction Products of β-Lactoglobulin Glycated with Several Sugars. J. Agric. Food Chem. 2001, 49, 5031–5038. [Google Scholar] [CrossRef]
- Diao, M.; Liang, Y.; Zhao, J.; Zhao, C.; Zhang, J.; Zhang, T. Enhanced cytotoxicity and antioxidant capacity of kaempferol complexed with α-lactalbumin. Food Chem. Toxicol. 2021, 153, 112265. Available online: https://www.sciencedirect.com/science/article/pii/S0278691521002982 (accessed on 12 January 2022). [CrossRef]
- Fagnani, R.; Mexia, M.M.; Puppio, A.A.N.; Battaglini, A.P.P. Sanitary aspects and technological challenges of whole milk microfiltration at low temperatures. Pesqui. Agropecuária Bras. 2016, 51, 990–997. [Google Scholar] [CrossRef] [Green Version]
- Corrochano, A.R.; Buckin, V.; Kelly, P.M.; Giblin, L. Invited review: Whey proteins as antioxidants and promoters of cellular antioxidant pathways. J. Dairy Sci. 2018, 101, 4747–4761. Available online: https://www.sciencedirect.com/science/article/pii/S0022030218302789 (accessed on 5 November 2021). [CrossRef] [Green Version]
- Shi, Y.-J.; Chiou, J.-T.; Huang, C.-H.; Lee, Y.-C.; Wang, L.-J.; Chang, L.-S. Modification of carboxyl groups converts α-lactalbumin into an active molten globule state with membrane-perturbing activity and cytotoxicity. Int. J. Biol. Macromol. 2020, 163, 1697–1706. Available online: https://www.sciencedirect.com/science/article/pii/S0141813020344548 (accessed on 5 November 2021). [CrossRef]
- Silveira, S.T.; Martínez-Maqueda, D.; Recio, I.; Hernández-Ledesma, B. Dipeptidyl peptidase-IV inhibitory peptides generated by tryptic hydrolysis of a whey protein concentrate rich in β-lactoglobulin. Food Chem. 2013, 141, 1072–1077. Available online: http://www.sciencedirect.com/science/article/pii/S0308814613003683 (accessed on 5 November 2021). [CrossRef]
- Nongonierma, A.B.; Cadamuro, C.; Le Gouic, A.; Mudgil, P.; Maqsood, S.; FitzGerald, R.J. Dipeptidyl peptidase IV (DPP-IV) inhibitory properties of a camel whey protein enriched hydrolysate preparation. Food Chem. 2018, 279, 70–79. Available online: http://www.sciencedirect.com/science/article/pii/S0308814618320892 (accessed on 5 November 2021). [CrossRef]
- Umezawa, H.; Aoyagi, T.; Ogawa, K.; Naganawa, H.; Hamada, M.; Takeuchi, T. Diprotins A and B, inhibitors of dipeptidyl aminopeptidase IV, produced by bacteria. J. Antibiot. 1984, 37, 422–425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farup, J.; Rahbek, S.K.; Storm, A.C.; Klitgaard, S.; Jørgensen, H.; Bibby, B.M.; Serena, A.; Vissing, K. Effect of degree of hy-drolysis of whey protein on in vivo plasma amino acid appearance in humans. Springerplus 2016, 5, 382. [Google Scholar] [CrossRef] [Green Version]
- Burcelin, R.; Dolci, W.; Thorens, B. Long-lasting antidiabetic effect of a dipeptidyl peptidase IV-resistant analog of glucagon-like peptide-1. Metab. Clin. Exp. 1999, 48, 252–258. [Google Scholar] [CrossRef]
- Voutetakis, A.; Cotrim, A.P.; Rowzee, A.; Zheng, C.; Rathod, T.; Yanik, T.; Loh, Y.P.; Baum, B.J.; Cawley, N.X. Systemic Delivery of Bioactive Glucagon-Like Peptide 1 after Adenoviral-Mediated Gene Transfer in the Murine Salivary Gland. Endocrinology 2010, 151, 4566–4572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, S.; Altaf Hussain, M.; Cheng, J.; Jiang, Z.; Geng, H.; Sun, Y.; Sun, C.; Hou, J. Effect of heat treatment on physicochemical and emulsifying properties of polymerized whey protein concentrate and polymerized whey protein isolate. LWT 2018, 98, 134–140. Available online: http://www.sciencedirect.com/science/article/pii/S0023643818306819 (accessed on 13 December 2021). [CrossRef]
- Damodaran, S.; Parkin, K.L.; Fennema, O.R. Fennema’s Food Chemistry, 4th ed.; CRC Press: New York, NY, USA, 1996; Volume 1. [Google Scholar]
- McGuffey, M.K.; Epting, K.L.; Kelly, R.M.; Foegeding, E.A. Denaturation and Aggregation of Three α-Lactalbumin Prepa-rations at Neutral pH. J. Agric. Food Chem. 2005, 53, 3182–3190. [Google Scholar] [CrossRef] [PubMed]
- Qian, F.; Sun, J.; Cao, D.; Tuo, Y.; Jiang, S.; Mu, G. Experimental and Modelling Study of the Denaturation of Milk Protein by Heat Treatment. Korean J. Food Sci. Anim. Resour. 2017, 37, 44–51. [Google Scholar] [CrossRef] [Green Version]




| Temperature (°C) | Types of Whey Protein Hydrolysates | |||
|---|---|---|---|---|
| WPI | β-LG | α-LA | Control | |
| 65 | 12.37 ± 0.06 Ba | 11.98 ± 0.08 Bb | 12.34 ± 0.02 Ba | |
| 75 | 12.43 ± 0.08 Aa | 12.20 ± 0.07 Ab | 12.41 ± 0.05 Aa | |
| 85 | 12.51 ± 0.06 Aa | 12.08 ± 0.02 Ab | 12.37 ± 0.07 Aa | |
| Control | 11.83 ± 0.06 Cc | |||
| Temperature (°C) | Types of Whey Protein Solutions | ||
|---|---|---|---|
| WPI | β-LG | α-LA | |
| 65 | 24.19 ± 0.85 Bb | 30.81 ± 0.89 Bb | 12.31 ± 0.52 Ba |
| 75 | 20.41 ± 1.64 Ab | 25.35 ± 1.10 Ab | 7.16 ± 0.82 Aa |
| 85 | 37.40 ± 0.36 Bb | 26.57 ± 0.70 Bb | 13.80 ± 0.87 Ba |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bunsroem, K.; Prinyawiwatkul, W.; Thaiudom, S. The Influence of Whey Protein Heating Parameters on Their Susceptibility to Digestive Enzymes and the Antidiabetic Activity of Hydrolysates. Foods 2022, 11, 829. https://doi.org/10.3390/foods11060829
Bunsroem K, Prinyawiwatkul W, Thaiudom S. The Influence of Whey Protein Heating Parameters on Their Susceptibility to Digestive Enzymes and the Antidiabetic Activity of Hydrolysates. Foods. 2022; 11(6):829. https://doi.org/10.3390/foods11060829
Chicago/Turabian StyleBunsroem, Kungnang, Witoon Prinyawiwatkul, and Siwatt Thaiudom. 2022. "The Influence of Whey Protein Heating Parameters on Their Susceptibility to Digestive Enzymes and the Antidiabetic Activity of Hydrolysates" Foods 11, no. 6: 829. https://doi.org/10.3390/foods11060829