1. Introduction
The poultry industry worldwide is in search of bioactive and cost-effective compounds that can improve product quality and human health-promoting attributes. The potential benefits for poultry production that can be derived from dietary supplementation with plant flavonoids have recently been under investigation with so far encouraging results, especially for fat quality and antioxidant function [
1]. Amongst flavonoids, hesperidin and naringin (flavanones that are abundant in citrus fruits) are potent antioxidants, possess anti-inflammatory properties, improve metabolic syndrome disease symptoms and modulate lipid metabolism [
2].
Some of the desirable properties of broiler meats as regards fat quality are reduced fat content and favorable fatty acid composition, e.g., increased poly-unsaturated/saturated fatty acid (PUFA/SFA) ratio and reduced omega n-6/n-3 ratio and atherogenicity (AI) and thrombogenicity (TI) indices. Hesperidin has been shown to decrease muscle fat content in broilers [
3], increase PUFA, improve n-6/n-3 and PUFA/SFA ratios in breast meat, and decrease serum and muscle cholesterol and triglyceride levels [
4]. Furthermore, hesperidin and naringin were found to reduce cholesterol content in layer hens’ egg yolk [
5,
6,
7] and to reduce serum cholesterol levels in layer hens [
6,
7]. In humans, flavonoids including hesperidin have been shown to improve metabolic syndrome health indices [
8].
Another important quality parameter for poultry meat is oxidative stability [
9]. Dietary supplementation with hesperidin has been shown to increase broiler meat antioxidant capacity during storage [
10,
11,
12,
13] and to improve antioxidant defense function in the plasma [
4] and liver [
3]. Naringin has also been found to reduce the oxidative deterioration of stored broiler meat [
11]. Naringin and/or hesperidin, or their aglycones naringenin and hesperetin, have been found to alleviate the symptoms of induced oxidative stress in rodents and rabbits, in the context of human disease animal model systems such as metabolic disorder, diabetes and liver injury, and in human cell lines [
14,
15,
16,
17,
18,
19,
20,
21].
Citrus flavanones are also known to possess anti-inflammatory properties [
2,
22]. In chicken, hesperidin has been found to exert immunomodulatory functions, increasing antibody titers following immunizations, improving heterophil adhesion, elevating responses to cutaneous basophilic hypersensitivity tests and increasing phagocytic activity following lipopolysaccharide challenge [
23,
24].
Hesperidin, naringin and their aglycones seem to mediate the effects described above on lipid metabolism, antioxidant defense and immune regulation via the modulation of genes involved in relevant pathways [
25,
26,
27]. Their effects on gene expression have been studied in many tissues and cell types, in vivo and in vitro, and under different physiological conditions, mainly in rodents, rabbits and human cell lines. The beneficial effects of the two flavonoids on metabolic disease have been linked to an increased hepatic expression of genes involved in fatty acid (FA) β-oxidation (such as
PPARα,
PPARγ,
ACOX,
CPT1A) and the decreased expression of genes involved in lipogenesis and lipid metabolism (e.g.,
FAS,
Srebf1,
ACC). Their antioxidant activity seems to be exerted via the downregulation of pro-apoptotic (e.g.,
Casp3,
Casp9,
BAX) and the upregulation of the anti-apoptotic (e.g.,
BCL-2) and antioxidant defense system (e.g.,
SOD,
CAT,
GSH-P,
GPx,
GR) genes. Their anti-inflammatory properties are linked to the modulation of genes involved in pro- and anti-inflammatory processes, such as
iNOS,
COX-2,
Nrf2,
NFκB,
TGFβ and
IL10. A comprehensive list of genes per functional category and corresponding references are presented in the
supplementary material (Supplementary Table S1).
In this study we investigated the effect of hesperidin and naringin supplementation on broiler meat’s FA profile. Furthermore, we provide novel data on the expression of genes related to the known effects of the two flavanones on antioxidant defense, lipid metabolism, and inflammation.
2. Materials and Methods
2.1. Animals and Experimental Design
In this study, 240-day-old Ross 308 broiler chickens, obtained from a commercial hatchery, as hatched, were housed in a controlled environment. Animal management and feed are described in [
11]. The 240 broiler chickens were equally allocated to 6 dietary treatment groups and 2 pens per treatment group. The six treatment groups were: N1 and N2, supplemented with 0.75 and 1.5 g naringin (Alfa Aesar GmbH & Co KG, Kandel, Germany) per kg of feed, respectively; E1 and E2, supplemented with 0.75 and 1.5 g hesperidin (TSI Europe NV, Zwijndrecht, Belgium) per kg of feed, respectively; control (C) with no feed additive; and vitamin E (VE) supplemented with 0.2 g a-tocopheryl acetate (vitamin E) (DSM Nutritional Products Hellas, Athens, Greece) per kg of feed. The VE treatment group served as a positive control for antioxidant activity and the level of supplementation was determined according to previously published data [
28]. Feed additives were supplemented from the 11th day of age until slaughter at 42 days.
2.2. Fatty Acid Profile Analysis
2.2.1. Lipid Extraction
Total lipids were extracted from abdominal adipose tissue (fat pad), the breast (
pectoralis major) and the thigh (
biceps femoris) muscle from 10 birds per treatment group according to Folch et al. [
29]. Afterwards, the organic phase was dried under reduced pressure with a rotary evaporator. The lipid extract was weighted, the percentage fat content was determined, and it was then subjected to transmethylation.
2.2.2. Transesterification and Gas Chromatographic Analysis
Direct transesterification on lipid extract was performed following [
30] with minor modification. Briefly, a quantity between 100 and 150 mg of lipid extract was directly methylated with 2 mL of 0.5 M sodium methylate at 50 °C for 30 min, followed by 2 mL of 140 g L
−1 boron trifluoride in methanol (BF3) at 50 °C for 30 min. Then, 2 mL of hexane was added and the upper hexane phase containing the fatty acid methyl esters (FAMEs) was transferred to gas–liquid chromatography (GLC) auto-sampler vials and analyzed in duplicate.
FAMEs were separated by GLC using a Shimadzu gas chromatograph (model GC-17A, Columbia, MD, USA) with a Shimadzu GC-2014 GC AOC-20i auto injector, equipped with a flame ionization detector (FID). The FA composition of the FAME was determined by capillary GC on a SP-2560 capillary column (75 m × 0.18 mm I.D., 0.14 μm; Supelco Inc., Bellefonte, PA, USA). The flow rate of carrier gas (Helium) was 1 mL∙min
−1, the injection temperature was 250 °C and the detector temperature was 270 °C. The injection volume was 1 μL (split 1:50). The temperature program was as follows: The initial temperature was held at 75 °C for 5 min after injection and then programmed to increase at 5 °C/min to 150 °C, to hold for 5 min, then to increase to 220 °C at 7 °C/min and hold for 20 min. Fatty acid peaks were recorded and integrated using a Shimadzu GC solution software (Shimadzu Corporation, Kyoto, Japan). Individual fatty acids were identified by comparing their retention times with known fatty acid methyl ester standards (Supelco 37 Component FAME Mix, purchased from Sigma-Aldrich, Taufkirchen, Germany). The individual FA content was expressed as weight percentage (g∙100 g
−1 of total FA). SFA, PUFA, MUFA, n-6 and n-3 were calculated as the sum of the percent content of all saturated, polyunsaturated, monounsaturated, n-6 and n-3 FA, respectively. PUFA/SFA and n-6/n-3 ratios were calculated by dividing PUFA by SFA and n-6 by n-3, respectively. The atherogenicity and thrombogenicity indices were calculated with the following formulas [
31]: AI = (12:0 + 4 · 14:0 + 16:0)/(Sum MUFA + Sum PUFA), TI = (14:0 + 16:0 + 18:0)/[0.5 · Sum MUFA + 0.5 · Sum (n-6) PUFA + 3 · Sum (n-3) PUFA + (n-3/n-6)].
2.3. RNA Extraction and cDNA Synthesis
Samples from the liver, breast (pectoralis major) muscle and abdominal adipose tissue (fat pad) from 4 (liver, fat pad) or 6 (muscle) animals per dietary group (C, E1, E2, N1, N2 and VE) were collected post-mortem at 42 d of age, snap-frozen in liquid nitrogen and stored at −80 °C until the extraction of RNA. RNA was extracted using the QIAzol® lysis reagent (Qiagen, Hilden, Germany) and according to the manufacturer’s instructions. Briefly, approximately 20 mg of frozen liver tissue was homogenized in 500 μL QIAzol® lysis reagent and spun at 12,000× g for 10 min at 4 °C. Supernatant was mixed with 0.1 mL chloroform and incubated at room temperature for 5 min. The mixture was spun at 12,000× g for 15 min at 4 °C and the upper aqueous phase was mixed with 0.25 mL isopropanol and incubated on ice for 2 min and at room temperature for 10 min, then was spun at 12,000× g for 10 min at 4 °C. The pellet was washed with 0.5 mL 70% ethanol and was then resuspended in 50 μL dH2O. The RNA preparations were then treated with DNAseI (TAKARA Bio INC, Shiga, Japan) according to the manufacturer’s recommendations to eliminate gDNA contamination. RNA concentration and purity were assessed by spectrophotometry on a Quawell Q5000 micro volume cuvette free spectrophotometer. The synthesis of first strand cDNA for the quantitative (q)PCR arrays was performed using 500 ng total RNA with the RT2 first strand kit (Qiagen, Hilden, Germany) and according to the manufacturer’s protocol. The synthesis of cDNA for single-gene qPCR was performed with the PrimeScript RT-PCR Reagent Kit (TAKARA Bio INC, Shiga, Japan) using 1 μg total RNA in 20 μL reactions according to the manufacturer’s instructions.
2.4. Quantitative (q)PCR and PCR Arrays
Custom RT
2 Profiler PCR arrays (Qiagen, Hilden, Germany) were designed to include 36 genes related to antioxidant activity and apoptosis, lipid metabolism, and anti-inflammatory responses, 5 housekeeping genes for normalization of the expression, 1 genomic DNA contamination-negative control, 3 reverse transcription, and 3 PCR-positive controls (
Table 1). The genes included in the array were chosen based on the available published data for the effect of hesperidin, naringin and their aglycones on the expression of genes in the liver (
Supplementary Table S1). Each well in a 96-well PCR plate contained the primers for the amplification of one of the 41 transcripts or controls. With each PCR plate, the gene expression in two liver samples was assayed. The RT
2 SYBR Green ROX qPCR mastermix (Qiagen, Hilden, Germany) was used to prepare 25 μL quantitative reverse transcription (qRT)-PCR reactions that were performed, according to the manufacturer’s guidelines, in an ABI 7500 thermal cycler (Applied Biosystems, ThermoFisher Scientific, Waltham, MA, USA) under the following thermal program: initial denaturation/activation for 10 min at 95 °C, 40 cycles of 15 s at 95 °C and 1 min at 60 °C. The cycles were followed by a melt curve analysis.
Threshold fluorescence was manually defined using the log view, above the background signal and within the lower half of the linear amplification phase of the amplification plot. Threshold cycle (CT) values were exported for all wells and quality control analysis was performed using the SABiosciences PCR array data analysis excel template. The expression of each gene (GOI) was normalized using the geometric mean of the expression of the housekeeping genes (HKG) (). Fold differences in expression levels for each gene in the treatment groups (expt), relative to the control group’s mean expression (ctrl), were determined using the formula: . values for each sample were extracted and submitted to statistical analysis using the SAS software (see “Statistical analysis” section).
Quantitative (q)RT-PCR was also performed to assess the expression of fatty acid synthase (FASN), peroxisome proliferator-activated receptor gamma (PPARγ), and adiponectin (ADIPOQ) in the breast muscle and fat pad. The actin beta (ACTB) gene was used as the internal control (housekeeping) gene for normalization. The primers used were as follows: ACTB—chACTB_F CGAGGCCCTCTTCCAGCCATCTTT and chACTB_R CACCAGACAGCACTGTGTTGGC; ADIPOQ—chAdipoQ_F CCAGGTCTACAAGGTGTCA and chAdipoQ_R CCATGTGTCCTGGAAATCCT; PPARγ—chPPARg_F TGTTGATTTTTCAAGCATTTCTTCACCACA and chPPARg_R AGGGAGGAGAAGGAGGCTCCAT; FASN—chFASN_F GGCTTGAGTTGGCACAGTGGCTA and chFASN_R CTTGGATTCCCAGCGCCTTCCA. All primers were designed so as to avoid genomic amplification (either across exon–exon boundaries or each primer of a pair was designed at different exons). Reactions were prepared with the KAPA SYBR FAST qPCR Master Mix (2X) Universal (KAPA Biosystems, Boston, Massachusetts, United States), at a 10 μL final volume using 1 μL cDNA (ca 20 ng total RNA) and 200 nM final concentration of each primer. The qPCR thermal protocol used was as follows: 1 cycle of 95 °C for 3 min, 40 cycles of 95 °C for 5 s, 60 °C for 30 s, and a final cycle at 60 °C for 30 s. This was followed by a melt–curve analysis to assess the specificity of the amplification.
The efficiencies of the reactions for all genes were between 90 and 110%, and the correlation coefficient between the threshold cycle and the log(Quantity) for the standard curve was >0.990. The raw data were analyzed with the ABI 7500 software and the mRNA abundance (quantity) was calculated relative to the standard curve obtained from serial dilutions, and was included in each qPCR run. The normalized expression levels of a target gene in each sample were estimated as the ratio of the test gene quantity divided by the respective quantity of the ACTB gene. The relative normalized expression in each sample was estimated as the ratio of the normalized expression divided by the mean normalized expression of the same gene in the control group.
2.5. Statistical Analysis
Statistical analysis was performed using SAS Studio (SAS University edition) and the mixed models task. The treatment effect on FA and the indices was assessed using each test parameter as the dependent variable and the treatment as the explanatory classification variable with fixed effect. Pairwise comparisons were performed between treatments and Tukey adjustment was used for multiple comparisons correction.
To assess the effect of hesperidin (E), naringin (N) and vitamin E (VE) on gene expression, the relative/normalized gene expression from the qPCR arrays, and the single gene qPCRs, the gene expression was assigned as the dependent variable, and E, N and VE were assigned as the classification variables with fixed effects. E and N had three class levels (0, 1, 2) and VE had two class levels (0, 1). The linear dose effects of E and N on FA and gene expression were assayed by assigning E and N as the explanatory continuous variables. Mean differences and treatment effects were considered significant for p < 0.05 and showed a trend towards difference at 0.10 > p > 0.05.
2.6. Ethics Statement
This study was carried out in strict accordance with the guidelines of “Council Directive 86/609/EEC regarding the protection of animals used for experimental and other scientific purposes”. The protocol was approved by the Research Ethics Committee of the Department of Animal Science and Aquaculture of the Agricultural University of Athens (approval document no 20/20032013). All efforts were made to minimize animal suffering.
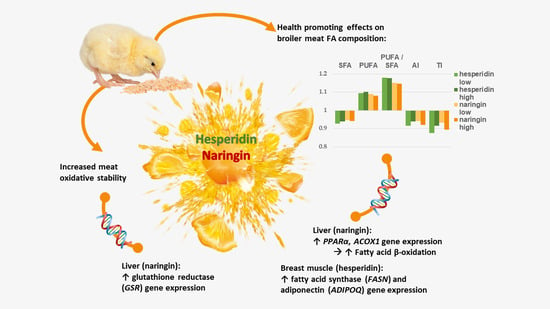
4. Discussion
There is mounting evidence for the benefits of using plant flavonoids in poultry production, including their positive effects on meat fatty acid composition and oxidative stability [
1,
32]. Here, we found that the citrus flavanones hesperidin and naringin beneficially modulated the fatty acid profile of broiler chicken meat and fat pads (
Table 3,
Table 4 and
Table 5). These effects were more pronounced in the breast meat and fat pad compared to thigh muscle. In the breast meat and fat pad, the PUFA and n-6 contents and PUFA/SFA ratio were increased. In addition, in the breast, the content of SFA was reduced. While the two compounds exhibited beneficial effects on meat FA composition, they had no adverse effects on growth performance [
11]. The present study is the first to report the effects of the two flavanones on the FA profile of the fat pad and thigh muscle. The FA composition and transcriptional activity were examined in the fat pad, as in chicken it is an important site of FA storage and reflects the fat content of the animal [
33,
34]. Similar effects to those found here on the FA profile of breast meat have been reported following the supplementation of broilers with a mixture of hesperidin and genistein [
4]. With increasing flavonoid mix concentrations, a reduction in SFA and MUFA was observed and an increase in PUFA in the breast meat was observed. Consistently with these results, the inclusion of orange (as yet unpublished data from our research group) or citrus [
35] pulp in the broiler diet has been reported to beneficially affect FA profile. Both orange and citrus pulp increased PUFA content, while citrus pulp additionally reduced SFA and increased the PUFA/SFA ratio in broiler breast meat [
35].
Metabolic disorders are currently a major human health issue worldwide. Apart from macronutrients, the quality of fat in the diet, including total SFA, PUFA and n-6 content, affects the development of metabolic disorder-related diseases. In addition to individual FA categories, PUFA/SFA, n-6/n-3 ratios and the atherogenicity and thrombogenicity indices have been proposed to predict the combined effects of different dietary factors on cardiovascular diseases [
31]. Furthermore, total SFA and AI in the diet have been found to be significantly correlated with the atherogenic index of the plasma [
36], an important index for atherosclerosis risk [
37]. Here, we report a significant and dose-dependent beneficial effect of hesperidin, naringin and vitamin E supplementation on AI and TI in the breast muscle. Thus, in the present study we found that both hesperidin and naringin had significant and dose-dependent beneficial effects on various factors of fat quality related to human health, mainly in the breast muscle and fat pad. Consistently with previous reports, many of these effects were also observed following vitamin E supplementation [
38,
39], but they were more pronounced in the case of the two flavanones.
In chicken, the liver is the main site of fatty acid biosynthesis [
40,
41]. To study the molecular mechanism underlying the observed effects on FA profile, we quantified the expressions of a number of genes involved in lipid metabolism in the liver (
Table 1 and
Table S2), breast muscle and fat pad (
Table 6). We found that the hepatic expression of two genes involved in FA β-oxidation,
PPARα and
ACOX1, was significantly increased by naringin supplementation. PPARα is a ligand-activated transcription factor that regulates lipid metabolism, and in particular peroxisomal β-oxidation of FA. The activation of PPARα promotes the uptake, utilization, and catabolism of FA via the upregulation of genes involved in FA transport, binding and activation [
42]. ACOX1, the first enzyme of the FA β-oxidation pathway, is a transcriptional target of PPARα. There are no reports on the effects of naringin on lipid metabolism-related genes in chicken. Nevertheless, consistently with our findings, quercetin, another plant flavonoid, was reported to increase the expression of PPARα in the livers of broilers [
43].
The supplementation of the diet of metabolic disorder-affected mice with naringin and/or its aglycone naringenin has been shown to reduce the hepatic expression of genes related to de novo FA synthesis, and increase the expression of FA β-oxidation genes [
44,
45,
46]. In these animal models, the effects of metabolic disease, such as hyperlipidemia and hypercholesterolemia, were attenuated by naringin/naringenin. Furthermore, similar effects were observed in the liver of naringin/naringenin-treated healthy mice [
47], rats [
48], and human and rat hepatic cell lines [
49]. In all the above cases, the activation of FA β-oxidation was evident by the upregulation of
PPARα and/or
ACOX1 gene expression, consistently with our findings, with the exception of the findings of Ke et al. [
45] who, despite observing an upregulation of FA oxidation, detected reduced
ACOX1 expression.
In the breast muscle, we found that
FASN expression was increased significantly by both hesperidin and naringin in a dose-dependent manner (
p-linear < 0.001 and 0.01, respectively). In contrast to our findings, the hepatic expression of
FASN has been shown to be downregulated in response to hesperidin and naringin supplementation [
44,
46,
49,
50,
51,
52]. Nevertheless, it has been observed that effects on
FASN expression may differ between the liver and skeletal muscle. A similar increase in
FASN expression in the muscle in conjunction with reduced SFA and increased PUFA content was detected in broilers treated with
Aspergillus awamori [
53]. Interestingly,
FASN mRNA expression was upregulated in the muscle tissue of naringenin-supplemented (3%
w/
w) mice relative to control, but did not reach significance (
p = 0.07) [
45]. The
FASN mRNA expression in our study was not affected in the fat pad (
Table 6), where it has been shown to be highly expressed [
54].
FASN expression has been positively linked with cell proliferation and rapid development, which is the case of the 42-day-old broiler chickens examined here. Thus, a plausible explanation for the increased
FASN expression observed could be that feed alone cannot meet the FA demands of actively proliferating tissues such as muscles, and as a consequence
FASN expression rises [
55].
Adiponectin is an adipokine produced mainly in the abdominal adipose tissue (fat pad), but also in the skeletal muscle [
56]. It is involved in many biological processes and plays a protective role in diabetes, obesity, atherosclerosis and other metabolic deregulations. Increased levels of plasma adiponectin and mRNA expression in the fat pad have been detected in response to hesperidin and naringin supplementation [
57,
58,
59]. Consistently with these findings, and in line with hesperidin’s beneficial effect on metabolic disorders and its anti-inflammatory properties, we detected increased
ADIPOQ expression in the breast muscle in the E1 treatment group.
Meat’s oxidative stability has been shown to improve as a result of hesperidin or naringin dietary supplementation [
11]. This effect could be attributed to the improvement of antioxidant defense status of the tissues. Indeed, we observed a significant upregulation of glutathione reductase (
GSR) gene expression in response to increasing naringin supplementation (
Figure 1 and
Supplementary Table S2). Hepatic
GSR expression was also increased in hesperidin-supplemented animals, but the difference from the control group was not significant. This is in line with the oxidative stability data, which showed that the antioxidant activity of naringin was more pronounced compared to hesperidin [
11].
GSR is an enzyme that plays an important role in resisting oxidative stress [
60]. It catalyzes the reduction of oxidized glutathione (GSSG) to the reduced glutathione (GSH), which is an essential molecule in resisting oxidative stress and preserving the reducing environment of all tissues. GSH can act as a scavenger for hydroxyl radicals, singlet oxygen, and various electrophiles [
61]. The GSSG/GSH ratio present in the cell is important for maintaining the oxidative balance of the cell [
62]. It is crucial that the cell reserves high levels of GSH and a low level of GSSG, and this ratio is regulated by GSR. Furthermore, GSH plays a pivotal role in the clearance and metabolism of xenobiotics, acts as a cofactor in specific detoxifying enzymes, participates in transport, and restores antioxidants such as vitamins C and E to their active forms [
63]. It can either scavenge hydroxyl radicals and singlet oxygen non enzymatically, or serve as an electron donor to several enzymes involved in reactive oxygen species (ROS) detoxification. Increasing GSH tissue levels may also provide benefits in terms of the improved meat quality of livestock animals. Post-mortem lipid hydroperoxidation and protein oxidation (e.g., oxymyoglobin to metmyoglobin) have significant effects on meat tenderness and color [
64,
65,
66]. GSH as an antioxidant may play a considerable role in preserving the shelf life and quality of meat products [
67]. This role may be compared with that of vitamin E [
68].
Consistent with our finding, Kapoor et al. [
69] showed that the in vitro addition of naringenin to glucose-stressed rat hepatocytes could prevent the generation of ROS and the decline in the cells’ antioxidant defense. In particular, they showed that naringenin restored GR expression in glucose-stressed rat hepatocytes to control levels.
Although vitamin E supplementation also improved oxidative stability, as expected [
11], no effect was observed on the hepatic expression of any of the antioxidant genes assessed here. This suggests that there are differences in the molecular mechanisms underlying the antioxidant activity of vitamin E and the two citrus flavanones. It could be argued that VE acts mainly via the direct scavenging of free radicals, while hesperidin and naringin also exert their antioxidant function via gene regulation, or alternatively that vitamin E regulates the expressions of genes not included in the array used in the present study.