Beneficial Anti-Platelet and Anti-Inflammatory Properties of Irish Apple Juice and Cider Bioactives
Abstract
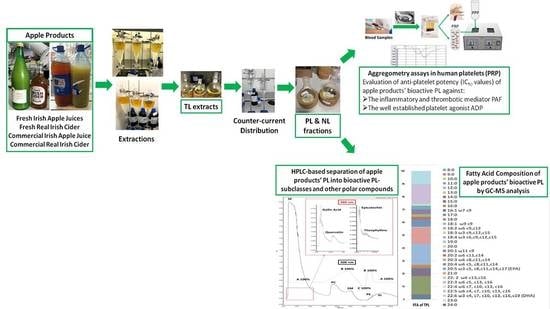
:1. Introduction
2. Materials and Methods
2.1. Materials, Reagents and Instrumentation
2.2. Apple Juice and Cider Samples Assessed
2.3. Extraction
2.4. Platelet Aggregometry Biological Assays
2.5. High-Performance Liquid Chromatography
2.6. Gas Chromatography–Mass Spectrometry (GC–MS)
2.7. Statistical Analysis
3. Results and Discussion
3.1. Yield of Extraction
3.2. Anti-Platelet Effects of Apple Juice and Cider Lipids
3.3. Fatty Acid Composition of Bioactive PL from Apple Juices and Their Fermented Cider Products
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AA and CA | apple juice and cider from apples with low content in tannins (Jonagold), respectively |
| AB and CB | apple juice and cider from apples with moderate content in tannins (Dabinett) |
| AE and CE | apple juice and cider from apples with high content in tannins (Aston Bitter) |
| AD | commercially found apple juice (relevant to and produced by pasteurization of the apple juice AA of the Jonagold variety) |
| CC | commercially found cider ‘Con’s Irish Cider®’ (produced by fermentation of a mixture of the aforementioned three different apple juice types in a ratio of 55% Jonagold, 35% Dabinett and 10% Aston bitter) |
| PL | polar lipids |
| TL | total lipids |
| NL | neutral lipids |
| HPLC | high-performance liquid chromatography |
| GCMS | gas chromatography mass spectra |
| PC | phosphatidylcholine |
| SM | sphyngomyelin |
| PE | phosphatidylethanolamine |
| PI | phosphatidylinositol |
| SFA | saturated fatty acids |
| MUFA | monounsaturated fatty acids |
| PUFA | polyunsaturated fatty acids |
| n3 | omega-3 PUFA |
| n6 | omega-6 PUFA |
| ND | non-detectable |
References
- Tierney, A.; Lordan, R.; Tsoupras, A.; Zabetakis, I. Chapter 8—Diet and cardiovascular disease: The Mediterranean diet. In The Impact of Nutrition and Statins on Cardiovascular Diseases; Zabetakis, I., Lordan, R., Tsoupras, A., Eds.; Academic Press: London, UK, 2019; pp. 267–288. [Google Scholar]
- Tsoupras, A.; Lordan, R.; Zabetakis, I. Inflammation, not cholesterol, is a cause of chronic disease. Nutrients 2018, 10, 604. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsoupras, A.B.; Iatrou, C.; Frangia, C.; Demopoulos, C.A. The Implication of platelet-activating factor in cancer growth and metastasis: Potent beneficial role of PAF-inhibitors and antioxidants. Infect. Disord. Drug Targets 2009, 9, 390–399. [Google Scholar] [CrossRef] [PubMed]
- Cousin, F.J.; Le Guellec, R.; Schlusselhuber, M.; Dalmasso, M.; Laplace, J.M.; Cretenet, M. Microorganisms in fermented apple beverages: Current knowledge and future directions. Microorganisms 2017, 5, 39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laaksonen, O.; Kuldjärv, R.; Paalme, T.; Virkki, M.; Yang, B. Impact of apple cultivar, ripening stage, fermentation type and yeast strain on phenolic composition of apple ciders. Food Chem. 2017, 233, 29–37. [Google Scholar] [CrossRef]
- Picinelli, L.A.; Diñeiro, G.Y.; Mangas-Sanchez, J.; Rodriguez, M.R.; Suarez, V.B. Phenolic and antioxidant composition of cider. J. Food Compos. Anal. 2009, 22, 644–648. [Google Scholar] [CrossRef]
- Koutsos, A.; Tuohy, K.M.; Lovegrove, J.A. Apples and Cardiovascular Health—Is the Gut Microbiota a Core Consideration? Nutrients 2015, 7, 3959–3998. [Google Scholar] [CrossRef] [PubMed]
- Fragopoulou, E.; Antonopoulou, S.; Tsoupras, A.; Tsantila, N.; Grypioti, A.; Gribilas, G.; Gritzapi, H.; Konsta, E.; Skandalou, E.; Papadopoulou, A. Antiatherogenic properties of red/white wine, musts, grape-skins, and yeast. In Proceedings of the 45th International Conference on the Bioscience of Lipids, Ioannina, Greece, 25–29 May 2004; p. 66. [Google Scholar]
- Tsoupras, A.; Lordan, R.; O’Keefe, E.; Shiels, K.; Saha, S.K.; Zabetakis, I. Structural Elucidation of Irish Ale Bioactive Polar Lipids with Antithrombotic Properties. Biomolecules 2020, 10, 1075. [Google Scholar] [CrossRef] [PubMed]
- Dutta-Roy, A.K.; Crosbie, L.; Gordon, M.J. Effects of tomato extract on human platelet aggregation in vitro. Platelets 2001, 12, 218–227. [Google Scholar] [PubMed]
- Lurie, S.; Ben-Arie, R. Microsomal membrane changes during the ripening of apple fruit. Plant. Physiol. 1983, 73, 636–638. [Google Scholar] [CrossRef] [Green Version]
- Tsoupras, A.; Lordan, R.; Demuru, M.; Shiels, K.; Saha, S.K.; Nasopoulou, C.; Zabetakis, I. Structural elucidation of irish organic farmed salmon (Salmo salar) polar lipids with antithrombotic activities. Mar. Drugs 2018, 16, 176. [Google Scholar] [CrossRef] [Green Version]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galanos, D.S.; Kapoulas, V.M. Isolation of polar lipids from triglyceride mixtures. J. Lipid Res. 1962, 3, 134–136. [Google Scholar] [CrossRef]
- Tsoupras, A.; Zabetakis, I.; Lordan, R. Platelet aggregometry assay for evaluating the effects of platelet agonists and antiplatelet compounds on platelet function in vitro. MethodsX 2019, 6, 63–70. [Google Scholar] [CrossRef]
- Lordan, R.; O’Keeffe, E.; Tsoupras, A.; Zabetakis, I. Total, neutral, and polar lipids of brewing ingredients, by-products and beer: Evaluation of antithrombotic activities. Foods 2019, 8, 171. [Google Scholar] [CrossRef] [Green Version]
- Tsoupras, A.; Lordan, R.; Harrington, J.; Pienaar, R.; Devaney, K.; Heaney, S.; Koidis, A.; Zabetakis, I. The effects of oxidation on the antithrombotic properties of tea lipids against PAF, thrombin, collagen, and ADP. Foods 2020, 9, 385. [Google Scholar] [CrossRef] [Green Version]
- Tsoupras, A.; O’Keeffe, E.; Lordan, R.; Redfern, S.; Zabetakis, I. Bioprospecting for antithrombotic polar lipids from salmon, herring, and boarfish by-products. Foods 2019, 8, 416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lordan, R.; Tsoupras, A.; Mitra, B.; Zabetakis, I. Dairy fats and cardiovascular disease: Do we really need to be concerned? Foods 2018, 7, 29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsoupras, A.B.; Demopoulos, C.A.; Pappas, K.M. Platelet-activating factor detection, metabolism and inhibitors in the ethanologenic bacterium Zymomonas mobilis. Eur. J. Lipid Sci. Technol. 2012, 114, 123–133. [Google Scholar] [CrossRef]
- Koukouraki, P.; Tsoupras, A.; Sotiroudis, G.; Demopoulos, C.A.; Sotiroudis, T.G. Antithrombotic properties of Spirulina extracts against platelet-activating factor and thrombin. Food Biosci. 2020, 37, 100686. [Google Scholar] [CrossRef]
- Pieszka, M.; Migda, W.; Gdsior, R.; Rudziska, M.; Bederska-Aojewska, D.; Pieszka, M.; Szczurek, P. Native oils from apple, blackcurrant, raspberry, and strawberry seeds as a source of polyenoic fatty acids, tocochromanols, and phytosterols: A health implication. J. Chem. 2015, 2015, 659541. [Google Scholar] [CrossRef] [Green Version]
- Radenkovs, V.; Kviesis, J.; Juhnevica-Radenkova, K.; Valdovska, A.; Püssa, T.; Klavins, M.; Drudze, I. Valorization of Wild Apple (Malus spp.) By-Products as a Source of Essential Fatty Acids, Tocopherols and Phytosterols with Antimicrobial Activity. Plants Basel Switz. 2018, 7, 90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simopoulos, A.P. The importance of the omega-6/omega-3 fatty acid ratio in cardiovascular disease and other chronic diseases. Exp. Biol. Med. 2008, 233, 674–688. [Google Scholar] [CrossRef] [PubMed]
- Tsoupras, A.; Fragopoulou, E.; Iatrou, C.; Demopoulos, C. In vitro protective effects of olive pomace polar lipids towards platelet activating factor metabolism in human renal cells. Curr. Top. Nutraceutical Res. 2011, 9, 105. [Google Scholar]
- Nasopoulou, C.; Tsoupras, A.B.; Karantonis, H.C.; Demopoulos, C.A.; Zabetakis, I. Fish polar lipids retard atherosclerosis in rabbits by down-regulating PAF biosynthesis and up-regulating PAF catabolism. Lipids Health Dis. 2011, 10, 1–18. [Google Scholar] [CrossRef] [Green Version]




| Peak | Standards | Molecular Class | RT (min) |
|---|---|---|---|
| 1 | Epicatechin (3 mg/mL) | Phenolic | 2–4 (280 nm) |
| 2 | Theophylline (3 mg/mL) | Phenolic | 3–5 (280 nm) |
| 3 | Gallic Acid (3 mg/mL) | Phenolic | 5–8 (280 nm) |
| 4 | Quercetin (3 mg/mL) | Phenolic | 10–16 (280 nm) |
| 5 | Cerebrosides (0.5 mg/mL) | Glycosphingolipid | 25–30 (208 nm) |
| 6 | L-α-Phosphatidylcholine (1.5 mg/mL) | Glycerol-based phospholipid | 35–40 (208 nm) |
| 7 | Sphyngomyelin (0.3 mg/mL) | sphingosine-based phospholipid | 45–50 (208 nm) |
| 8 | L-α-Lysophosphatidylcholine from Glycine max (soybean) (0.3 mg/mL) | Glycerol-based phospholipid | 50–53 (208 nm) |
| 9 | Digalactosyldiglyceride (0.3 mg/mL) | Glycerol-based Glycolipid | 53–56 (208 nm) |
| 10 | Sulfatides (0.3 mg/mL) | Glycerol-based Glyco-Sulfo-lipid | 56–59 (208 nm) |
| 11 | L-α-Phosphatidylethanolamine from Glycine max (soybean) (1.2 mg/mL) | Glycerol-based phospholipid | 62–67 (208 nm) |
| 12 | L-α-Phosphatidylinositol sodium salt from Glycine max (soybean) (0.9 mg/mL) | Glycerol-based phospholipid | 75–78 (208 nm) |
| Samples | TL (g/100 g) | NL (g/100 g) | PL (g/100 g) |
|---|---|---|---|
| Apple Juice AA | 0.072 ± 0.002 | 0.016 ± 0.002 | 0.056 ± 0.004 |
| Apple Juice AB | 0.042 ± 0.005 | 0.010 ± 0.005 | 0.031 ± 0.003 |
| Apple Juice AE | 0.221 ± 0.143 | 0.030 ± 0.035 | 0.191 ± 0.116 |
| Commercial Apple Juice AD | 0.150 ± 0.051 | 0.070 ± 0.058 | 0.080 ± 0.016 |
| Cider CA | 0.033 ± 0.012 | 0.005 ± 0.002 | 0.028 ± 0.004 |
| Cider CB | 0.025 ± 0.009 | 0.004 ± 0.001 | 0.021 ± 0.003 |
| Cider CE | 0.046 ± 0.013 | 0.006 ± 0.001 | 0.040 ± 0.005 |
| Commercial Cider CC | 0.042 ± 0.005 | 0.007 ± 0.003 | 0.035 ± 0.002 |
| Fatty Acid | AA | AB | AD | AE | CA | CB | CE | CC |
|---|---|---|---|---|---|---|---|---|
| C12:0 | 0.10 ± 0.04 | ND | ND | ND | 0.417 ± 0.246 | ND | 0.64 ± 0.06 | ND |
| C14:0 | 2.46 ± 0.32 | 3.824 ± 0.845 | 0.648 ± 0.183 | 0.147 ± 0.054 | 1.637 ± 1.056 | ND | 1.56 ± 0.09 | 4.52 ± 0.48 |
| C15:0 | 0.28 ± 0.02 | 0.383 ± 0.118 | 0.12 ± 0.033 | 0.118 ± 0.014 | 0.179 ± 0.119 | ND | 0.22 ± 0.02 | 0.45 ± 0.12 |
| C16:0 | 21.83 ± 2.43 | 24.928 ± 2.95 | 14.39 ± 1.62 | 17.38 ± 0.88 | 15.80 ± 6.88 | 10.05 ± 3.71 | 20.62 ± 2.96 | 22.07 ± 3.38 |
| C16:1 c9 | 0.55 ± 0.12 | 1.32 ± 0.22 | 0.09 ± 0.01 | 0.19 ± 0.05 | 0.30 ± 0.17 | 0.13 ± 0.30 | 1.03 ± 0.12 | 0.39 ± 0.33 |
| C17:0 | 0.43 ± 0.10 | 0.51 ± 0.03 | 0.38 ± 0.05 | 0.39 ± 0.07 | 0.22 ± 0.27 | 0.87 ± 1.51 | 0.39 ± 0.08 | 0.58 ± 0.81 |
| C18:0 | 5.57 ± 0.08 | 9.42 ± 0.26 | 3.58 ± 0.03 | 4.63 ± 0.44 | 6.64 ± 1.51 | 4.34 ± 0.43 | 5.80 ± 0.64 | 9.04 ± 1.39 |
| C18:1 c9 | 5.71 ± 0.78 | 8.923 ± 0.30 | 3.82 ± 0.07 | 2.01 ± 0.67 | 8.04 ± 1.58 | 2.44 ± 0.89 | 8.73 ± 0.93 | 4.14 ± 1.00 |
| C18:2 c9,12 | 42.28 ± 3.71 | 25.81 ± 0.74 | 59.43 ± 1.67 | 36.10 ± 1.51 | 18.44 ± 4.84 | 16.21 ± 4.90 | 20.17 ± 3.61 | 10.30 ± 1.41 |
| C18:3 c9,12,15 | 8.07 ± 1.31 | 6.39 ± 0.84 | 9.80 ± 0.32 | 16.80 ± 2.04 | 5.34 ± 2.42 | 8.79 ± 3.30 | 4.26 ± 0.55 | 1.61 ± 0.68 |
| C20:0 | 2.60 ± 0.15 | 2.10 ± 0.29 | 3.08 ± 0.33 | 2.59 ± 0.34 | 2.956 ± 0.24 | 3.30 ± 1.14 | 3.26 ± 0.08 | 2.56 ± 1.25 |
| C20:1 c11 | 1.42 ± 0.17 | 3.94 ± 0.23 | 0.74 ± 0.12 | 1.54 ± 0.26 | 2.25 ± 1.21 | 6.21 ± 2.16 | 3.17 ± 0.57 | 2.63 ± 1.27 |
| C20:2 c11,14 | 0.51 ± 0.14 | 0.53 ± 0.18 | 0.51 ± 0.40 | 0.72 ± 0.07 | 0.87 ± 0.35 | 2.44 ± 1.04 | 0.47 ± 0.16 | 1.34 ± 0.52 |
| C20:3 c8,11,14 | 0.23 ± 0.15 | 0.15 ± 0.03 | 0.09 ± 0.03 | 0.30 ± 0.14 | 0.67 ± 0.15 | 1.10 ± 0.09 | 0.36 ± 0.22 | 2.20 ± 0.82 |
| C20:4 c5,8,11,14 | 0.92 ± 0.38 | 0.21 ± 0.12 | 0.15 ± 0.12 | 0.05 ± 0.01 | 2.87 ± 2.42 | 2.57 ± 2.60 | 0.08 ± 0.05 | 2.19 ± 2.24 |
| C20:5 c5,11,14,17 | 0.40 ± 0.34 | 0.22 ± 0.12 | 0.07 ± 0.02 | 0.11 ± 0.09 | 0.49 ± 0.39 | 4.66 ± 4.23 | 0.11 ± 0.08 | 3.51 ± 3.98 |
| C22:0 | 0.29 ± 0.12 | 0.25 ± 0.05 | 0.08 ± 0.09 | 0.23 ± 0.12 | 1.37 ± 1.20 | 4.88 ± 3.83 | 1.33 ± 0.53 | 2.90 ± 1.06 |
| C20:4 c5,8,11,14 | 0.46 ± 0.39 | 0.19 ± 0.16 | 0.17 ± 0.10 | 2.56 ± 0.92 | 2.99 ± 1.09 | 2.52 ± 1.79 | 0.32 ± 0.22 | 3.23 ± 1.51 |
| C20:5 c5,8,11,14,17 | 1.64 ± 0.41 | 1.83 ± 1.03 | 2.02 ± 0.73 | 0.50 ± 0.37 | 5.26 ± 1.71 | 5.08 ± 4.53 | 2.69 ± 0.58 | 4.38 ± 1.31 |
| C22:1 c13 | 1.94 ± 0.31 | 4.96 ± 0.09 | 0.10 ± 0.06 | 3.89 ± 0.71 | 4.92 ± 3.11 | 6.06 ± 3.10 | 20.2 ± 9.08 | 4.12 ± 1.01 |
| C22:4 c7,10,13,16 | 0.46 ± 0.23 | 0.77 ± 0.24 | 0.22 ± 0.03 | 4.22 ± 4.18 | 8.47 ± 4.89 | 8.10 ± 2.84 | 1.76 ± 0.93 | 6.40 ± 2.14 |
| C22:5 c7,10,13,16,19 | 1.25 ± 0.58 | 1.86 ± 0.88 | 0.31 ± 0.064 | 3.32 ± 0.71 | 2.49 ± 1.27 | 5.10 ± 1.82 | 1.67 ± 0.81 | 7.24 ± 3.14 |
| C22:6 c4,7,10,13,16,19 | 0.62 ± 0.71 | 1.49 ± 1.95 | 0.25 ± 0.12 | 1.43 ± 0.73 | 7.41 ± 3.61 | 5.17 ± 1.45 | 1.16 ± 0.39 | 4.69 ± 1.81 |
| SFA | 33.54 ± 2.52 | 41.41 ± 2.99 | 22.27 ± 1.64 | 25.48 ± 1.68 | 29.22 ± 8.79 | 23.44 ± 0.83 | 33.85 ± 3.70 | 41.76 ± 3.34 |
| MUFA | 9.62 ± 0.90 | 19.14 ± 0.48 | 4.75 ± 0.10 | 7.64 ± 1.14 | 15.50 ± 2.83 | 14.84 ± 3.86 | 33.11 ± 7.53 | 11.14 ± 3.15 |
| PUFA | 56.84 ± 2.48 | 39.45 ± 3.38 | 72.98 ± 1.73 | 66.99 ± 2.72 | 55.29 ± 5.97 | 61.73 ± 4.43 | 33.04 ± 3.95 | 47.10 ± 5.54 |
| n6 | 44.40 ± 3.34 | 27.47 ± 0.74 | 60.37 ± 1.52 | 42.28 ± 2.93 | 31.32 ± 3.12 | 30.40 ± 5.06 | 22.84 ± 3.54 | 22.43 ± 2.86 |
| n3 | 12.44 ± 1.22 | 11.98 ± 3.96 | 12.61 ± 0.31 | 24.71 ± 0.28 | 23.97 ± 3.14 | 31.33 ± 8.92 | 10.21 ± 0.50 | 24.66 ± 6.07 |
| n6/n3 | 3.61 ± 0.64 | 2.46 ± 0.88 | 4.79 ± 0.24 | 1.71 ± 0.14 | 1.31 ± 0.30 | 1.08 ± 0.49 | 2.23 ± 0.46 | 0.96 ± 0.36 |
| Fatty Acid | Fraction 3 (PC) of AA | Fraction 3 (PC) of AD | Fraction 5 (PE) of CA | Fraction 5 (PE) of CC |
|---|---|---|---|---|
| C12:0 | 1.45 ± 0.12 | ND | ND | ND |
| C14:0 | 3.36 ± 0.10 | 4.40 ± 0.79 | 7.24 ± 2.61 | 4.34 ± 0.97 |
| C15:0 | 3.36 ± 0.12 | ND | ND | ND |
| C16:0 | 24.55 ± 4.28 | 28.08 ± 3.61 | 30.75 ± 6.52 | 31.41 ± 5.88 |
| C16:1 c9 | 2.65 ± 0.30 | 2.27 ± 0.20 | 3.45 ± 0.10 | 1.06 ± 0.15 |
| C17:0 | 1.27 ± 0.12 | ND | ND | ND |
| C18:0 | 33.33 ± 8.61 | 19.20 ± 1.05 | 24.39 ± 3.79 | 18.72 ± 2.67 |
| C18:1 c9 | 5.31 ± 1.90 | 13.33 ± 2.81 | 15.36 ± 2.91 | 14.17 ± 3.60 |
| C18:2 c9,12 | 2.54 ± 2.04 | 5.80 ± 1.42 | 4.43 ± 1.02 | 5.80 ± 4.32 |
| C18:3 c9,12,15 | 5.17 ± 0.85 | 5.13 ± 3.78 | 4.06 ± 1.02 | 3.85 ± 2.19 |
| C20:0 | 2.93 ± 2.89 | 2.17 ± 0.86 | 1.27 ± 0.70 | 2.51 ± 1.25 |
| C20:1 c11 | 3.78 ± 0.90 | 3.60 ± 1.08 | 0.85 ± 0.37 | 2.28 ± 1.24 |
| C20:5 c5,8,11,14,17 | 2.17 ± 0.71 | 3.25 ± 0.60 | 1.36 ± 0.63 | 2.53 ± 1.19 |
| C22:1 c13 | 3.69 ± 1.19 | 2.81 ± 0.91 | 1.55 ± 0.90 | 3.98 ± 1.14 |
| C22:5 c7,10,13,16,19 | 3.74 ± 1.56 | 4.98 ± 1.58 | 2.29 ± 0.95 | 3.60 ± 0.97 |
| C22:6 c4,7,10,13,16,19 | 3.84 ± 1.05 | 4.98 ± 0.30 | 1.57 ± 0.27 | 2.57 ± 1.54 |
| SFA | 67.10 ± 6.69 | 53.85 ± 6.32 | 60.81 ± 12.60 | 56.98 ± 4.64 |
| MUFA | 15.43 ± 2.89 | 22.01 ± 2.31 | 19.77 ± 3.36 | 21.49 ± 4.87 |
| PUFA | 17.47 ± 5.44 | 24.14 ± 5.44 | 13.71 ± 3.40 | 22.33 ± 1.26 |
| n6 | 2.54 ± 2.03 | 5.80 ± 1.42 | 4.43 ± 1.02 | 5.80 ± 4.45 |
| n3 | 14.93 ± 3.49 | 18.34 ± 4.06 | 9.28 ± 2.57 | 12.55 ± 3.67 |
| n6/n3 | 0.16 ± 0.16 | 0.32 ± 0.14 | 0.49 ± 0.24 | 0.57 ± 0.61 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsoupras, A.; Moran, D.; Pleskach, H.; Durkin, M.; Traas, C.; Zabetakis, I. Beneficial Anti-Platelet and Anti-Inflammatory Properties of Irish Apple Juice and Cider Bioactives. Foods 2021, 10, 412. https://doi.org/10.3390/foods10020412
Tsoupras A, Moran D, Pleskach H, Durkin M, Traas C, Zabetakis I. Beneficial Anti-Platelet and Anti-Inflammatory Properties of Irish Apple Juice and Cider Bioactives. Foods. 2021; 10(2):412. https://doi.org/10.3390/foods10020412
Chicago/Turabian StyleTsoupras, Alexandros, Donal Moran, Hayley Pleskach, Maria Durkin, Con Traas, and Ioannis Zabetakis. 2021. "Beneficial Anti-Platelet and Anti-Inflammatory Properties of Irish Apple Juice and Cider Bioactives" Foods 10, no. 2: 412. https://doi.org/10.3390/foods10020412