The Inclusion of the Food Microstructural Influence in Predictive Microbiology: State-of-the-Art
Abstract
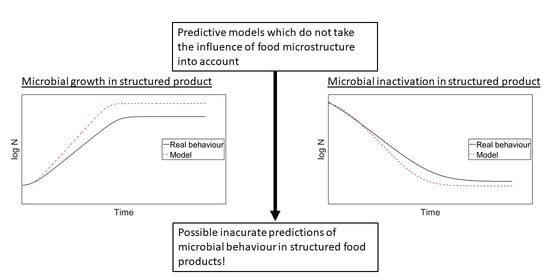
:1. Introduction
2. Historical Overview on the Inclusion of Food Microstructure in Predictive Models
2.1. The Absence of Food Microstructure in the Early Days of Predictive Microbiology
2.2. More Attention to Food Microstructure in the Last Decades
3. Growth Models Incorporating Food Microstructure
3.1. Macroscale Secondary Models Including Food Microstructural Factors
3.2. Semi-Mechanistic Microscale Models
4. Inactivation Models Incorporating Food Microstructure
4.1. Macroscale Secondary Models Including Food Microstructural Factors
4.2. Semi-Mechanistic Microscale Models
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- McMeekin, T.A.; Olley, J.; Ratkowsky, D.A.; Ross, T. Predictive microbiology: Towards the interface and beyond. Int. J. Food Microbiol. 2002, 73, 395–407. [Google Scholar] [CrossRef]
- Stavropoulou, E.; Bezirtzoglou, E. Predictive modeling of microbial behavior in food. Foods 2019, 8, 654. [Google Scholar] [CrossRef] [Green Version]
- Lopatkin, A.J.; Collins, J.J. Predictive biology: Modelling, understanding and harnessing microbial complexity. Nat. Rev. Microbiol. 2020, 18, 507–520. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Rodríguez, F.; Carrasco, E.; Pradhan, A.K.; Sant’Ana, A.S.; Valdramidis, V.P.; Valero, A. Special issue on 10th international conference of predictive modelling in foods: Towards a new paradigm in predictive microbiology. Int. J. Food Microbiol. 2019, 291, 65–66. [Google Scholar] [CrossRef]
- McDonald, K.; Sun, D.-W. Predictive food microbiology for the meat industry: A review. Int. J. Food Microbiol. 1999, 52, 1–27. [Google Scholar] [CrossRef]
- Heertje, I. Structure and function of food products: A review. Food Struct. 2014, 1, 3–23. [Google Scholar] [CrossRef] [Green Version]
- Aguilera, J.M. Why food microstructure? J. Food Eng. 2005, 67, 3–11. [Google Scholar] [CrossRef]
- Bhopatkar, D.; Hamaker, B.R.; Campanella, O.H. Micro to macro level structures of food materials. In Food Materials Science and Engineering; Bhandari, B., Roos, Y.H., Eds.; Blackwell Publishing Ltd.: Hoboken, NJ, USA, 2012; pp. 26–51. [Google Scholar]
- Ubbink, J.; Burbridge, A.; Mezzenga, R. Food structure and functionality: A soft matter perspective. Soft Matter 2008, 4, 1569–1581. [Google Scholar] [CrossRef] [PubMed]
- Wilson, P.D.G.; Brocklehurst, T.F.; Arino, S.; Thualt, D.; Jakobsen, M.; Lange, M.; Farkas, J.; Wimpenny, J.W.T.; Van Impe, J.F. Modelling microbial growth in structured foods: Towards a unified approach. Int. J. Food Microbiol. 2002, 73, 275–289. [Google Scholar] [CrossRef]
- Verheyen, D.; Bolívar, A.; Pérez-Rodríguez, F.; Baka, M.; Skåra, T.; Van Impe, J.F. Effect of food microstructure on growth dynamics of Listeria monocytogenes in fish-based model systems. Int. J. Food Microbiol. 2018, 283, 7–13. [Google Scholar] [CrossRef]
- Wimpenny, J.W.T.; Leistner, L.; Thomas, L.V.; Mitchell, A.J.; Katsaras, K.; Peetz, P. Submerged bacterial colonies within food and model systems: Their growth, distribution and interactions. Int. J. Food Microbiol. 1995, 28, 299–315. [Google Scholar] [CrossRef]
- Verheyen, D.; Xu, X.M.; Govaert, M.; Baka, M.; Van Impe, J.F. Food microstructure and fat content affect growth morphology, growth kinetics, and preferred phase for cell growth of Listeria monocytogenes in fish-based model systems. Appl. Environ. Microbiol. 2019, 85, e00707-19. [Google Scholar] [CrossRef] [Green Version]
- Mertens, L.; Geeraerd, A.H.; Dang, T.D.T.; Vermeulen, A.; Serneels, K.; Van Derlinden, E.; Cappuyns, A.M.; Moldenaers, P.; Debevere, J.; Devlieghere, F.; et al. Design of an experimental viscoelastic food model system for studying Zygosaccharomyces bailii spoilage in acidic sauces. Appl. Environ. Microbiol. 2009, 75, 7060–7069. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baka, M.; Noriega, E.; Van Langendonck, K.; Van Impe, J.F. Influence of food intrinsic complexity on Listeria monocytogenes growth in/on vacuum-packed model systems at suboptimal temperatures. Int. J. Food Microbiol. 2016, 235, 17–27. [Google Scholar] [CrossRef]
- Pérez-Rodríguez, F.; Valero, A. Predictive Microbiology in Foods; SpringerBriefs in Food, Health, and Nutrition; Springer: New York, NY, USA, 2013. [Google Scholar]
- Theys, T. Modelling the (Boundaries of) Microbial Growth in Structured Media: Effect of pH, Water Activity and Gelatin on the Growth of Salmonella Typhimurium. Ph.D. Thesis, KU Leuven, Leuven, Belgium, 2009. [Google Scholar]
- Velliou, E.G.; Noriega, E.; Van Derlinden, E.; Mertens, L.; Boons, K.; Geeraerd, A.H.; Devlieghere, F.; Van Impe, J.F. The effect of colony formation on the heat inactivation dynamics of Escherichia coli K12 and Salmonella typhimurium. Food Res. Int. 2013, 54, 1746–1752. [Google Scholar] [CrossRef]
- Juneja, V.K.; Eblen, B.S.; Marks, H.M. Modeling non-linear survival curves to calculate thermal inactivation of Salmonella in poultry of different fat levels. Int. J. Food Microbiol. 2001, 70, 37–51. [Google Scholar] [CrossRef]
- Murphy, R.Y.; Marks, B.P.; Johnson, E.R.; Johnson, M.G. Thermal inactivation kinetics of Salmonella and Listeria in ground chicken breast meat and liquid medium. J. Food Sci. 2000, 65, 706–710. [Google Scholar] [CrossRef]
- Verheyen, D. Micro- and Macroscopic Investigation of the Food Microstructural Influence on Microbial Dynamics: Case Study in/on Fish Products. Ph.D. Thesis, KU Leuven, Leuven, Belgium, 2020. [Google Scholar]
- Smet, C.; Noriega, E.; Van Mierlo, J.; Valdramidis, V.P.; Van Impe, J.F. Influence of the growth morphology on the behaviour of Salmonella Typhimurium and Listeria monocytogenes under osmotic stress. Food Res. Int. 2015, 77, 515–526. [Google Scholar] [CrossRef]
- Robins, M.M.; Wilson, P.D.G. Food structure and microbial growth. Trends Food Sci. Technol. 1994, 5, 289–293. [Google Scholar] [CrossRef]
- Antwi, M.; Bernaerts, K.; Van Impe, J.F.; Geeraerd, A.H. Modelling the combined effects of structured food model system and lactic acid on Listeria innocua and Lactococcus lactis growth in mono- and coculture. Int. J. Food Microbiol. 2007, 120, 71–84. [Google Scholar] [CrossRef]
- Skandamis, P.N.; Jeanson, S. Colonial vs. Planktonic type of growth: Mathematical modelling of microbial dynamics on surfaces and in liquid, semi-liquid and solid foods. Front. Microbiol. 2015, 6, 1178. [Google Scholar] [CrossRef] [Green Version]
- Buchanan, R.L. Predictive food microbiology. Trends Food Sci. Technol. 1993, 4, 6–11. [Google Scholar] [CrossRef]
- Theys, T.E.; Geeraerd, A.H.; Verhulst, A.; Poot, K.; Van Bree, I.; Devlieghere, F.; Moldenaers, P.; Wilson, D.; Brocklehurst, T.; Van Impe, J.F. Effect of pH, water activity and gel micro-structure, including oxygen profiles and rheological characterization, on the growth kinetics of Salmonella Typhimurium. Int. J. Food Microbiol. 2008, 128, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Genigeorgis, C.; Martin, S.; Franti, C.E.; Riemann, H. Initiation of Staphylococcal growth in laboratory media. Appl. Microbiol. 1971, 21, 934–939. [Google Scholar] [CrossRef] [PubMed]
- Nixon, P.A. Temperature integration as a means of assessing storage conditions. In Report on Quality in Fish Products, Seminar No. 3; Fishing Industry Board: Wellington, New Zealand, 1971; pp. 34–44. [Google Scholar]
- Spencer, R.; Baines, C.R. The effect of temperature on the spoilage of wet fish: I. Storage at constant temperature between −1 °C and 25 °C. Food Technol. Champ. 1964, 18, 769–772. [Google Scholar]
- Gompertz, B. On the nature of the function expressive of the law of human mortality, and on a new mode of determining the value of life contingencies. Philos. Trans. R. Soc. Lond. 1825, 115, 513–585. [Google Scholar]
- Zwietering, M.H.; Jongenburger, I.; Rombouts, F.M.; van’t Riet, K. Modeling of the bacterial growth curve. Appl. Environ. Microbiol. 1990, 56, 1875–1881. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baranyi, J.; Roberts, T.A. A dynamic approach to predicting bacterial growth in food. Int. J. Food Microbiol. 1994, 23, 277–294. [Google Scholar] [CrossRef]
- Bhaduri, S.; Turner-Jones, C.O.; Buchanan, R.L.; Phillips, J.G. Response surface model of the effect of pH, sodium chloride and sodium nitrite on growth of Yersinia enterocolitica at low temperatures. Int. J. Food Microbiol. 1994, 23, 333–343. [Google Scholar] [CrossRef]
- George, S.M.; Richardson, L.C.C.; Peck, M.W. Predictive models of the effect of temperature, pH and acetic and lactic acid on the growth of Listeria monocytogenes. Int. J. Food Microbiol. 1996, 32, 73–90. [Google Scholar] [CrossRef]
- Ng, T.M.; Schaffner, D.W. Mathematical models for the effects of pH, temperature, and sodium chloride on the growth of Bacillus stearothermophilus in salty carrots. Appl. Environ. Microbiol. 1997, 63, 1237–1243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sutherland, J.P.; Bayliss, A.J.; Braxton, D.S. Predictive modelling of growth of Escherichia coli O157:H7: The effects of temperature, pH and sodium chloride. Int. J. Food Microbiol. 1995, 25, 29–49. [Google Scholar] [CrossRef]
- Augustin, J.-C.; Carlier, V. Modelling the growth rate of Listeria monocytogenes with a multiplicative type model including interactions between environmental factors. Int. J. Food Microbiol. 2000, 56, 53–70. [Google Scholar] [CrossRef]
- Le Marc, Y.; Huchet, V.; Bourgeois, C.M.; Guyonnet, J.P.; Mafart, P.; Thuault, D. Modelling the growth kinetics of Listeria as a function of temperature, pH and organic acid concentration. Int. J. Food Microbiol. 2002, 73, 219–237. [Google Scholar] [CrossRef]
- Panagou, E.Z.; Skandamis, P.N.; Nychas, G.-J.E. Modelling the combined effect of temperature, pH and aw on the growth rate of Monascus ruber, a heat-resistant fungus isolated from green table olives. J. Appl. Microbiol. 2003, 94, 146–156. [Google Scholar] [CrossRef]
- Little, C.L.; Knøchel, S. Growth and survival of Yersinia enterocolotica, Salmonella and Bacillus cereus in Brie stored at 4, 8 and 20 °C. Int. J. Food Microbiol. 1994, 24, 137–145. [Google Scholar] [CrossRef]
- Meldrum, R.J.; Brocklehurst, T.F.; Wilson, D.R.; Wilson, P.D.G. The effects of cell immobilization, pH and sucrose on the growth of Listeria monocytogenes Scott A at 10 °C. Food Microbiol. 2003, 20, 97–103. [Google Scholar] [CrossRef]
- Ongeng, D.; Ryckeboer, J.; Vermeulen, A.; Devlieghere, F. The effect of micro-architectural structure of cabbage substratum and or background bacterial flora on the growth of Listeria monocytogenes. Int. J. Food Microbiol. 2007, 119, 291–299. [Google Scholar] [CrossRef]
- Esty, J.R.; Meyer, K.F. The heat resistance of the spore of B. botulinus and allied anaerobes XI. J. Infect. Dis. 1922, 31, 650–663. [Google Scholar] [CrossRef]
- Bigelow, W.D. The logarithmic nature of thermal death time curves. J. Infect. Dis. 1921, 29, 528–536. [Google Scholar] [CrossRef]
- Bevilacqua, A.; Speranza, B.; Sinigaglia, M.; Corbo, M.R. A focus on the death kinetics in predictive microbiology: Benefits and limits of the most important models and some tools dealing with their application in foods. Foods 2015, 4, 565–580. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Desriac, N.; Vergos, M.; Achberger, V.; Coroller, L.; Couvert, O. Predicting heat process efficiency in thermal processes when bacterial inactivation is not log-linear. Int. J. Food Microbiol. 2019, 290, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Ball, C.O.; Olson, F.C.W. Sterilization in Food Technology: Theory, Practice and Calculation; McGraw-Hill: New York, NY, USA, 1957. [Google Scholar]
- Garrett, E.R. Prediction of stability in pharmaceutical preparation II. Vitamin stability in liquid multivitamin preparations. J. Am. Pharm. Assoc. 1956, 45, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Levine, S. Determination of the thermal death rate of bacteria. Food Res. 1956, 21, 295–301. [Google Scholar] [CrossRef]
- Davey, K.R. Linear-Arrhenius models for bacterial growth and death and vitamin denaturations. J. Ind. Microbiol. 1993, 12, 172–179. [Google Scholar] [CrossRef]
- Cerf, O.; Davey, K.R.; Sadoudi, A.K. Thermal inactivation of bacteria—A new predictive model for the combined effect of three environmental factors: Temperature, pH and water activity. Food Res. Int. 1996, 29, 219–226. [Google Scholar] [CrossRef]
- Davey, K.R.; Lin, S.H.; Wood, D.G. The effect of pH on continuous high-temperature/short-time sterilization of liquid. Am. Inst. Chem. Eng. J. 1978, 3, 537–540. [Google Scholar] [CrossRef]
- Blackburn, C.d.W.; Curtis, L.M.; Humpheson, L.; Billon, C.; McClure, P.J. Development of thermal inactivation models for Salmonella enteritidis and Escherichia coli O157:H7 with temperature, pH and NaCl as controlling factors. Int. J. Food Microbiol. 1997, 38, 31–44. [Google Scholar] [CrossRef]
- Zanoni, B.; Peri, C.; Garzaroli, C. A dynamic mathematical model of the thermal inactivation of Enterococcus faecium during Bologna Sausage Cooking. Lebensm. Wiss. Technol. 1997, 30, 727–734. [Google Scholar] [CrossRef]
- Albert, I.; Mafart, P. A modified Weibull model for bacterial inactivation. Int. J. Food Microbiol. 2005, 100, 197–211. [Google Scholar] [CrossRef]
- Baranyi, J.; Jones, A.; Walker, C.; Kaloti, A.; Robinson, T.P.; Mackey, B.M. A combined model for growth and subsequent thermal inactivation of Brochothrix thermosphacta. Appl. Environ. Microbiol. 1996, 62, 1029–1035. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Casolari, A. Microbial death. In Physiological Models in Microbiology 2; Bazin, M.J., Prosser, J.I., Eds.; CRC Press: Boca Raton, FL, USA, 2009; pp. 1–44. [Google Scholar]
- Chiruta, J.; Davey, K.R.; Thomas, C.J. Combined effect of temperature and pH on microbial death in continuous pasteurisation of liquids. In Engineering and Food at ICEF7; Jowitt, R., Ed.; Sheffield Academic Press: Sheffield, UK, 1997; pp. A109–A112. [Google Scholar]
- Daugthry, B.J.; Davey, K.R.; Thomas, C.J.; Verbyla, A.P. Food processing–A new model for the thermal destruction of contaminating bacteria. In Engineering and Food at ICEF7; Jowitt, R., Ed.; Sheffield Academic Press: Sheffield, UK, 1997; pp. A113–A116. [Google Scholar]
- Geeraerd, A.H.; Herremans, C.H.; Van Impe, J.F. Structural model requirements to describe microbial inactivation during a mild heat treatment. Int. J. Food Microbiol. 2000, 59, 185–209. [Google Scholar] [CrossRef]
- Sapru, V.; Teixeira, A.A.; Smerage, G.H.; Lindsay, J.A. Predicting thermophilic spore population dynamics for UHT sterilization processes. J. Food Sci. 1992, 575, 1248–1252. [Google Scholar] [CrossRef]
- Whiting, R.C. Modeling bacterial survival in unfavorable environments. J. Ind. Micro 1993, 12, 240–246. [Google Scholar] [CrossRef]
- Xiong, R.; Xie, G.; Edmondson, A.E.; Sheard, M.A. A mathematical model for bacterial inactivation. Int. J. Food Microbiol. 1999, 46, 45–55. [Google Scholar] [CrossRef]
- Aggelis, G.; Samelis, J.; Metaxopoulos, J. A novel modelling approach for predicting microbial growth in a raw cured meat product stored at 3 °C and at 12 °C in air. Int. J. Food Microbiol. 1998, 43, 39–52. [Google Scholar] [CrossRef]
- Baker, D.A.; Genigeorgis, C. Predicting the safe storage of fresh fish under modified atmospheres with respect to Clostridium botulinum toxigenesis by modeling length of the lag phase of growth. J. Food Prot. 1990, 53, 131–140. [Google Scholar] [CrossRef]
- Cauchie, E.; Delhalle, L.; Baré, G.; Tahiri, A.; Taminiau, B.; Korsak, N.; Burteau, S.; Fall, P.A.; Farnir, F.; Daube, G. Modeling the growth and interaction between Brochothrix thermosphacta, Pseudomonas spp., and Leuconostoc gelidum in minced pork samples. Front. Microbiol. 2020, 11, 639. [Google Scholar] [CrossRef] [Green Version]
- Choi, W.-S.; Son, N.; Cho, J.-I.; Joo, I.-S.; Han, J.-A.; Kwak, H.-S.; Hong, J.-H.; Suh, S.H. Predictive model of Staphylococcus aureus growth on egg products. Food Sci. Biotechnol. 2019, 28, 913–922. [Google Scholar] [CrossRef]
- Chung, K.-H.; Park, M.S.; Kim, H.-Y.; Bahk, G.J. Growth prediction and time–temperature criteria model of Vibrio parahaemolyticus on traditional Korean raw crab marinated in soy sauce (ganjang-gejang) at different storage temperatures. Food Control 2019, 98, 187–193. [Google Scholar] [CrossRef]
- Dickson, J.S.; Siragusa, G.R.; Wray, J.E., Jr. Predicting the growth of Salmonella typhimurium on beef by using the temperature function integration technique. Appl. Environ. Microbiol. 1992, 58, 3482–3487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giannuzzi, L.; Pinotti, A.; Zaritzky, N. Mathematical modelling of microbial growth in packaged refrigerated beef stored at different temperatures. Int. J. Food Microbiol. 1998, 39, 101–110. [Google Scholar] [CrossRef]
- Hwang, C.-A.; Huang, L. Growth and survival of Bacillus cereus from spores in cooked rice–One-step dynamic analysis and predictive modelling. Food Control 2019, 96, 403–409. [Google Scholar] [CrossRef]
- Hyun, J.-E.; Yoon, J.-H.; Lee, S.-Y. Response surface modeling for the inactivation of Bacillus cereus on cooked spinach by natural antimicrobial at various temperatures. J. Food Saf. 2018, 38, e12484. [Google Scholar] [CrossRef]
- Juneja, V.K.; Mishra, A.; Pradhan, A.K. Dynamic predictive model for growth of Bacillus cereus from spores in cooked beans. J. Food Prot. 2018, 81, 308–315. [Google Scholar] [CrossRef] [PubMed]
- Juneja, V.K.; Golden, C.E.; Mishra, A.; Harrison, M.A.; Mohr, T.B. Predictive model for growth of Bacillus cereus at temperature applicable to cooling of cooked pasta. J. Food Sci. 2019, 84, 590–598. [Google Scholar] [CrossRef]
- Kowalik, J.; Lobacz, A.; Zulewska, J.; Dec, B. Analysis and mathematical modelling of the behaviour of Escherichia coli in the mascarpone cheese during cold storage. Int. J. Food Sci. Technol. 2018, 53, 1541–1548. [Google Scholar] [CrossRef]
- Li, M.; Huang, L.; Zhu, Y.; Wei, Q. Growth of Clostridium perfringens in roasted chicken and braised beef during cooling–One-step dynamics analysis and modelling. Food Control 2019, 106, 106739. [Google Scholar] [CrossRef]
- Longhi, D.A.; da Silva, N.B.; Martins, W.F.; Carciofi, B.A.M.; de Aragão, G.M.F.; Laurindo, J.B. Optimal experimental design to model spoilage bacteria growth in vacuum-packaged ham. J. Food Eng. 2018, 216, 20–26. [Google Scholar] [CrossRef]
- Oscar, T.P. Response surface models for effects of temperature and previous growth sodium chloride on growth kinetics of Salmonella typhimurium on cooked chicken breast. J. Food Prot. 1999, 62, 1470–1474. [Google Scholar] [CrossRef]
- Park, S.Y.; Ha, S.-D. Predictive growth model of the effects of temperature on the growth kinetics of generic Escherichia coli in the Korean traditional rice cake product “Garaetteok”. J. Food Sci. Technol. 2018, 55, 506–512. [Google Scholar] [CrossRef]
- Park, S.Y.; Choi, S.-Y.; Ha, S.-D. Predictive modeling for the growth of Aeromonas hydrophila on lettuce as a function of combined storage temperature and relative humidty. Foodborne Pathog. Dis. 2019, 16, 376–383. [Google Scholar] [CrossRef] [PubMed]
- Roberts, T.A.; Gibson, A.M.; Robinson, A. Prediction of toxin production by Clostridium botulinum in pasteurized pork slurry. J. Food Technol. 1981, 16, 337–355. [Google Scholar] [CrossRef]
- Taoukis, P.S.; Koutsoumanis, K.; Nychas, G.J.E. Use of time-temperature integrators and predictive modelling for shelf life control of chilled fish under dynamic storage conditions. Int. J. Food Microbiol. 1999, 53, 21–31. [Google Scholar] [CrossRef] [Green Version]
- Tarlak, F.; Johannessen, G.; Villegas, I.B.; Bolívar, A.; Posada-Izquierdo, G.D.; Pérez-Rodríguez, F. Modelling of the behaviour of Salmonella enterica serovar Reading on commercial fresh-cut iceberg lettuce stored at different temperatures. Foods 2020, 9, 946. [Google Scholar] [CrossRef]
- Vankerschaver, K.; Willocx, F.; Smout, C.; Hendrickx, M.; Tobback, P. The influence of temperature and gas mixtures on the growth of the intrinsic micro-organisms on cut endive: Predictive versus actual growth. Food Microbiol. 1996, 13, 427–440. [Google Scholar] [CrossRef]
- Zamora, M.C.; Zaritzky, N.E. Modeling of microbial growth in refrigerated packaged beef. J. Food Sci. 1985, 50, 1003–1006. [Google Scholar] [CrossRef]
- Costa, J.C.C.P.; Bover-Cid, S.; Bolívar, A.; Zurera, G.; Pérez-Rodríguez, F. Modelling the interaction of the sakacin-producing Lactobacillus sakei CTC494 and Listeria monocytogenes in filleted gilthead sea bream (Sparus aurata) under modified atmosphere packaging at isothermal and non-isothermal conditions. Int. J. Food Microbiol. 2019, 297, 72–84. [Google Scholar] [CrossRef]
- Cuggino, S.G.; Bascón-Villegas, I.; Rincón, F.; Pérez, M.A.; Posada-Izquierdo, G.; Marugán, J.; Carro, C.P.; Pérez-Rodríguez, F. Modelling the combined effect of choline, benzyl isothiocyanate, exposure time and cut size on the reduction of Salmonella in fresh-cut lettuce during washing process. Food Microbiol. 2020, 86, 103346. [Google Scholar] [CrossRef]
- Jaczynski, J.; Park, J.W. Predictive models for microbial inactivation and texture degradation in surimi seafood during thermal processing. J. Food Sci. 2003, 68, 1025–1030. [Google Scholar] [CrossRef]
- Jia, Z.; Li, C.; Fang, T.; Chen, J. Predictive modeling of the effect of ε-polylysine hydrochloride on growth and thermal inactivation of Listeria monocytogenes in fish balls. J. Food Sci. 2019, 84, 127–132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Juneja, V.K.; Gonzales-Barron, U.; Butler, F.; Yadav, A.S.; Friedman, M. Predictive thermal inactivation model for the combined effect of temperature, cinnamaldehyde and carvacrol on starvation-stressed multiple Salmonella serotypes in ground chicken. Int. J. Food Microbiol. 2013, 265, 184–199. [Google Scholar] [CrossRef] [PubMed]
- Juneja, V.K.; Garcia-Dávila, J.; Lopez-Romero, J.C.; Pena-Ramos, E.A.; Camou, J.P.; Valenzuela-Melendres, M. Modeling the effects of temperature, sodium chloride, and green tea and their interactions on the thermal inactivation of Listeria monocytogenes in Turkey. J. Food Prot. 2014, 77, 1696–1702. [Google Scholar] [CrossRef] [PubMed]
- Koutsoumanis, K.; Lambropoulou, K.; Nychas, G.-J.E. A predictive model for the non-thermal inactivation of Salmonella enteritidis in a food model system supplemented with a natural antimicrobial. Int. J. Food Microbiol. 1999, 49, 63–74. [Google Scholar] [CrossRef]
- Nazarowec-White, M.; McKellar, R.C.; Piyasena, P. Predictive modelling of Enterobacter sakazakii inactivation in bovine milk during high-temperature short-time pasteurization. Food Res. Int. 1999, 32, 375–379. [Google Scholar] [CrossRef]
- Portela, J.B.; Coimbra, P.T.; Cappato, L.P.; Alvarenga, V.O.; Oliveira, R.B.A.; Pereira, K.S.; Azeredo, D.R.P.; Sant’Ana, A.S.; Nascimento, J.S.; Cruz, A.G. Predictive model for inactivation of Salmonella in infant formula during microwave heating processing. Food Control 2019, 104, 308–312. [Google Scholar] [CrossRef]
- Santillana Farakos, S.M.; Pouillot, R.; Anderson, N.; Johnson, R.; Son, I.; Van Doren, J. Modeling the survival kinetics of Salmonella in tree nuts for use in risk assessment. Int. J. Food Microbiol. 2016, 227, 41–50. [Google Scholar] [CrossRef] [Green Version]
- Sheen, S.; Huang, C.-Y.; Ramos, R.; Chien, S.-Y.; Scullen, J.; Sommers, C. Lethality prediction for Escherichia Coli O157:H7 and uropathogenic E. coli in ground chicken treated with high pressure processing and trans-cinnamaldehyde. J. Food Sci. 2018, 83, 740–749. [Google Scholar] [CrossRef]
- Noriega, E.; Laca, A.; Díaz, M. Modelling of diffusion-limited growth to predict Listeria distribution in structured model foods. J. Food Eng. 2008, 87, 247–256. [Google Scholar] [CrossRef]
- Broughall, J.M.; Anslow, P.A.; Kilsby, D.C. Hazard analysis applied to microbial growth in foods: Development of mathematical models describing the effect of water activity. J. Appl. Bacteriol. 1983, 55, 101–110. [Google Scholar] [CrossRef]
- Devlieghere, F.; Geeraerd, A.H.; Versyck, K.J.; Vandewaetere, J.; Van Impe, J.; Debevere, J. Growth of Listeria monocytogenes in modified atmosphere packed cooked meat products: A predictive model. Food Microbiol. 2001, 18, 53–66. [Google Scholar] [CrossRef]
- Gibson, A.M.; Bratchell, N.; Roberts, T.A. Predicting microbial growth: Growth responses of salmonellae in a laboratory medium as affected by pH, sodium chloride and storage temperature. Int. J. Food Microbiol. 1988, 6, 155–178. [Google Scholar] [CrossRef]
- Juneja, V.K.; Marmer, B.S.; Phillips, J.G.; Miller, A.J. Influence of the intrinsic properties of food on thermal inactivation of spores of nonproteolytic Clostridium botulinum: Development of a predictive model. J. Food Saf. 1995, 15, 349–364. [Google Scholar] [CrossRef]
- Aspridou, Z.; Moschakis, T.; Biliaderis, C.G.; Koutsoumanis, K.P. Effect of the substrate’s microstructure on the growth of Listeria monocytogenes. Food Res. Int. 2014, 64, 683–691. [Google Scholar] [CrossRef] [PubMed]
- Boons, K.; Van Derlinden, E.; Mertens, L.; Peeters, V.; Van Impe, J.F. Effect of immobilization and salt concentration on the growth dynamics of Escherichia coli K12 and Salmonella typhimurium. J. Food Sci. 2013, 78, 567–574. [Google Scholar] [CrossRef]
- Costello, K.M.; Gutierrez-Merino, J.; Bussemaker, M.; Ramaioli, M.; Baka, M.; Van Impe, J.F.; Velliou, E.G. Modelling the microbial dynamics and antimicrobial resistance development of Listeria in viscoelastic food model systems of various structural complexities. Int. J. Food Microbiol. 2018, 286, 15–30. [Google Scholar] [CrossRef]
- Kabanova, N.; Stulova, I.; Vilu, R. Microcalorimetric study of the growth of bacterial colonies of Lactococcus lactis IL1403 in agar gels. Food Microbiol. 2012, 29, 67–79. [Google Scholar] [CrossRef]
- Prachaiyo, P.; McLandsborough, L.A. Oil-in-water emulsion as a model system to study the growth of Escherichia coli O157:H7 in a heterogeneous food system. J. Food Sci. 2003, 68, 1018–1024. [Google Scholar] [CrossRef]
- Zalazar, A.L.; Gliemmo, M.F.; Campos, C.A. Effect of stabilizers, oil level and structure on the growth of Zygosaccharomyces bailii and on physical stability of model systems simulating acid sauces. Food Res. Int. 2016, 85, 200–208. [Google Scholar] [CrossRef]
- Castro, M.P.; Rojas, A.M.; Campos, C.A.; Gerschenson, L.N. Effect of preservatives, tween 20, oil content and emulsion structure on the survival of Lactobacillus fructivorans in model salad dressings. LWT Food Sci. Technol. 2009, 42, 1428–1434. [Google Scholar] [CrossRef]
- Verheyen, D.; Baka, M.; Akkermans, S.; Skåra, T.; Van Impe, J.F. Effect of microstructure and initial cell conditions on thermal inactivation kinetics and sublethal injury of Listeria monocytogenes in fish-based food model systems. Food Microbiol. 2019, 84, 103267. [Google Scholar] [CrossRef] [PubMed]
- Verheyen, D.; Govaert, M.; Seow, T.K.; Ruvina, J.; Mukherjee, V.; Baka, M.; Skåra, T.; Van Impe, J.F. The complex effect of food matrix fat content on thermal inactivation of Listeria monocytogenes: Case study in emulsion and gelled emulsion model systems. Front. Microbiol. 2020, 10, 3149. [Google Scholar] [CrossRef] [PubMed]
- Bellara, S.R.; Fryer, P.J.; McFarlane, C.M.; Thomas, C.R.; Hocking, P.M.; Mackey, B.M. Visualization and modelling of the thermal inactivation of bacteria in a model food. Appl. Environ. Microbiol. 1999, 65, 3095–3099. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamoud-Agha, M.M.; Curet, S.; Simonin, H.; Boillereaux, L. Microwave inactivation of Escherichia coli K12 CIP 54.117 in a gel medium: Experimental and numerical study. J. Food Eng. 2013, 116, 315–323. [Google Scholar] [CrossRef]
- Mackey, B.M.; Kelly, A.F.; Colvin, J.A.; Robbins, P.T.; Fryer, P.J. Predicting the thermal inactivation of bacteria in a solid matrix: Simulation studies on the relative effects of microbial thermal resistance parameters and process conditions. Int. J. Food Microbiol. 2006, 107, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Noriega, E.; Laca, A.; Díaz, M. Modelling of diffusion-limited growth for food safety in simulated cheeses. Food Bioprod. Process. 2008, 86, 122–129. [Google Scholar] [CrossRef]
- Mertens, L.; Van Derlinden, E.; Dang, T.D.T.; Cappuyns, A.M.; Vermeulen, A.; Debevere, J.; Moldenaers, P.; Devlieghere, F.; Geeraerd, A.H.; Van Impe, J.F. On the critical evaluation of growth/no growth assessment of Zygosaccharomyces bailii with optical density measurements: Liquid versus structured media. Food Microbiol. 2011, 28, 736–745. [Google Scholar] [CrossRef]
- Ter Steeg, P.F.; Otten, G.D.; Alderliesten, M.; De Weijer, R.; Naaktgeboren, G.; Bijl, J.; Vasbinder, A.J.; Kershof, I.; Van Duijvendijk, A.M. Modelling the effects of (green) antifungals, droplet size distribution and temperature on mould outgrowth in water-in-oil emulsions. Int. J. Food Microbiol. 2001, 67, 227–239. [Google Scholar] [CrossRef]
- Kapetanakou, A.E.; Ampavi, A.; Yanniotis, S.; Drosinos, E.H.; Skandamis, P.N. Development of a model describing the effect of temperature, water activity and (gel) structure on growth and ochratoxin A production by Aspergillus carbonarius in vitro and evaluation in food matrices of different viscosity. Food Microbiol. 2011, 28, 727–735. [Google Scholar] [CrossRef]
- Dens, E.J.; Van Impe, J.F. On the need for another type of predictive model in structured foods. Int. J. Food Microbiol. 2001, 64, 247–260. [Google Scholar] [CrossRef]
- De Bonis, M.V.; Ruocco, G. A heat and mass transfer perspective of microbial behavior modelling in a structured vegetable food. Int. J. Food Eng. 2016, 190, 72–79. [Google Scholar] [CrossRef]
- Ferrier, R.; Hezard, B.; Lintz, A.; Stahl, V.; Augustin, J.-C. Combining individual-based modelling and food microenvironment descriptions to predict the growth of Listeria monocytogenes on smear soft cheese. Appl. Environ. Microbiol. 2013, 19, 5870–5881. [Google Scholar] [CrossRef] [Green Version]
- Augustin, J.-C.; Ferrier, R.; Hezard, B.; Lintz, A.; Stahl, V. Comparison of individual-based modeling and population approaches for prediction of foodborne pathogens growth. Food Microbiol. 2015, 45, 205–215. [Google Scholar] [CrossRef] [PubMed]
- Vereecken, K.M.; Devlieghere, F.; Bockstaele, A.; Debevere, J.; Van Impe, J.F. A model for lactic acid-induced inhibition of Yersinia enterocolitica in mono- and coculture with Lactobacillus sakei. Food Microbiol. 2003, 20, 701–713. [Google Scholar] [CrossRef]
- Ross, T.; Ratkowsky, D.A.; Mellefont, L.A.; McMeekin, T.A. Modelling the effects of temperature, water activity, pH and lactic acid concentration on the growth rate of Escherichia coli. Int. J. Food Microbiol. 2003, 82, 33–43. [Google Scholar] [CrossRef]
- Reiner, M. Über die strömung einer elastichen flüssigkeit durch eine kapillare. Kolloid, Z. 1926, 39, 80–87. [Google Scholar] [CrossRef]
- Verheyen, D.; Bolívar, A.; Pérez-Rodríguez, F.; Baka, M.; Skåra, T.; Van Impe, J.F. Isolating the effect of fat content on Listeria monocytogenes growth dynamics in fish-based emulsion and gelled emulsion systems. Food Control 2020, 108, 106874. [Google Scholar] [CrossRef]
- Van Impe, J.F.; Poschet, F.; Geeraerd, A.H.; Vereecken, K.M. Towards a novel class of predictive microbial growth models. Int. J. Food Microbiol. 2005, 100, 97–105. [Google Scholar] [CrossRef]
- Tack, I. Metabolic Differentiation in Microbial Colonies and Biofilms: A Multiscale Modelling Approach. Ph.D. Thesis, KU Leuven, Leuven, Belgium, 2016. [Google Scholar]
- Ayala, F.J.; Gilpin, M.J.; Ehrenfield, J.G. Competition between species: Theoretical models and experimental results. Theor. Pop Biol. 1973, 4, 331–356. [Google Scholar] [CrossRef] [Green Version]
- Bailey, J.E.; Ollis, D.F. Biochemical Engineering Fundamentals, 2nd ed.; McGraw-Hill: New York, NY, USA, 1986. [Google Scholar]
- Baranyi, J.; Robinson, T.P.; Kaloti, A.; Mackey, B.M. Predicting the growth of Brochothrix thermosphacta at changing temperature. Int. J. Food Microbiol. 1995, 27, 61–75. [Google Scholar] [CrossRef]
- Ratkowsky, D.A.; Olley, J.; McMeekin, T.A.; Ball, A. Relationship between temperature and growth rate of bacterial cultures. J. Bacteriol. 1982, 149, 1–5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gorochowski, T.E.; Matyjaszkiewicz, A.; Todd, T.; Oak, N.; Kowalska, K.; Reid, S.; Tsaneva-Atanasova, K.T.; Savery, N.J.; Grierson, C.S.; di Bernardo, M. BSim: An agent-based tool for modeling bacterial populations in systems and synthetic biology. PLoS ONE 2012, 7, e42790. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- González-Cabaleiro, R.; Mitchell, A.M.; Smith, W.; Wipat, A.; Ofiteru, I.D. Heterogeneity in pure microbial systems: Experimental measurements and modeling. Front. Microbiol. 2017, 8, 1813. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kreft, J.-U.; Booth, G.; Wimpenny, J.W.T. BacSim, a simulator for individual-based modelling of bacterial colony growth. Microbiology 1998, 144, 3275–3287. [Google Scholar] [CrossRef] [Green Version]
- Ginovart, M.; López, D.; Valls, J. INDISIM, an individual-based discrete simulation model to study bacterial cultures. J. Theor. Biol. 2002, 214, 305–319. [Google Scholar] [CrossRef]
- Verhulst, A.J.; Cappuyns, A.M.; Van Derlinden, E.; Bernaerts, K.; Van Impe, J.F. Analysis of the lag phase to exponential growth transition by incorporating inoculum characteristics. Food Microbiol. 2011, 28, 656–666. [Google Scholar] [CrossRef]
- Tack, I.L.M.M.; Nimmegeers, P.; Akkermans, S.; Hashem, I.; Van Impe, J.F.M. Simulation of Escherichia coli dynamics in biofilms and submerged colonies with an individual-based model including metabolic network information. Front. Microbiol. 2017, 8, 2509. [Google Scholar] [CrossRef]
- Lardon, L.A.; Merkey, B.V.; Martins, S.; Dötsch, A.; Picioreanu, C.; Kreft, J.-U.; Smets, B.F. iDynoMiCS: Next-generation individual-based modelling of biofilms. Environ. Microbiol. 2011, 13, 2416–2434. [Google Scholar] [CrossRef]
- Hellweger, F.L.; Clegg, R.J.; Clark, J.R.; Plugge, C.M.; Kreft, J.U. Advancing microbial sciences by individual-based modelling. Nat. Rev. Microbiol. 2016, 14, 461–471. [Google Scholar] [CrossRef]
- Possas, A.; Pérez-Rodríguez, F.; Valero, A.; Rincón, F.; García-Gimeno, R.M. Mathematical approach for the Listeria monocytogenes inactivation during high hydrostatic pressure processing of a simulated meat medium. Innov. Food Sci. Emerg. Technol. 2018, 47, 271–278. [Google Scholar] [CrossRef]
- Chhabra, A.T.; Carter, W.H.; Linton, R.H.; Cousin, M.A. A predictive model to determine the effects of pH, milkfat, and temperature on thermal inactivation of Listeria monocytogenes. J. Food Prot. 1999, 62, 1143–1149. [Google Scholar] [CrossRef]
- Santillana Farakos, S.M.; Frank, J.F.; Schaffner, D.W. Modeling the influence of temperature, water activity and water mobility on the persistence of Salmonella in low-moisture foods. Int. J. Food Microbiol. 2013, 166, 280–293. [Google Scholar] [CrossRef] [Green Version]
- Trimble, L.M.; Frank, J.F.; Schaffner, D.W. Modification of a predictive model to include the influence of fat content on Salmonella inactivation in low-water-activity foods. J. Food Prot. 2020, 83, 801–815. [Google Scholar] [CrossRef]
- Albuquerque, C.D.D.; Curet, S.; Boillereaux, L. A 3D-CFD-heat-transfer-based model for the microbial inactivation of pasteurized food products. Innov. Food Sci. Emerg. Technol. 2019, 54, 172–181. [Google Scholar] [CrossRef]
- Gil, M.M.; Miller, F.A.; Brandão, T.R.S.; Silva, C.L.M. On the use of the Gompertz model to predict microbial thermal inactivation under isothermal and non-isothermal conditions. Food Eng. Rev. 2011, 3, 17–25. [Google Scholar] [CrossRef]
- Mafart, P.; Couvert, O.; Gaillard, S.; Leguerinel, I. On calculating sterility in thermal preservation methods: Application of the Weibull frequency distribution model. Int. J. Food Microbiol. 2002, 72, 107–113. [Google Scholar] [CrossRef] [Green Version]
- Huang, L. Thermal inactivation of Listeria monocytogenes in ground beef under isothermal and dynamic temperature conditions. J. Food Eng. 2009, 90, 380–387. [Google Scholar] [CrossRef]
- Chen, H.-H.; Kang, H.-Y.; Chen, S.-D. The effects of ingredients and water content on the rheological properties of batters and physical properties of crusts in fried foods. J. Food Eng. 2008, 88, 45–54. [Google Scholar] [CrossRef]
- Rao, M.A. Rheology of Fluid and Semifluid Foods: Principles and Applications; Springer Science+Business Media, LLC: New York, NY, USA, 2007. [Google Scholar]
- Whiting, R.C.; Sackitey, S.; Calderone, S.; Morely, K.; Phillips, J.G. Model for the survival of Staphylococcus aureus in nongrowth environments. Int. J. Food Microbiol. 1996, 31, 231–243. [Google Scholar] [CrossRef]
- Bhuvaneswari, E.; Anandharamakrishnan, C. Heat transfer analysis of pasteurization of bottled beer in a tunnel pasteurizer using computational fluid dynamics. Innov. Food Sci. Emerg. Technol. 2014, 23, 156–163. [Google Scholar] [CrossRef]
- Erdogdu, F.; Tutar, M.; Sarghini, F.; Skipnes, D. Effects of viscosity and agitation rate on temperature and flow field in cans during reciprocal agitation. J. Food Eng. 2017, 213, 76–88. [Google Scholar] [CrossRef]
- Topcam, H.; Karatas, O.; Erol, B.; Erdogdu, F. Effect of rotation on temperature uniformity of microwave processed low-high viscosity liquids: A computational study with experimental validation. Innov. Food Sci. Emerg. Technol. 2020, 60, 102306. [Google Scholar] [CrossRef]
- Wu, H.; Karayiannis, T.G.; Tassou, S.A. A two-dimensional frying model for the investigation and optimisation of continuous industrial frying systems. Appl. Therm. Eng. 2013, 51, 926–936. [Google Scholar] [CrossRef]
- Bedane, T.F.; Erdogdu, F.; Lyng, J.G.; Marra, F. Effects of geometry and orientation of food products on heating uniformity during radio frequency heating. Food Bioprod. Process. 2021, 125, 149–160. [Google Scholar] [CrossRef]
- Erdogdu, F.; Karatas, O.; Sarghini, F. A short update on heat transfer modelling for computational food processing in conventional and innovative processing. Curr. Opin. Food Sci. 2018, 23, 113–119. [Google Scholar] [CrossRef]
- Aspridou, A.; Koutsoumanis, K.P. Individual cell heterogeneity as variability source in population dynamics of microbial inactivation. Food Microbiol. 2015, 45, 216–221. [Google Scholar] [CrossRef] [PubMed]
- Abe, H.; Koyama, K.; Kawamura, S.; Koseki, S. Stochastic evaluation of Salmonella enterica lethality during thermal inactivation. Int. J. Food Microbiol. 2018, 285, 129–135. [Google Scholar] [CrossRef]
- Koseki, S.; Koyama, K.; Abe, H. Recent advances in predictive microbiology: Theory and application of conversion from population dynamics to individual cell heterogeneity during inactivation process. Curr. Opin. Food Sci. 2021, 42, 60–67. [Google Scholar] [CrossRef]


| Year | Type | Microorganism(s) | Food Product | Ref. |
|---|---|---|---|---|
| 1981 | Growth | Clostridium botulinum | Pork slurry | [82] |
| 1985 | Growth | Various background microflora | Beef | [86] |
| 1990 | Growth | Clostridium botulinum | Fish filets | [66] |
| 1992 | Growth | Salmonella Typhimurium | Beef | [70] |
| 1996 | Growth | Various background microflora | Cut endive | [85] |
| 1997 | Inactivation | Enterococcus faecium | Bologna sausage | [55] |
| 1998 | Growth | Various background microflora | Sausage | [65] |
| 1998 | Growth | Various background microflora | Beef | [71] |
| 1999 | Inactivation | Salmonella enteritidis | Tarama salad | [93] |
| 1999 | Inactivation | Enterobacter sakazakii | Bovine whole milk | [94] |
| 1999 | Growth | Salmonella Typhimurium | Cooked chicken breast | [79] |
| 1999 | Growth | Pseudomonas spp. and Shewanella putrefaciens | Fresh bogue fish | [83] |
| 2003 | Inactivation | Staphylococcus aureus | Surimi seafood sticks | [89] |
| 2013 | Inactivation | Salmonella | Ground chicken | [91] |
| 2014 | Inactivation | Listeria monocytogenes | Ground turkey | [92] |
| 2016 | Inactivation | Salmonella | Tree nuts | [96] |
| 2018 | Growth | Bacillus cereus | Cooked spinach | [73] |
| 2018 | Growth | Bacillus cereus (spores) | Cooked beans | [74] |
| 2018 | Growth | Escherichia coli | Mascarpone cheese | [76] |
| 2018 | Growth | Weissella viridescens | Vacuum-packaged ham | [78] |
| 2018 | Growth | Escherichia coli | Korean rice cake | [80] |
| 2018 | Inactivation | Escherichia coli | Ground chicken | [97] |
| 2019 | Growth | Staphylococcus aureus | Egg products | [68] |
| 2019 | Growth | Vibrio parahaemolyticus | Korean raw crab marinated in soy sauce | [69] |
| 2019 | Inactivation | Listeria monocytogenes | Gilthead sea bream fillets | [87] |
| 2019 | Growth | Bacillus cereus | Cooked rice | [72] |
| 2019 | Growth/inactivation | Listeria monocytogenes | Fish balls | [90] |
| 2019 | Growth | Bacillus cereus (spores) | Cooked pasta | [75] |
| 2019 | Growth | Clostridium perfringens | Roasted chicken and braised beef | [77] |
| 2019 | Growth | Aeromonas hydrophila | Lettuce | [81] |
| 2019 | Inactivation | Salmonella | Infant formula | [95] |
| 2020 | Growth | Brochothrix thermosphacta, Leuconostoc gelidum and Pseudomonas spp. | Minced pork | [67] |
| 2020 | Inactivation | Salmonella Thompson | Iceberg lettuce | [88] |
| 2020 | Growth | Salmonella Reading and lactic acid bacteria | Iceberg lettuce | [84] |
| Macroscale Secondary Models | |||
|---|---|---|---|
| Model Description | Microstructural Factors | Non-Microstructural Factors | Ref. |
| Listeria innocua and Lactococcus lactis growth (mono- and co-culture) in a gelled system | Gelatine concentration | Undissociated lactic acid concentration, pH, physiological state of the cells (for lag phase) | [24] |
| Aspergillus carbonarius growth in broth | Gelatine concentration | Temperature, water activity, physiological state of the cells (for lag phase) | [118] |
| Salmonella Typhimurium growth in broth | Gelatine concentration | Water activity, pH, physiological state of the cells (for lag phase) | [27] |
| Semi-Mechanistic Microscale Models | |||
| Model Description | Included Environmental Factors | Ref. | |
| Mixed population growth model for homogeneous food products, with 2-dimensional space dependency | Food structure (via firmness of the food), biomass transport (via diffusion) | [119] | |
| Listeria innocua growth in solid or paste foods | Dissolved oxygen concentration (and diffusion), biomass transport (via diffusion) | [98,115] | |
| Escherichia coli growth in a (3D)-structured leafy product during handling and storage | Temperature/heat transfer, biomass transfer (via diffusion), leafy structure (via inter-leaf contact points and entrapped air pockets) | [120] | |
| Listeria monocytogenes growth on the surface of smear soft cheese and vacuum-packed cold-smoked salmon | Local pH, local water activity, temperature, structural environment (e.g., hollows, crests) | [121,122] | |
| Macroscale Secondary Models | |||
| Model Description | Microstructural Factors | Non-Microstructural Factors | Ref. |
| Listeria monocytogenes inactivation in homogenised milk model systems | Fat content | Temperature, pH | [142] |
| Salmonella inactivation in whey protein powder model systems | Water mobility | Temperature, water activity | [143] |
| Salmonella inactivation in whey protein–peanut oil powders model systems | Fat content | Temperature, water activity | [144] |
| Semi-Mechanistic Microscale Models | |||
| Model Description | Included Environmental Factors | Ref. | |
| Escherichia coli K12 thermal inactivation (microwave) in calcium alginate gels | Local temperature (via microwave dielectric heating and heat transfer, taking thermophysical properties of the gels into account) | [113] | |
| Escherichia coli K12 thermal inactivation in pre-packed ground beef in water baths | Fluid flow in water bath, local temperature (via heat transfer, taking thermophysical properties of the ground beef into account) | [145] | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Verheyen, D.; Van Impe, J.F.M. The Inclusion of the Food Microstructural Influence in Predictive Microbiology: State-of-the-Art. Foods 2021, 10, 2119. https://doi.org/10.3390/foods10092119
Verheyen D, Van Impe JFM. The Inclusion of the Food Microstructural Influence in Predictive Microbiology: State-of-the-Art. Foods. 2021; 10(9):2119. https://doi.org/10.3390/foods10092119
Chicago/Turabian StyleVerheyen, Davy, and Jan F. M. Van Impe. 2021. "The Inclusion of the Food Microstructural Influence in Predictive Microbiology: State-of-the-Art" Foods 10, no. 9: 2119. https://doi.org/10.3390/foods10092119