Cold Microfiltration as an Enabler of Sustainable Dairy Protein Ingredient Innovation
Abstract
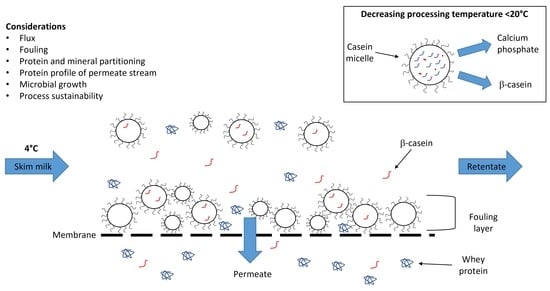
:1. Introduction to Microfiltration
2. Choice of Membranes
2.1. Membrane Structures and Configurations
2.2. Choice of Membrane Material
2.2.1. Polymeric
2.2.2. Ceramic
3. Cold Microfiltration in Dairy Processing
3.1. Temperature Range
3.2. Membrane Performance and Fouling
3.3. Composition of the Fouling Layer
3.4. Microbial Impact
3.5. Protein Partitioning
3.6. Mineral Partitioning
3.7. Enzyme Partitioning
4. Microfiltration as an Enabler of Dairy Ingredient Innovation
4.1. Warm Microfiltration Applications in the Dairy Industry
4.2. Cold Microfiltration
4.2.1. Influence of Temperature on β-Casein Dissociation
4.2.2. Production of β-Casein-Enriched Ingredients via Cold Microfiltration
5. Sustainability
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Pouliot, Y. Membrane processes in dairy technology—From a simple idea to worldwide panacea. Int. Dairy J. 2008, 18, 735–740. [Google Scholar] [CrossRef]
- Saboya, L.V.; Maubois, J.L. Current developments of microfiltration technology in the dairy industry. Lait 2000, 80, 541–553. [Google Scholar] [CrossRef] [Green Version]
- Gésan-Guiziou, G. Removal of bacteria, spores and somatic cells from milk by centrifugation and microfiltration techniques. In Improving the Safety and Quality of Milk: Milk Production and Processing; Griffiths, M.W., Ed.; Woodhead Publishing Ltd.: Cambridge, UK, 2010; Volume 1, pp. 349–372. ISBN 9781845694388. [Google Scholar]
- Wang, D.; Fritsch, J.; Moraru, C.I. Shelf life and quality of skim milk processed by cold microfiltration with a 1.4-μm pore size membrane, with or without heat treatment. J. Dairy Sci. 2019, 102, 8798–8806. [Google Scholar] [CrossRef]
- Cheryan, M. Ultrafiltration and Microfiltration Handbook; Technomic Publishing Co., Inc.: Lancaster, PA, USA, 1998. [Google Scholar]
- Strathmann, H.; Giorno, L.; Drioli, E. Introduction to Membrane Science and Technology; Wiley-VCH: Weinheim, Germany, 2011. [Google Scholar]
- Smith, K. Development of membrane processes. In Membrane Processing. Dairy and Beverage Applications, 1st ed.; Tamime, A.Y., Ed.; Wiley-Blackwell: West Sussex, UK, 2013; pp. 16–29. ISBN 9781118457023. [Google Scholar]
- Wang, H.; Zhou, H. Understand the basics of membrane filtration. Chem. Eng. Prog. 2013, 109, 33–40. [Google Scholar]
- Smith, K. Commercial membrane technology. In Membrane Processing. Dairy and Beverage Applications, 1st ed.; Tamime, A.Y., Ed.; Wiley-Blackwell: West Sussex, UK, 2013; pp. 61–79. ISBN 9781118457023. [Google Scholar]
- Espina, V.S.; Jaffrin, M.Y.; Ding, L.H. Comparison of rotating ceramic membranes and polymeric membranes in fractionation of milk proteins by microfiltration. Desalination 2009, 245, 714–722. [Google Scholar] [CrossRef]
- Karasu, K.; Glennon, N.; Lawrence, N.D.; Stevens, G.W.; O’Connor, A.J.; Barber, A.R.; Yoshikawa, S.; Kentish, S.E. A comparison between ceramic and polymeric membrane systems for casein concentrate manufacture. Int. J. Dairy Technol. 2010, 63, 284–289. [Google Scholar] [CrossRef]
- Zulewska, J.; Newbold, M.; Barbano, D.M. Efficiency of serum protein removal from skim milk with ceramic and polymeric membranes at 50 °C. J. Dairy Sci. 2009, 92, 1361–1377. [Google Scholar] [CrossRef] [PubMed]
- Carter, B.; DiMarzo, L.; Pranata, J.; Barbano, D.M.; Drake, M. Efficiency of removal of whey protein from sweet whey using polymeric microfiltration membranes. J. Dairy Sci. 2021, 104, 8630–8643. [Google Scholar] [CrossRef] [PubMed]
- Baruah, G.L.; Nayak, A.; Belfort, G. Scale-up from laboratory microfiltration to a ceramic pilot plant: Design and performance. J. Memb. Sci. 2006, 274, 56–63. [Google Scholar] [CrossRef]
- Liu, F.; Hashim, N.A.; Liu, Y.; Abed, M.R.M.; Li, K. Progress in the production and modification of PVDF membranes. J. Memb. Sci. 2011, 375, 1–27. [Google Scholar] [CrossRef]
- Carter, B.G.; Cheng, N.; Kapoor, R.; Meletharayil, G.H.; Drake, M.A. Invited review: Microfiltration-derived casein and whey proteins from milk. J. Dairy Sci. 2021, 104, 2465–2479. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Ramchandran, L.; Vasiljevic, T. Lower ultrafiltration temperature improves membrane performance and emulsifying properties of milk protein concentrates. Dairy Sci. Technol. 2015, 95, 15–31. [Google Scholar] [CrossRef] [Green Version]
- Steinhauer, T.; Hanély, S.; Bogendörfer, K.; Kulozik, U. Temperature dependent membrane fouling during filtration of whey and whey proteins. J. Memb. Sci. 2015, 492, 364–370. [Google Scholar] [CrossRef]
- McCarthy, N.A.; Wijayanti, H.B.; Crowley, S.V.; O’Mahony, J.A.; Fenelon, M.A. Pilot-scale ceramic membrane filtration of skim milk for the production of a protein base ingredient for use in infant milk formula. Int. Dairy J. 2017, 73, 57–62. [Google Scholar] [CrossRef]
- O’Mahony, J.A.; Smith, K.E.; Lucey, J.A. Purification of Beta Casein from Milk. U.S. Patent US8889208 B2, 18 November 2014. [Google Scholar]
- O’Mahony, J.A.; Tuohy, J.J. Further applications of membrane filtration in dairy processing. In Membrane Processing. Dairy and Beverage Applications, 1st ed.; Tamime, A.Y., Ed.; Wiley-Blackwell: West Sussex, UK, 2013; pp. 225–262. ISBN 9781118457023. [Google Scholar]
- Hurt, E.E.; Adams, M.C.; Barbano, D.M. Microfiltration of skim milk and modified skim milk using a 0.1-μm ceramic uniform transmembrane pressure system at temperatures of 50, 55, 60, and 65 °C. J. Dairy Sci. 2015, 98, 765–780. [Google Scholar] [CrossRef]
- Crowley, S.V.; Caldeo, V.; McCarthy, N.A.; Fenelon, M.A.; Kelly, A.L.; O’Mahony, J.A. Processing and protein-fractionation characteristics of different polymeric membranes during filtration of skim milk at refrigeration temperatures. Int. Dairy J. 2015, 48, 23–30. [Google Scholar] [CrossRef]
- Tan, T.J.; Wang, D.; Moraru, C.I. A physicochemical investigation of membrane fouling in cold microfiltration of skim milk. J. Dairy Sci. 2014, 97, 4759–4771. [Google Scholar] [CrossRef] [PubMed]
- Hartinger, M.; Heidebrecht, H.-J.; Schiffer, S.; Dumpler, J.; Kulozik, U. Milk protein fractionation by means of spiral-wound microfiltration membranes: Effect of the pressure adjustment mode and temperature on flux and protein permeation. Foods 2019, 8, 180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ng, K.S.Y.; Dunstan, D.E.; Martin, G.J.O. Influence of processing temperature on flux decline during skim milk ultrafiltration. Sep. Purif. Technol. 2018, 195, 322–331. [Google Scholar] [CrossRef]
- Barukčić, I.; Božanić, R.; Kulozik, U. Effect of pore size and process temperature on flux, microbial reduction and fouling mechanisms during sweet whey cross-flow microfiltration by ceramic membranes. Int. Dairy J. 2014, 39, 8–15. [Google Scholar] [CrossRef]
- Schiffer, S.; Kulozik, U. Effect of temperature-dependent bacterial growth during milk protein fractionation by means of 0.1 µm microfiltration on the length of possible production cycle times. Membranes 2020, 10, 326. [Google Scholar] [CrossRef] [PubMed]
- de Wit, J.N. Thermal behaviour of bovine β-lactoglobulin at temperatures up to 150 °C. A review. Trends Food Sci. Technol. 2009, 20, 27–34. [Google Scholar] [CrossRef]
- Beckman, S.L.; Barbano, D.M. Effect of microfiltration concentration factor on serum protein removal from skim milk using spiral-wound polymeric membranes. J. Dairy Sci. 2013, 96, 6199–6212. [Google Scholar] [CrossRef]
- Koh, L.L.A.; Ashokkumar, M. Kentish, S.E. Membrane fouling, cleaning and disinfection. In Membrane Processing. Dairy and Beverage Applications, 1st ed.; Tamime, A.Y., Ed.; Wiley-Blackwell: West Sussex, UK, 2013; pp. 208–224. ISBN 9781118457023. [Google Scholar]
- James, B.J.; Jing, Y.; Chen, X.D. Membrane fouling during filtration of milk—A microstructural study. J. Food Eng. 2003, 60, 431–437. [Google Scholar] [CrossRef]
- France, T.C.; Bot, F.; Kelly, A.L.; Crowley, S.V.; O’Mahony, J.A. The influence of temperature on filtration performance and fouling during cold microfiltration of skim milk. Sep. Purif. Technol. 2021, 262, 118256. [Google Scholar] [CrossRef]
- Schmidt, V.S.J.; Kaufmann, V.; Kulozik, U.; Scherer, S.; Wenning, M. Microbial biodiversity, quality and shelf life of microfiltered and pasteurized extended shelf life (ESL) milk from Germany, Austria and Switzerland. Int. J. Food Microbiol. 2012, 154, 1–9. [Google Scholar] [CrossRef]
- Li, F.; Hunt, K.; Van Hoorde, K.; Butler, F.; Jordan, K.; Tobin, J.T. Occurrence and identification of spore-forming bacteria in skim-milk powders. Int. Dairy J. 2019, 97, 176–184. [Google Scholar] [CrossRef]
- Burgess, S.A.; Lindsay, D.; Flint, S.H. Thermophilic bacilli and their importance in dairy processing. Int. J. Food Microbiol. 2010, 144, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Seale, B.; Bremer, P.; Flint, S.; Brooks, J.; Palmer, J. Overview of the problems resulting from biofilm contamination in the dairy industry. In Biofilms in the Dairy Industry; Teh, K.H., Flint, S., Brooks, J., Knight, G., Eds.; John Wiley & Sons: West Sussex, UK, 2015; pp. 49–64. ISBN 9781118876213. [Google Scholar]
- Seale, B.; Burgess, S.; Flint, S.; Brooks, J.; Bremer, P.; Parkar, S. Thermophilic spore-forming bacilli in the dairy industry. In Biofilms in the Dairy Industry; Teh, K.H., Flint, S., Brooks, J., Knight, G., Eds.; John Wiley & Sons: West Sussex, UK, 2015; pp. 112–137. ISBN 9781118876213. [Google Scholar]
- Chamberland, J.; Messier, T.; Dugat-Bony, E.; Lessard, M.-H.; Labrie, S.; Doyen, A.; Pouliot, Y. Influence of feed temperature to biofouling of ultrafiltration membrane during skim milk processing. Int. Dairy J. 2019, 93, 99–105. [Google Scholar] [CrossRef]
- Samaržija, D.; Zamberlin, Š.; Pogačić, T. Psychrotrophic bacteria and milk and dairy products quality. Mljekarstvo 2012, 62, 77–95. [Google Scholar] [CrossRef]
- Jarto, I.; Lucey, J.A.; Smith, K.E. Impact of processing temperature on production of milk protein permeate during microfiltration of skim or whole milk. Int. J. Dairy Technol. 2018, 71, 844–848. [Google Scholar] [CrossRef]
- Beckman, S.L.; Zulewska, J.; Newbold, M.; Barbano, D.M. Production efficiency of micellar casein concentrate using polymeric spiral-wound microfiltration membranes. J. Dairy Sci. 2010, 93, 4506–4517. [Google Scholar] [CrossRef] [Green Version]
- Fox, P.F.; Uniacke-Lowe, T.; McSweeney, P.L.H.; O’Mahony, J.A. Salts of Milk. In Dairy Chemistry and Biochemistry, 2nd ed.; Springer: Cham, Switzerland, 2015; pp. 241–270. ISBN 9783319148922. [Google Scholar]
- France, T.C.; Kelly, A.L.; Crowley, S.V.; O’Mahony, J.A. The effects of temperature and transmembrane pressure on protein, calcium and plasmin partitioning during microfiltration of skim milk. Int. Dairy J. 2021, 114, 104930. [Google Scholar] [CrossRef]
- Lin, M.J.; Grandison, A.S.; Lewis, M.J. Partitioning of calcium and magnesium (total divalent cations) during membrane filtration of milk. J. Food Eng. 2015, 149, 153–158. [Google Scholar] [CrossRef]
- Zulewska, J.; Kowalik, J.; Lobacz, A.; Dec, B. Short communication: Calcium partitioning during microfiltration of milk and its influence on rennet coagulation time. J. Dairy Sci. 2018, 101, 10860–10865. [Google Scholar] [CrossRef]
- Crowley, S.V.; Burlot, E.; Silva, J.V.C.; McCarthy, N.A.; Wijayanti, H.B.; Fenelon, M.A.; O’Mahony, J.A. Rehydration behaviour of spray-dried micellar casein concentrates produced using microfiltration of skim milk at cold or warm temperatures. Int. Dairy J. 2018, 81, 72–79. [Google Scholar] [CrossRef]
- Dauphas, S.; Mouhous-Riou, N.; Metro, B.; MacKie, A.R.; Wilde, P.J.; Anton, M.; Riaublanc, A. The supramolecular organisation of β-casein: Effect on interfacial properties. Food Hydrocoll. 2005, 19, 387–393. [Google Scholar] [CrossRef]
- Crowley, S.V.; Kelly, A.L.; O’Mahony, J.A.; Lucey, J.A. Colloidal properties of protein complexes formed in β-casein concentrate solutions as influenced by heating and cooling in the presence of different solutes. Colloids Surf. B Biointerfaces 2019, 174, 343–351. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Auty, M.A.E.; Crowley, S.V.; Kelly, A.L.; O’Mahony, J.A.; Brodkorb, A. Self-association of bovine β-casein as influenced by calcium chloride, buffer type and temperature. Food Hydrocoll. 2019, 88, 190–198. [Google Scholar] [CrossRef]
- Huppertz, T. Chemistry of the caseins. In Advanced Dairy Chemistry. Vol. 1A Proteins: Basic Aspects, 4th ed.; Fox, P.F., McSweeney, P.L.H., Eds.; Springer: Boston, MA, USA, 2013; pp. 135–160. ISBN 978-1-4614-4714-6. [Google Scholar]
- Korycha-Dahl, M.; Dumas, B.R.; Chene, N.; Martal, J. Plasmin Activity in Milk. J. Dairy Sci. 1983, 66, 704–711. [Google Scholar] [CrossRef]
- Richardson, B. Proteinases of bovine milk and the effect of pasteurization on their activity. N. Z. J. Dairy Sci. 1983, 18, 233–245. [Google Scholar]
- Aaltonen, T.; Ollikainen, P. Effect of microfiltration of milk on plasmin activity. Int. Dairy J. 2011, 21, 193–197. [Google Scholar] [CrossRef]
- Levin, M.A.; Burrington, K.J.; Hartel, R.W. Whey protein phospholipid concentrate and delactosed permeate: Applications in caramel, ice cream, and cake. J. Dairy Sci. 2016, 99, 6948–6960. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maubois, J.L. Membrane microfiltration; a tool for a new approach in dairy technology. Aust. J. Dairy Technol. 2002, 57, 92–96. [Google Scholar]
- Lindquist, A. A Method for the Production of Sterile Skimmed Milk. Patent PCT WO No. 57549, 23 December 1998. [Google Scholar]
- Fritsch, J.; Moraru, C.I. Development and optimization of a carbon dioxide-aided cold microfiltration process for the physical removal of microorganisms and somatic cells from skim milk. J. Dairy Sci. 2008, 91, 3744–3760. [Google Scholar] [CrossRef] [PubMed]
- Griep, E.R.; Cheng, Y.; Moraru, C.I. Efficient removal of spores from skim milk using cold microfiltration: Spore size and surface property considerations. J. Dairy Sci. 2018, 101, 9703–9713. [Google Scholar] [CrossRef] [Green Version]
- Hansen, S.F.; Hogan, S.A.; Tobin, J.; Rasmussen, J.T.; Larsen, L.B.; Wiking, L. Microfiltration of raw milk for production of high-purity milk fat globule membrane material. J. Food Eng. 2020, 276, 109887. [Google Scholar] [CrossRef]
- Heino, A.T.; Uusi-Rauva, J.O.; Rantamäki, P.R.; Tossavainen, O. Functional properties of native and cheese whey protein concentrate powders. Int. J. Dairy Technol. 2007, 60, 277–285. [Google Scholar] [CrossRef]
- Evans, J.; Zulewska, J.; Newbold, M.; Drake, M.A.; Barbano, D.M. Comparison of composition, sensory, and volatile components of thirty-four percent whey protein and milk serum protein concentrates. J. Dairy Sci. 2009, 92, 4773–4791. [Google Scholar] [CrossRef]
- Jäger, R.; Kerksick, C.M.; Campbell, B.I.; Cribb, P.J.; Wells, S.D.; Skwiat, T.M.; Purpura, M.; Ziegenfuss, T.N.; Ferrando, A.A.; Arent, S.M.; et al. International society of sports nutrition position stand: Protein and exercise. J. Int. Soc. Sports Nutr. 2017, 14, 1–25. [Google Scholar] [CrossRef] [Green Version]
- Soodam, K.; Guinee, T.P. The case for milk protein standardisation using membrane filtration for improving cheese consistency and quality. Int. J. Dairy Technol. 2018, 71, 277–291. [Google Scholar] [CrossRef]
- De Kruif, C.G.; Holt, C. Casein micelle structure, functions and interactions. In Advanced Dairy Chemistry-1 Proteins, 3rd ed.; Fox, P.F., McSweeney, P.L.H., Eds.; Kluwer Academic/Plenum Publishers: New York, NY, USA, 2003; pp. 233–276. ISBN 978-1-4419-8602-3. [Google Scholar]
- Creamer, L.K.; Berry, G.P.; Mills, O.E. A study of the dissociation of β-casein from the bovine casein micelle at low temperature. N. Z. J. Dairy Sci. Technol. 1977, 12, 58–66. [Google Scholar]
- Payens, T.A.J.; Van Markwijk, B.W. Some features of the association of β-casein. BBA–Biochim. Biophys. Acta 1963, 71, 517–530. [Google Scholar] [CrossRef]
- Andrews, A.L.; Atkinson, D.; Evans, M.T.A.; Finer, E.G.; Green, J.P.; Phillips, M.C.; Robertson, R.N. The conformation and aggregation of bovine β-casein A. I. Molecular aspects of thermal aggregation. Biopolymers 1979, 18, 1105–1121. [Google Scholar] [CrossRef]
- Farrell, H.M., Jr.; Wickham, E.D.; Unruh, J.J.; Qi, P.X.; Hoagland, P.D. Secondary structural studies of bovine caseins: Temperature dependence of β-casein structure as analyzed by circular dichroism and FTIR spectroscopy and correlation with micellization. Food Hydrocoll. 2001, 15, 341–354. [Google Scholar] [CrossRef]
- Schiffer, S.; Vannieuwenhuyse, L.; Susianto, C.; Hartinger, M.; Kulozik, U. Influence of pH and calcium concentration on milk protein fractionation by 0.1 μm microfiltration at low temperatures. Int. Dairy J. 2021, 118, 105048. [Google Scholar] [CrossRef]
- Rose, D. Relation between Micellar and Serum Casein in Bovine Milk. J. Dairy Sci. 1968, 51, 1897–1902. [Google Scholar] [CrossRef]
- Philippe, M.; Gaucheron, F.; Le Graet, Y.; Michel, F.; Garem, A. Physicochemical characterization of calcium-supplemented skim milk. Lait 2003, 83, 45–59. [Google Scholar] [CrossRef]
- Le Berre, O.; Daufin, G. Fouling and selectivity of membranes during separation of β-casein. J. Memb. Sci. 1994, 88, 263–270. [Google Scholar] [CrossRef]
- Schäfer, J.; Schubert, T.; Atamer, Z. Pilot-scale β-casein depletion from micellar casein via cold microfiltration in the diafiltration mode. Int. Dairy J. 2019, 97, 222–229. [Google Scholar] [CrossRef]
- van der Schaaf, J.M.; Crowley, S.V.; Kelly, A.L.; O’Mahony, J.A. Manufacture of Milk and Whey Products: Fractionated Casein Ingredients—β-Casein. In Reference Module in Food Science; Elsevier, 2021. [Google Scholar] [CrossRef]
- Atamer, Z.; Post, A.E.; Schubert, T.; Holder, A.; Boom, R.M.; Hinrichs, J. Bovine β-casein: Isolation, properties and functionality. A review. Int. Dairy J. 2017, 66, 115–125. [Google Scholar] [CrossRef]
- Chen, C.W. Guidance on the conceptual design of sustainable product-service systems. Sustainability 2018, 10, 2452. [Google Scholar] [CrossRef] [Green Version]
- Kamali, M.; Suhas, D.P.; Costa, M.E.; Capela, I.; Aminabhavi, T.M. Sustainability considerations in membrane-based technologies for industrial effluents treatment. Chem. Eng. J. 2019, 368, 474–494. [Google Scholar] [CrossRef]
- Anand, S.; Singh, D. Resistance of the constitutive microflora of biofilms formed on whey reverse-osmosis membranes to individual cleaning steps of a typical clean-in-place protocol. J. Dairy Sci. 2013, 96, 6213–6222. [Google Scholar] [CrossRef] [Green Version]
- Méthot-Hains, S.; Benoit, S.; Bouchard, C.; Doyen, A.; Bazinet, L.; Pouliot, Y. Effect of transmembrane pressure control on energy efficiency during skim milk concentration by ultrafiltration at 10 and 50 °C. J. Dairy Sci. 2016, 99, 8655–8664. [Google Scholar] [CrossRef] [Green Version]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
France, T.C.; Kelly, A.L.; Crowley, S.V.; O’Mahony, J.A. Cold Microfiltration as an Enabler of Sustainable Dairy Protein Ingredient Innovation. Foods 2021, 10, 2091. https://doi.org/10.3390/foods10092091
France TC, Kelly AL, Crowley SV, O’Mahony JA. Cold Microfiltration as an Enabler of Sustainable Dairy Protein Ingredient Innovation. Foods. 2021; 10(9):2091. https://doi.org/10.3390/foods10092091
Chicago/Turabian StyleFrance, Thomas C., Alan L. Kelly, Shane V. Crowley, and James A. O’Mahony. 2021. "Cold Microfiltration as an Enabler of Sustainable Dairy Protein Ingredient Innovation" Foods 10, no. 9: 2091. https://doi.org/10.3390/foods10092091