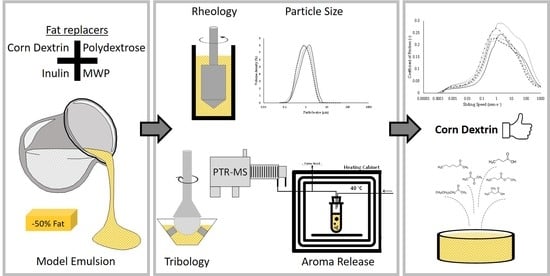
Influence of Fat Replacers on the Rheological, Tribological, and Aroma Release Properties of Reduced-Fat Emulsions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of the Model Emulsions
2.3. Preparation of the Aroma Solutions
2.4. Compositional Analysis
2.5. Particle Size Distribution
2.6. Rheological Properties
2.7. Tribological Properties
2.8. Aroma Release Analysis by Proton-Transfer-Reaction Mass Spectrometry (PTR-MS)
2.9. Statistical Analysis
3. Results and Discussion
3.1. Composition
3.2. Particle Size Distribution
3.3. Rheological Properties
3.4. Tribological Properties
3.5. Aroma Release
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. Obesity: Preventing and Managing the Global Epidemic: Report on a WHO Consultation; World Health Organization: Geneva, Switzerland, 2000; ISBN 9241208945. [Google Scholar]
- Lucca, P.A.; Tepper, B.J. Fat replacers and the functionality of fat in foods. Trends Food Sci. Technol. 1994, 5, 12–19. [Google Scholar] [CrossRef]
- Peng, X.; Yao, Y. Carbohydrates as fat replacers. Annu. Rev. Food Sci. Technol. 2017, 8, 331–351. [Google Scholar] [CrossRef] [PubMed]
- Akoh, C.C. Fat replacers. Food Technol. 1998, 52, 47–53. [Google Scholar]
- Srisuvor, N.; Chinprahast, N.; Prakitchaiwattana, C.; Subhimaros, S. Effects of inulin and polydextrose on physicochemical and sensory properties of low-fat set yoghurt with probiotic-cultured banana purée. Lebensm. Wiss. Technol. 2013, 51, 30–36. [Google Scholar] [CrossRef]
- Chung, C.; Degner, B.; McClements, D.J. Reduced calorie emulsion-based foods: Protein microparticles and dietary fiber as fat replacers. Food Res. Int. 2014, 64, 664–676. [Google Scholar] [CrossRef] [PubMed]
- Felisberto, M.H.F.; Galvão, M.T.E.L.; Picone, C.S.F.; Cunha, R.L.; Pollonio, M.A.R. Effect of prebiotic ingredients on the rheological properties and microstructure of reduced-sodium and low-fat meat emulsions. Lebensm. Wiss. Technol. 2015, 60, 148–155. [Google Scholar] [CrossRef] [Green Version]
- Schädle, C.N.; Eisner, P.; Bader-Mittermaier, S. The combined effects of different fat replacers and rennet casein on the properties of reduced-fat processed cheese. J. Dairy Sci. 2020, 103, 3980–3993. [Google Scholar] [CrossRef]
- López-Castejón, M.L.; Bengoechea, C.; Espinosa, S.; Carrera, C. Characterization of prebiotic emulsions stabilized by inulin and β-lactoglobulin. Food Hydrocoll. 2019, 87, 382–393. [Google Scholar] [CrossRef]
- Oberg, E.N.; Oberg, C.J.; Motawee, M.M.; Martini, S.; McMahon, D.J. Increasing stringiness of low-fat mozzarella string cheese using polysaccharides. J. Dairy Sci. 2015, 98, 4243–4254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Temiz, H.; Kezer, G. Effects of fat replacers on physicochemical, microbial and sensorial properties of Kefir made using mixture of cow and goat’s milk. J. Food Process. Preserv. 2015, 39, 1421–1430. [Google Scholar] [CrossRef]
- Shi, D.; Li, C.; Stone, A.K.; Guldiken, B.; Nickerson, M.T. Recent Developments in Processing, Functionality, and Food Applications of Microparticulated Proteins. Food Rev. Int. 2021, 1–24. [Google Scholar] [CrossRef]
- Tiefenbacher, K.F. The Technology of Wafers and Waffles; Elsevier: London, UK, 2017; ISBN 978-0-12-809438-9. [Google Scholar]
- Roller, S.; Jones, S.A. Handbook of Fat Replacers; CRC Press: Boca Raton, FL, USA, 1996; ISBN 0849325129. [Google Scholar]
- Singer, N.S.; Dunn, J.M. Protein microparticulation: The principle and the process. J. Am. Coll. Nutr. 1990, 9, 388–397. [Google Scholar] [CrossRef]
- Chavan, R.S.; Khedkar, C.D.; Bhatt, S. Fat replacer. In Encyclopedia of Food and Health; Caballero, B., Finglas, P.M., Toldrá, F., Eds.; Academic Press: Oxford, UK; Waltham, MA, USA, 2016; pp. 589–595. ISBN 978-0-12-384947-2. [Google Scholar]
- McClements, D.J. Food emulsions: Principles, Practices, and Techniques, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2016; ISBN 978-1-4987-2669-6. [Google Scholar]
- Chung, C.; Smith, G.; Degner, B.; McClements, D.J. Reduced fat food emulsions: Physicochemical, sensory, and biological aspects. Crit. Rev. Food Sci. Nutr. 2016, 56, 650–685. [Google Scholar] [CrossRef] [PubMed]
- Ognean, C.F.; Darie, N.; Ognean, M. Fat replacers-Review. J. Agroaliment. Proc. Technol. 2006, 12, 433–442. [Google Scholar]
- Stokes, J.R. Oral Tribology. In Food Oral Processing: Fundamentals of Eating and Sensory Perception; Chen, J., Engelen, L., Eds.; Wiley Blackwell A John Wiley & Sons Ltd. Publication: Chichester, UK; Ames, IA, USA, 2012; pp. 265–288. ISBN 1444360914. [Google Scholar]
- Liu, K.; Tian, Y.; Stieger, M.; van der Linden, E.; van de Velde, F. Evidence for ball-bearing mechanism of microparticulated whey protein as fat replacer in liquid and semi-solid multi-component model foods. Food Hydrocoll. 2016, 52, 403–414. [Google Scholar] [CrossRef]
- Joyner, H.S.; Pernell, C.W.; Daubert, C.R. Impact of Oil-in-Water Emulsion Composition and Preparation Method on Emulsion Physical Properties and Friction Behaviors. Tribol. Lett. 2014, 56, 143–160. [Google Scholar] [CrossRef]
- Hamilton, I.E.; Norton, I.T. Modification to the lubrication properties of xanthan gum fluid gels as a result of sunflower oil and triglyceride stabilised water in oil emulsion addition. Food Hydrocoll. 2016, 55, 220–227. [Google Scholar] [CrossRef]
- Anvari, M.; Joyner, H.S. Effect of formulation on structure-function relationships of concentrated emulsions: Rheological, tribological, and microstructural characterization. Food Hydrocoll. 2017, 72, 11–26. [Google Scholar] [CrossRef]
- Malone, M.; Appelqvist, I.; Norton, I. Oral behaviour of food hydrocolloids and emulsions. Part 1. Lubrication and deposition considerations. Food Hydrocoll. 2003, 17, 763–773. [Google Scholar] [CrossRef]
- de Wijk, R.A.; Prinz, J.F. The role of friction in perceived oral texture. Food Qual. Prefer. 2005, 16, 121–129. [Google Scholar] [CrossRef]
- de Wijk, R.A.; Prinz, J.F. Mechanisms underlying the role of friction in oral texture. J. Texture Stud. 2006, 37, 413–427. [Google Scholar] [CrossRef]
- Lee, S.K.; Anema, S.G.; Klostermeyer, H. The influence of moisture content on the rheological properties of processed cheese spreads. Int. J. Food Sci. Technol. 2004, 39, 763–771. [Google Scholar] [CrossRef]
- Lee, S.; Heuberger, M.; Rousset, P.; Spencer, N.D. A Tribological Model for Chocolate in the Mouth: General Implications for Slurry-Lubricated Hard/Soft Sliding Counterfaces. Tribol. Lett. 2004, 16, 239–249. [Google Scholar] [CrossRef]
- Bellamy, M.; Godinot, N.; Mischler, S.; Martin, N.; Hartmann, C. Influence of emulsion composition on lubrication capacity and texture perception. Int. J. Food Sci. Technol. 2009, 44, 1939–1949. [Google Scholar] [CrossRef]
- Terpstra, M.E.; Jellema, R.H.; Janssen, A.M.; de Wijk, R.A.; Prinz, J.F.; van der Linden, E. Prediction of texture perception of mayonnaises from rheological and novel instrumental measurements. J. Texture Stud. 2009, 40, 82–108. [Google Scholar] [CrossRef]
- Guichard, E. Interaction of aroma compounds with food matrices. In Flavour Development, Analysis and Perception in Food and Beverages; Parker, J.K., Elmore, J.S., Methven, L., Eds.; WP Woodhead Publishin: Shaston, UK; Elsevier: Amsterdam, The Netherlands, 2015; pp. 273–295. ISBN 9781782421030. [Google Scholar]
- Seuvre, A.-M.; Voilley, A. Physico-Chemical Interactions in the Flavor-Release Process. In Springer Handbook of Odor; Buettner, A., Ed.; Springer International Publishing: Cham, Switzerland, 2017; pp. 35–36. ISBN 978-3-319-26930-6. [Google Scholar]
- Frank, D.; Appelqvist, I.; Piyasiri, U.; Wooster, T.J.; Delahunty, C. Proton transfer reaction mass spectrometry and time intensity perceptual measurement of flavor release from lipid emulsions using trained human subjects. J. Agric. Food Chem. 2011, 59, 4891–4903. [Google Scholar] [CrossRef]
- van Ruth, S.M.; King, C.; Giannouli, P. Influence of lipid fraction, emulsifier fraction, and mean particle diameter of oil-in-water emulsions on the release of 20 aroma compounds. J. Agric. Food Chem. 2002, 50, 2365–2371. [Google Scholar] [CrossRef]
- Delarue, J.; Giampaoli, P. Carbohydrate–flavour interactions. In Flavour in Food; Voilley, A., Ed.; CRC Press: Boca Raton, FL, USA, 2006; pp. 208–228. ISBN 9781855739604. [Google Scholar]
- Guichard, E. Interactions between flavor compounds and food ingredients and their influence on flavor perception. Food Rev. Int. 2002, 18, 49–70. [Google Scholar] [CrossRef]
- Guichard, E. Flavour retention and release from protein solutions. Biotechnol. Adv. 2006, 24, 226–229. [Google Scholar] [CrossRef]
- Hansen, A.P. A review of the interactions between milk proteins and dairy flavor compounds. Adv. Exp. Med. Biol. 1997, 415, 67–76. [Google Scholar] [CrossRef]
- Kühn, J.; Considine, T.; Singh, H. Interactions of Milk Proteins and Volatile Flavor Compounds: Implications in the Development of Protein Foods. J. Food Sci. 2006, 71, R72–R82. [Google Scholar] [CrossRef]
- Tromelin, A.; Andriot, I.; Guichard, E. Protein–flavour interactions. In Flavour in Food; Voilley, A., Ed.; CRC Press: Boca Raton, FL, USA, 2006; pp. 172–207. ISBN 9781855739604. [Google Scholar]
- McClements, D.J. Modulation of globular protein functionality by weakly interacting cosolvents. Crit. Rev. Food Sci. Nutr. 2002, 42, 417–471. [Google Scholar] [CrossRef] [PubMed]
- Tromelin, A.; Guichard, E. 2D- and 3D-QSAR Models of Interaction between Flavor Compounds and beta-Lactoglobulin Using Catalyst and Cerius2. QSAR Comb. Sci. 2004, 23, 214–233. [Google Scholar] [CrossRef]
- Meynier, A.; Rampon, V.; Dalgalarrondo, M.; Genot, C. Hexanal and t-2-hexenal form covalent bonds with whey proteins and sodium caseinate in aqueous solution. Int. Dairy J. 2004, 14, 681–690. [Google Scholar] [CrossRef]
- Mottram, D.S.; Szauman-Szumski, C.; Dodson, A. Interaction of Thiol and Disulfide Flavor Compounds with Food Components. J. Agric. Food Chem. 1996, 44, 2349–2351. [Google Scholar] [CrossRef]
- Tarrega, A.; Yven, C.; Semon, E.; Mielle, P.; Salles, C. Effect of Oral Physiology Parameters on In-Mouth Aroma Compound Release Using Lipoprotein Matrices: An In Vitro Approach. Foods 2019, 8, 106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andriot, I.; Harrison, M.; Fournier, N.; Guichard, E. Interactions between methyl ketones and beta-lactoglobulin: Sensory analysis, headspace analysis, and mathematical modeling. J. Agric. Food Chem. 2000, 48, 4246–4251. [Google Scholar] [CrossRef] [PubMed]
- Jasinski, E.; Kilara, A. Flavor binding by whey proteins. Milchwissenschaft 1985, 40, 569–599. [Google Scholar]
- Lesme, H.; Rannou, C.; Famelart, M.-H.; Bouhallab, S.; Prost, C. Yogurts enriched with milk proteins: Texture properties, aroma release and sensory perception. Trends Food Sci. Technol. 2020, 98, 140–149. [Google Scholar] [CrossRef]
- Houde, M.; Khodaei, N.; Karboune, S. Assessment of interaction of vanillin with barley, pea and whey proteins: Binding properties and sensory characteristics. Lebensm. Wiss. Technol. 2018, 91, 133–142. [Google Scholar] [CrossRef]
- Liu, X.; Powers, J.R.; Swanson, B.G.; Hill, H.H.; Clark, S. High Hydrostatic Pressure Affects Flavor-binding Properties of Whey Protein Concentrate. J. Food Sci. 2005, 70, C581–C585. [Google Scholar] [CrossRef]
- Viry, O.; Boom, R.; Avison, S.; Pascu, M.; Bodnár, I. A predictive model for flavor partitioning and protein-flavor interactions in fat-free dairy protein solutions. Food Res. Int. 2018, 109, 52–58. [Google Scholar] [CrossRef]
- Smit, G.; Smit, B.A.; Engels, W.J.M. Flavour formation by lactic acid bacteria and biochemical flavour profiling of cheese products. FEMS Microbiol. Rev. 2005, 29, 591–610. [Google Scholar] [CrossRef]
- Curioni, P.; Bosset, J.O. Key odorants in various cheese types as determined by gas chromatography-olfactometry. Int. Dairy J. 2002, 12, 959–984. [Google Scholar] [CrossRef]
- Niimi, J.; Eddy, A.I.; Overington, A.R.; Heenan, S.P.; Silcock, P.; Bremer, P.J.; Delahunty, C.M. Aroma–taste interactions between a model cheese aroma and five basic tastes in solution. Food Qual Prefer. 2014, 31, 1–9. [Google Scholar] [CrossRef]
- Nursten, H.E. The flavour of milk and dairy products: I. Milk of different kinds, milk powder, butter and cream. Int. J. Dairy Technol. 1997, 50, 48–56. [Google Scholar] [CrossRef]
- Kilcawley, K.N. Cheese Flavour. In Fundamentals of Cheese Science, 2nd ed.; Fox, P.F., Guinee, T.P., Cogan, T.M., McSweeney, P.L.H., Eds.; Springer: Boston, MA, USA, 2017; pp. 443–474. ISBN 978-1-4899-7679-6. [Google Scholar]
- Ellis, A.M.; Mayhew, C.A. (Eds.) PTR-MS in the Food Sciences. In Proton Transfer Reaction Mass Spectrometry; John Wiley & Sons, Ltd.: Chichester, UK, 2014; pp. 221–265. ISBN 9781118682883. [Google Scholar]
- Lenze, S.; Wolfschoon-Pombo, A.; Schrader, K.; Kulozik, U. Effect of the compositional factors and processing conditions on the creaming reaction during process cheese manufacturing. Food Bioproc. Tech. 2019, 56, 1224. [Google Scholar] [CrossRef]
- AOAC. Method 925.10. Solids (total) and moisture in flour. In Official Methods of Analysis of the Association of Official Analytical Chemists (AOAC); Latimer, G.W., Horwitz, W., Eds.; AOAC: Gaithersburg, MD, USA, 2005. [Google Scholar]
- AOAC. Method 968.06. Protein (crude) in animal feed. In Official Methods of Analysis of the Association of Official Analytical Chemists (AOAC); Latimer, G.W., Horwitz, W., Eds.; AOAC: Gaithersburg, MD, USA, 2005. [Google Scholar]
- DGF. Deutsche Einheitsmethoden: Zur Untersuchung von Fetten, Fettprodukten, Tensiden und Verwandten Stoffen; 23. Aktualisierungslieferung zur 2. Aufl.; Wissenschaftliche Verlagsgesellschaft: Stuttgart, Germany, 2018; ISBN 978-3-8047-3808-9. [Google Scholar]
- Senturk Parreidt, T.; Schott, M.; Schmid, M.; Müller, K. Effect of presence and concentration of plasticizers, vegetable oils, and surfactants on the properties of sodium-alginate-based edible coatings. Int. J. Mol. Sci. 2018, 19, 742. [Google Scholar] [CrossRef] [Green Version]
- Drioli, E.; Giorno, L. Encyclopedia of Membranes; Springer: Berlin/Heidelberg, Germany, 2016; ISBN 978-3-662-44323-1. [Google Scholar]
- Liu, H.; Xu, X.M.; Guo, S. Rheological, texture and sensory properties of low-fat mayonnaise with different fat mimetics. Lebensm. Wiss. Technol. 2007, 40, 946–954. [Google Scholar] [CrossRef]
- Mezger, T.G. Das Rheologie-Handbuch: Für Anwender von Rotations- und Oszillations-Rheometern; 2., überarb. Aufl.; Vincentz Network: Hannover, Germany, 2006; ISBN 978-3-87870-175-0. [Google Scholar]
- Arancibia, C.; Costell, E.; Bayarri, S. Impact of Structural Differences on Perceived Sweetness in Semisolid Dairy Matrices. J. Texture Stud. 2013, 44, 346–356. [Google Scholar] [CrossRef]
- Shama, F.; Sherman, P. Identification of stimuli controlling the sensory evaluation of viscosity: II. Oral methods. J. Texture Stud. 1973, 4, 111–118. [Google Scholar] [CrossRef]
- Tárrega, A.; Durán, L.; Costell, E. Flow behaviour of semi-solid dairy desserts. Effect of temperature. Int. Dairy J. 2004, 14, 345–353. [Google Scholar] [CrossRef]
- Krzeminski, A.; Tomaschunas, M.; Köhn, E.; Busch-Stockfisch, M.; Weiss, J.; Hinrichs, J. Relating creamy perception of whey protein enriched yogurt systems to instrumental data by means of multivariate data analysis. J. Food Sci. 2013, 78, S314–S319. [Google Scholar] [CrossRef] [PubMed]
- Hahn, C.; Nöbel, S.; Maisch, R.; Rösingh, W.; Weiss, J.; Hinrichs, J. Adjusting rheological properties of concentrated microgel suspensions by particle size distribution. Food Hydrocoll. 2015, 49, 183–191. [Google Scholar] [CrossRef]
- Stokes, J.R. ‘Oral’ Rheology. In Food Oral Processing: Fundamentals of Eating and Sensory Perception; Chen, J., Engelen, L., Eds.; Wiley Blackwell: Chichester, UK; A John Wiley & Sons, Ltd., Publication: Ames, IA, USA, 2012; pp. 227–264. ISBN 1444360914. [Google Scholar]
- Schädle, C.N.; Bader-Mittermaier, S.; Sanahuja, S. Characterization of Reduced-Fat Mayonnaise and Comparison of Sensory Perception, Rheological, Tribological, and Textural Analyses. Foods 2022, 11, 806. [Google Scholar] [CrossRef]
- Miller, J.L.; Watkin, K.L. The influence of bolus volume and viscosity on anterior lingual force during the oral stage of swallowing. Dysphagia 1996, 11, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Siefarth, C.; Tyapkova, O.; Beauchamp, J.; Schweiggert, U.; Buettner, A.; Bader, S. Influence of polyols and bulking agents on flavour release from low-viscosity solutions. Food Chem. 2011, 129, 1462–1468. [Google Scholar] [CrossRef]
- IFA. GESTIS-Stoffdatenbank. Available online: Gestis.dguv.de (accessed on 10 February 2022).
- Bordiga, M. Food Aroma Evolution: During Food Processing, Cooking and Aging; CRC Press LLC: Milton, UK, 2020; ISBN 978-1-138-33824-1. [Google Scholar]
- Gu, Y.S.; Decker, A.E.; McClements, D.J. Production and characterization of oil-in-water emulsions containing droplets stabilized by multilayer membranes consisting of beta-lactoglobulin, iota-carrageenan and gelatin. Langmuir 2005, 21, 5752–5760. [Google Scholar] [CrossRef] [PubMed]
- McClements, D.J.; Demetriades, K. An integrated approach to the development of reduced-fat food emulsions. Crit. Rev. Food Sci. Nutr. 1998, 38, 511–536. [Google Scholar] [CrossRef]
- Renard, D.; Robert, P.; Faucheron, S.; Sanchez, C. Rheological properties of mixed gels made of microparticulated whey proteins and β-lactoglobulin. Colloids Surf. B Biointerfaces 1999, 12, 113–121. [Google Scholar] [CrossRef]
- Liu, R.; Tian, Z.; Song, Y.; Wu, T.; Sui, W.; Zhang, M. Optimization of the Production of Microparticulated Egg White Proteins as Fat Mimetic in Salad Dressings Using Uniform Design. FSTR 2018, 24, 817–827. [Google Scholar] [CrossRef] [Green Version]
- Kokini, J.L.; Kadane, J.B.; Cussler, E.L. Liquid texture perceived in the mouth. J. Texture Stud. 1977, 8, 195–218. [Google Scholar] [CrossRef]
- Bayarri, S.; Smith, T.; Hollowood, T.A.; Hort, J. The role of rheological behaviour in flavour perception in model oil/water emulsions. Eur. Food Res. Technol. 2007, 226, 161–168. [Google Scholar] [CrossRef]
- Cook, D.J.; Linforth, R.S.T.; Taylor, A.J. Effects of hydrocolloid thickeners on the perception of savory flavors. J. Agric. Food Chem. 2003, 51, 3067–3072. [Google Scholar] [CrossRef] [PubMed]
- Torres, I.C.; Mutaf, G.; Larsen, F.H.; Ipsen, R. Effect of hydration of microparticulated whey protein ingredients on their gelling behaviour in a non-fat milk system. J. Food Eng. 2016, 184, 31–37. [Google Scholar] [CrossRef]
- Gabriele, A.; Spyropoulos, F.; Norton, I.T. A conceptual model for fluid gel lubrication. Soft Matter 2010, 6, 4205. [Google Scholar] [CrossRef]
- Ipsen, R. Microparticulated whey proteins for improving dairy product texture. Int. Dairy J. 2017, 67, 73–79. [Google Scholar] [CrossRef]
- Wang, Q.; Zhu, Y.; Ji, Z.; Chen, J. Lubrication and Sensory Properties of Emulsion Systems and Effects of Droplet Size Distribution. Foods 2021, 10, 3024. [Google Scholar] [CrossRef] [PubMed]
- Flamminii, F.; Di Mattia, C.D.; Sacchetti, G.; Neri, L.; Mastrocola, D.; Pittia, P. Physical and Sensory Properties of Mayonnaise Enriched with Encapsulated Olive Leaf Phenolic Extracts. Foods 2020, 9, 997. [Google Scholar] [CrossRef] [PubMed]
- Shewan, H.M.; Pradal, C.; Stokes, J.R. Tribology and its growing use toward the study of food oral processing and sensory perception. J. Texture Stud. 2020, 51, 7–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Selway, N.; Stokes, J.R. Insights into the dynamics of oral lubrication and mouthfeel using soft tribology: Differentiating semi-fluid foods with similar rheology. Food Res. Int. 2013, 54, 423–431. [Google Scholar] [CrossRef]
- Laguna, L.; Farrell, G.; Bryant, M.; Morina, A.; Sarkar, A. Relating rheology and tribology of commercial dairy colloids to sensory perception. Food Funct. 2017, 8, 563–573. [Google Scholar] [CrossRef]
- Sarkar, A.; Soltanahmadi, S.; Chen, J.; Stokes, J.R. Oral tribology: Providing insight into oral processing of food colloids. Food Hydrocoll. 2021, 117, 106635. [Google Scholar] [CrossRef]
- Bayarri, S.; Taylor, A.J.; Hort, J. The role of fat in flavor perception: Effect of partition and viscosity in model emulsions. J. Agric. Food Chem. 2006, 54, 8862–8868. [Google Scholar] [CrossRef] [PubMed]
- Lauverjat, C.; Déléris, I.; Tréléa, I.C.; Salles, C.; Souchon, I. Salt and Aroma Compound Release in Model Cheeses in Relation to Their Mobility. J. Agric. Food Chem. 2009, 57, 9878–9887. [Google Scholar] [CrossRef]
- Lesme, H.; Alleaume, C.; Bouhallab, S.; Famelart, M.-H.; Marzin, C.; Lopez-Torres, L.; Prost, C.; Rannou, C. Aroma-retention capacities of functional whey protein aggregates: Study of a strawberry aroma in solutions and in fat-free yogurts. Food Res. Int. 2020, 136, 109491. [Google Scholar] [CrossRef]
- Miettinen, S.-M.; Tuorila, H.; Piironen, V.; Vehkalahti, K.; Hyvönen, L. Effect of Emulsion Characteristics on the Release of Aroma As Detected by Sensory Evaluation, Static Headspace Gas Chromatography, and Electronic Nose. J. Agric. Food Chem. 2002, 50, 4232–4239. [Google Scholar] [CrossRef]
- Charles, M.; Rosselin, V.; Beck, L.; Sauvageot, F.; Guichard, E. Flavor Release from Salad Dressings: Sensory and Physicochemical Approaches in Relation with the Structure. J. Agric. Food Chem. 2000, 48, 1810–1816. [Google Scholar] [CrossRef]
- Roberts, D.D.; Pollien, P.; Antille, N.; Lindinger, C.; Yeretzian, C. Comparison of nosespace, headspace, and sensory intensity ratings for the evaluation of flavor absorption by fat. J. Agric. Food Chem. 2003, 51, 3636–3642. [Google Scholar] [CrossRef] [PubMed]
- Weel, K.G.C.; Boelrijk, A.E.M.; Burger, J.J.; Verschueren, M.; Gruppen, H.; Voragen, A.G.J.; Smit, G. New device to simulate swallowing and in vivo aroma release in the throat from liquid and semiliquid food systems. J. Agric. Food Chem. 2004, 52, 6564–6571. [Google Scholar] [CrossRef] [PubMed]
- Bylaite, E.; Meyer, A.S.; Adler-Nissen, J. Changes in macroscopic viscosity do not affect the release of aroma aldehydes from a pectinaceous food model system of low sucrose content. J. Agric. Food Chem. 2003, 51, 8020–8026. [Google Scholar] [CrossRef] [PubMed]




| Full Fat | Wat | Lac | Inu | Poly | CD | MWP | |
|---|---|---|---|---|---|---|---|
| (g/100 g) | (g/100 g) | (g/100 g) | (g/100 g) | (g/100 g) | (g/100 g) | (g/100 g) | |
| Buffer solution | 65.7 | 80.7 | 65.7 | 65.7 | 65.7 | 65.7 | 65.7 |
| Fat | 30 | 15 | 15 | 15 | 15 | 15 | 15 |
| Lac/Inu/Poly/CD/MWP | 0 | 0 | 15 | 15 | 15 | 15 | 15 |
| Sodium casein | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| Sunflower lecithin | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Aroma solution 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Aroma solution 2 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 |
| Potassium sorbate | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 |
| Aroma Solution | Aroma Compound | Concentration in Aroma Solution (µg∙g−1) | Concentration in Emulsion (µg∙g−1) |
|---|---|---|---|
| 1 | Butane-2,3-dione | 243 | 2.4 |
| Butanoic acid | 8484 | 84.8 | |
| 3-Methylbutanoic acid | 24,097 | 241.0 | |
| Ethyl butanoate | 1514 | 15.1 | |
| 2 | Heptan-2-one | 5237 | 5.2 |
| Nonan-2-one | 56,923 | 56.9 |
| Aroma Compound | Molecular Weight | Major Product Ion | Sensitivity Factor | Log p-Value [76] | Odor Quality [77] | CAS |
|---|---|---|---|---|---|---|
| (g∙mol−1) | (m/z) | (ncps∙ppbv−1) | ||||
| Butane-2,3-dione | 86 | 87 | 3.98 | −1.34 | Buttery | 431-03-8 |
| Butanoic acid | 88 | 89 | 1.64 | 0.79 | Rancid, cheesy, sharp | 107-92-6 |
| 3-Methylbutanoic acid | 102 | 103 | 1.81 | 1.16 | Sharp, sweaty, sweet, fruity | 503-74-2 |
| Ethyl butanoate | 116 | 117 | 0.67 | 1.71 | Pineapple, banana, fruity | 105-54-4 |
| Heptan-2-one | 114 | 115 | 2.86 | 1.98 | Blue cheese, fruity, sweet | 110-43-0 |
| Nonan-2-one | 142 | 143 | 3.91 | 3.16 | Fruity, musty, rose, tea-like | 821-55-6 |
| Formulation | DM | Fat (abs) | Fat (in DM) | Protein (abs) | Protein (in DM) | Energy Density 1 | Energy Density 1 |
|---|---|---|---|---|---|---|---|
| per 100 g | (g) | (g) | (g) | (g) | (g) | (kJ) | (kcal) |
| Full fat | 33.6 ± 0.3 a | 30.0 ± 0.1 a | 89.3 ± 0.9 a | 1.6 ± 0.1 b | 4.8 ± 0.4 c | 1178 | 286 |
| Wat | 18.3 ± 0.8 b | 15.8 ± 0.5 c | 86.5 ± 6.0 a | 1.7 ± 0.1 b | 9.2 ± 0.9 b | 623 | 151 |
| Lac | 32.4 ± 0.3 a | 17.8 ± 0.4 bc | 54.9 ± 0.7 bc | 1.8 ± 0.0 b | 5.6 ± 0.1 c | 865 | 208 |
| Inu | 33.1 ± 0.7 a | 15.7 ± 0.4 c | 47.6 ± 1.1 c | 1.7 ± 0.2 b | 5.2 ± 0.6 c | 698 | 169 |
| Poly | 33.1 ± 0.1 a | 17.0 ± 0.6 bc | 51.4 ± 1.6 bc | 1.8 ± 0.0 b | 5.3 ± 0.1 c | 623 | 151 |
| CD | 32.5 ± 0.2 a | 18.3 ± 2.2 bc | 56.2 ± 7.0 bc | 1.8 ± 0.0 b | 5.5 ± 0.0 c | 748 | 181 |
| MWP | 32.4 ± 0.1 a | 19.1 ± 0.5 b | 58.8 ± 1.6 b | 9.8 ± 0.4 a | 30.2 ± 1.2 a | 863 | 208 |
| Formulation | dv,0.1 | dv,0.5 | dv,0.9 | d4,3 | d3,2 | Span |
|---|---|---|---|---|---|---|
| (µm) | (µm) | (µm) | (µm) | (µm) | (µm) | |
| Full fat | 0.38 ± 0.01 a | 1.09 ± 0.06 a | 2.46 ± 0.21 b | 1.28 ± 0.09 a | 0.78 ± 0.03 a | 1.90 ± 0.09 e |
| Wat | 0.33 ± 0.02 b | 0.90 ± 0.04 c | 2.15 ± 0.11 cd | 1.10 ± 0.05 b | 0.67 ± 0.04 c | 2.02 ± 0.06 d |
| Lac | 0.32 ± 0.01 c | 0.76 ± 0.06 e | 1.79 ± 0.18 e | 0.93 ± 0.09 d | 0.61 ± 0.03 d | 1.93 ± 0.10 e |
| Inu | 0.30 ± 0.02 d | 0.80 ± 0.05 d | 2.23 ± 0.25 c | 1.08 ± 0.08 b | 0.61 ± 0.04 d | 2.41 ± 0.19 a |
| Poly | 0.30 ± 0.01 d | 0.81 ± 0.05 d | 2.05 ± 0.15 d | 1.03 ± 0.07 c | 0.61 ± 0.03 d | 2.14 ± 0.07 c |
| CD | 0.31 ± 0.01 cd | 0.78 ± 0.03 de | 1.88 ± 0.09 e | 0.96 ± 0.04 d | 0.61 ± 0.02 d | 2.03 ± 0.07 d |
| MWP | 0.34 ± 0.02 b | 1.03 ± 0.09 b | 2.68 ± 0.22 a | 1.31 ± 0.11 a | 0.72 ± 0.05 b | 2.28 ± 0.03 b |
| Formulation | Viscosity η at 10 s−1 (mPa∙s) | Viscosity η at 100 s−1 (mPa∙s) | Yield Stress τ0 (mPa) | Consistency K (mPa∙sn) | Flow Index n (–) |
|---|---|---|---|---|---|
| Full fat | 25.7 ± 0.9 b | 20.5 ± 0.1 b | 20.0 ± 3.0 a | 30.5 ± 1.7 b | 0.91 ± 0.01 d |
| Wat | 5.7 ± 0.1 d | 5.6 ± 0.1 e | 15.0 ± 0.6 bc | 4.3 ± 0.1 b | 1.05 ± 0.00 a |
| Lac | 12.2 ± 1.3 cd | 11.6 ± 1.0 d | 16.0 ± 5.6 ab | 10.5 ± 1.1 b | 1.02 ± 0.01 b |
| Inu | 16.6 ± 0.9 bc | 15.6 ± 0.8 cd | 11.4 ± 2.6 cd | 16.2 ± 1.3 b | 0.99± 0.01 c |
| Poly | 15.0 ± 0.6 bcd | 14.2 ± 0.5 cd | 13.3 ± 2.1 bc | 14.1 ± 0.8 b | 1.00 ± 0.00 c |
| CD | 18.9 ± 0.9 bc | 18.0 ± 0.7 bc | 8.1 ± 2.6 d | 18.3 ± 1.4 b | 0.99 ± 0.01 c |
| MWP | 127.6 ± 21.1 a | 79.2 ± 8.82 a | not detected | 260.2 ± 66.0 a | 0.75 ± 0.02 e |
| Formulation | Butane-2,3-dione (ppbv) | Butanoic Acid (ppbv) | 3-Methylbutanoic Acid (ppbv) | Heptan-2-one (ppbv) | Ethyl Butanoate (ppbv) | Nonan-2-one (ppbv) |
|---|---|---|---|---|---|---|
| Full fat | 459.6 ± 21.1 c | 1384.4 ± 114.7 d | 216.1 ± 10.1 c | 472.2 ± 22.8 d | 1758.0 ± 141.1 d | 397.0 ± 19.0 d |
| Wat | 462.4 ± 26.7 c | 2514.9 ± 392.1 a | 195.6 ± 9.6 c | 863.5 ± 72.7 a | 3228.9 ± 550.0 a | 769.6 ± 73.0 a |
| Lac | 508.4 ± 12.6 a | 2014.8 ± 297.1 b | 277.1 ± 35.3 a | 735.2 ± 47.9 b | 2587.3 ± 402.3 bc | 665.8 ± 81.3 b |
| Inu | 517.9 ± 25.3 a | 2016.5 ± 105.7 b | 247.3 ± 10.3 b | 710.7 ± 32.7 b | 2749.9 ± 220.8 b | 618.6 ± 27.9 b |
| Poly | 472.2 ± 18.4 bc | 1927.4 ± 118.5 bc | 253.0 ± 12.5 b | 707.4 ± 57.4 b | 2658.1 ± 212.1 b | 635.2 ± 68.6 b |
| CD | 498.6 ± 21.7 ab | 1621.9 ± 245.0 cd | 253.5 ± 7.6 ab | 698.4 ± 30.7 b | 2145.0 ± 235.4 cd | 624.5 ± 22.2 b |
| MWP | 347.9 ± 31.4 d | 1539.0 ± 195.6 d | 139.8 ± 12.0 d | 580.9 ± 88.7 c | 2118.7 ± 295.3 d | 523.6 ± 104.1 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schädle, C.N.; Sanahuja, S.; Bader-Mittermaier, S. Influence of Fat Replacers on the Rheological, Tribological, and Aroma Release Properties of Reduced-Fat Emulsions. Foods 2022, 11, 820. https://doi.org/10.3390/foods11060820
Schädle CN, Sanahuja S, Bader-Mittermaier S. Influence of Fat Replacers on the Rheological, Tribological, and Aroma Release Properties of Reduced-Fat Emulsions. Foods. 2022; 11(6):820. https://doi.org/10.3390/foods11060820
Chicago/Turabian StyleSchädle, Christopher N., Solange Sanahuja, and Stephanie Bader-Mittermaier. 2022. "Influence of Fat Replacers on the Rheological, Tribological, and Aroma Release Properties of Reduced-Fat Emulsions" Foods 11, no. 6: 820. https://doi.org/10.3390/foods11060820