Myalgic Encephalomyelitis or What? The International Consensus Criteria
Abstract
:1. Introduction
1.1. ME (1938–1990)
1.2. CFS (1988–2018)
1.3. ME-ICC (2011)
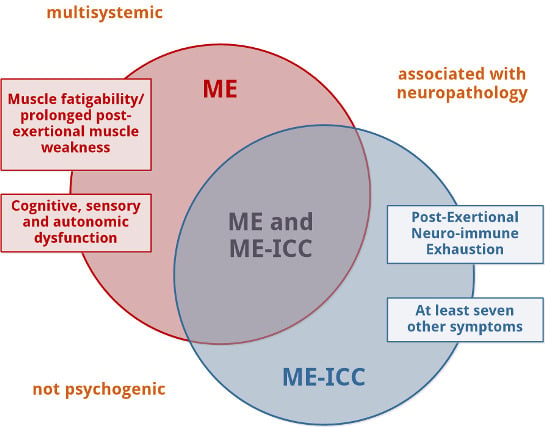
2. ME vs. ME-ICC: Similarities and Differences
2.1. Similarities
2.1.1. ME Is a Neurological Disease
2.1.2. ME Is a Multisystemic Disease
2.1.3. ME Is Not a Psychogenic Disorder
2.1.4. ME Is Assumed to Be Associated with Neuropathology
2.2. Differences
2.2.1. Muscle Fatigability or Prolonged Post-Exertional Muscle Weakness, Mandatory for the Diagnosis of ME, Is an Optional Element for the Diagnosis of ME-ICC
2.2.2. Post-Exertional Neuro-Immune Exhaustion, Mandatory for ME-ICC, Is Not a Mandatory Feature of ME
2.2.3. The Diagnosis of ME-ICC Requires Many More Symptoms Than the Diagnosis of ME
2.2.4. Autonomic, Sensory, and Cognitive Dysfunction (Mandatory for the Diagnosis of ME) Are Not Compulsory to Meet the ME-ICC Requirements for Neurological Impairments
3. Summary
4. Discussion
5. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Dowsett, E.G.; Ramsay, A.M.; McCartney, R.A.; Bell, E.J. Myalgic Encephalomyelitis—A persistent enteroviral infection? Postgrad. Med. J. 1990, 66, 526–530. [Google Scholar] [CrossRef] [PubMed]
- Ramsay, A.M.; Dowsett, E.G. Myalgic Encephalomyelitis: Then and now, an epidemiological introduction. In The Clinical and Scientific Basis of Myalgic Encephalomyelitis/Chronic Fatigue Syndrome; Hyde, B.M., Goldstein, J., Levine, P., Eds.; Nightingale Research Foundation: Ottawa, ON, Canada, 1992. [Google Scholar]
- Holmes, G.P.; Kaplan, J.E.; Gantz, N.M.; Komaroff, A.L.; Schonberger, L.B.; Straus, S.E.; Jones, J.F.; Dubois, A.E.; Cunningham-Rundles, C.; Pahwa, S.; et al. Chronic fatigue syndrome: A working case definition. Ann. Intern. Med. 1988, 108, 387–389. [Google Scholar] [CrossRef]
- Fukuda, K.; Straus, S.E.; Hickie, I.; Sharpe, M.; Dobbins, J.G.; Komaroff, A.L. The chronic fatigue syndrome: A comprehensive approach to its definition and study. Ann. Intern. Med. 1994, 121, 953–959. [Google Scholar] [CrossRef] [PubMed]
- Twisk, F.N.M. Myalgic Encephalomyelitis, chronic fatigue syndrome, and Systemic Exertion Intolerance Disease: Three distinct clinical entities. Challenges 2018, 9, 19. [Google Scholar] [CrossRef]
- Carruthers, B.M.; van de Sande, M.I.; de Meirleir, K.L.; Klimas, N.G.; Broderick, G.; Mitchell, T.; Staines, D.; Powles, A.C.P.; Speight, N.; Vallings, R.; et al. Myalgic encephalomyelitis: International consensus criteria. J. Intern. Med. 2011, 270, 327–338. [Google Scholar] [CrossRef]
- Gilliam, A.G. Epidemiological study on an epidemic, diagnosed as poliomyelitis, occurring among the personnel of Los Angeles County General Hospital during the summer of 1934; US Government Printing Office: Washington, DC, USA, 1938; Volume 240, pp. 1–90.
- The Medical Staff of The Royal Free Hospital. An outbreak of Encephalomyelitis in the Royal Free Hospital Group, London, in 1955. Br. Med. J. 1957, 2, 895–904. [Google Scholar] [CrossRef]
- Sigurdsson, B.; Sigurjonsson, J.; Sigurdsson, J.H.; Thorkelsson, J.; Gudmundsson, K.R. A disease epidemic in Iceland simulating poliomyelitis. Am. J. Hyg. 1950, 52, 222–238. [Google Scholar] [CrossRef] [PubMed]
- Parish, J.G. Early outbreaks of ‘epidemic neuromyasthenia’. Postgrad. Med. J. 1978, 54, 711–717. [Google Scholar] [CrossRef] [PubMed]
- Acheson, E.D. The clinical syndrome variously called benign myalgic encephalomyelitis, Iceland disease and epidemic neuromyasthenia. Am. J. Med. 1959, 26, 569–595. [Google Scholar] [CrossRef] [Green Version]
- Ramsay, A.M. Encephalomyelitis in north west London; an endemic infection simulating poliomyelitis and hysteria. Lancet 1957, 270, 1196–1200. [Google Scholar] [CrossRef]
- Acheson, D.E. A new clinical entity? Lancet 1956, 267, 789–790. [Google Scholar] [CrossRef]
- Institute of Medicine. Beyond Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: Redefining an Illness; National Academies Press: Washington, DC, USA, 2015. [Google Scholar]
- Twisk, F.N.M. The status of and future research into Myalgic Encephalomyelitis and chronic fatigue syndrome: The need of accurate diagnosis, objective assessment, and acknowledging biological and clinical subgroups. Front. Physiol. 2014, 5, 109. [Google Scholar] [CrossRef]
- Twisk, F.N.M. Replacing Myalgic Encephalomyelitis and chronic fatigue syndrome with systemic exercise intolerance disease is not the way forward. Diagnostics 2016, 6, E10. [Google Scholar] [CrossRef]
- Twisk, F.N.M. Myalgic Encephalomyelitis (ME) or what? An operational definition. Diagnostics 2018, 8, E64. [Google Scholar] [CrossRef]
- Carruthers, B.M.; van de Sande, M.I. Myalgic Encephalomyelitis—Adult & Paediatric: International Consensus Primer for Medical Practioners; Carruthers and van de Sande: Vancouver/Calgary, BC/AB, Canada, 2012. [Google Scholar]
- Nakatomi, Y.; Mizuno, K.; Ishii, A.; Wada, Y.; Tanaka, M.; Tazawa, S.; Onoe, K.; Fukuda, S.; Kawabe, J.; Takahashi, K.; et al. Neuroinflammation in patients with chronic fatigue syndrome/Myalgic Encephalomyelitis: An 11C-(R.)-PK11195 PET study. J. Nucl. Med. 2014, 55, 945–950. [Google Scholar] [CrossRef]
- Ramsay, A.M. Postviral fatigue syndrome—The saga of Royal Free Disease, 1st ed.; Gower Medical Publishing: London, UK, 1986. [Google Scholar]
- Wilson, A.; Hickie, I.; Hadzi-Pavlovic, D.; Wakefield, D.; Parker, G.; Straus, S.E.; Dale, J.; McCluskey, D.; Hinds, G.; Brickman, A.; et al. What is chronic fatigue syndrome? Heterogeneity within an international multicentre study. Aust. N. Z. J. Psychiatry 2001, 35, 520–527. [Google Scholar] [CrossRef]
- Carruthers, B.M.; Jain, A.K.; de Meirleir, K.; Peterson, D.L.; Klimas, N.G.; Lerner, A.M.; Bested, A.C.; Flor-Henry, P.; Joshi, P.; Peter Powles, A.C.; et al. Myalgic encephalomyelitis/chronic fatigue syndrome: Clinical working case definition, diagnostic and treatment protocols. J. Chronic Fatigue Syndr. 2003, 11, 7–115. [Google Scholar] [CrossRef]
- Twisk FNM. Accurate diagnosis of myalgic encephalomyelitis and chronic fatigue syndrome based upon objective test methods for characteristic symptoms. World J. Methodol. 2015, 5, 68–87. [Google Scholar] [CrossRef] [PubMed]
- Hornig, M.; Montoya, J.G.; Klimas, N.G.; Levine, S.; Felsenstein, D.; Bateman, L.; Peterson, P.L.; Gottschalk, C.G.; Schultz, A.F.; Che, X.; et al. Distinct plasma immune signatures in ME/CFS are present early in the course of illness. Sci. Adv. 2015, 1, e1400121. [Google Scholar] [CrossRef]
- Smylie, A.L.; Broderick, G.; Fernandes, H.; Razdan, S.; Barnes, Z.; Collado, F.; Sol, C.; Fletcher, M.A.; Klimas, N. A comparison of sex-specific immune signatures in Gulf War illness and chronic fatigue syndrome. BMC Immunol. 2013, 14, 29. [Google Scholar] [CrossRef] [PubMed]
- Richardson, A.M.; Lewis, D.P.; Kita, B.; Ludlow, H.; Groome, N.P.; Hedger, M.P.; de Kretser, D.M.; Lidbury, B.A. Weighting of orthostatic intolerance time measurements with standing difficulty score stratifies ME/CFS symptom severity and analyte detection. J. Transl. Med. 2018, 16, 97. [Google Scholar] [CrossRef] [PubMed]

| ME [1,2] | ME-ICC [6,18] |
|---|---|
| The pathognomonic features (of ME): 1. a complaint of general or local muscular fatigue following minimal exertion with prolonged recovery time a; 2. neurological disturbance, especially of cognitive, autonomic, and sensory functions; 3. variable involvement of cardiac and other systems; 4. prolonged relapsing course. “Other characteristics include [...] variation in intensity of symptoms within and between episodes, tending to chronicity.” [1] | A. Post-exertional neuro-immune exhaustion (PENE): mandatory. B. Neurological impairments (at least one symptom from three of the four symptom categories): 1. Neurocognitive impairments a. Difficulty processing information: slowed thought, impaired concentration b. Short-term memory loss 2. Pain a. Headaches b. Significant pain in muscles, muscle-tendon junctions, joints, abdomen, or chest 3. Sleep disturbance a. Disturbed sleep patterns b. Unrefreshing sleep 4. Neurosensory, perceptual, and motor disturbances a. Neurosensory and perceptual symptoms b. Motor dysfunction C. Immune, gastro-intestinal, and genitourinary impairments (at least one symptom from three of five symptom categories): 1. Flu-like symptoms (recurrent or chronic, which typically activate or worsen with exertion) 2. Susceptibility to viral infections with prolonged recovery periods 3. Gastro-intestinal symptoms 4. Genitourinary symptoms 5. Sensitivities to food, medications, odors, or chemicals D. Energy production or transportation impairments (at least one of four symptoms): 1. Cardiovascular symptoms 2. Respiratory symptoms 3. Loss of thermostatic stability 4. Intolerance of extremes of temperature |
© 2018 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Twisk, F. Myalgic Encephalomyelitis or What? The International Consensus Criteria. Diagnostics 2019, 9, 1. https://doi.org/10.3390/diagnostics9010001
Twisk F. Myalgic Encephalomyelitis or What? The International Consensus Criteria. Diagnostics. 2019; 9(1):1. https://doi.org/10.3390/diagnostics9010001
Chicago/Turabian StyleTwisk, Frank. 2019. "Myalgic Encephalomyelitis or What? The International Consensus Criteria" Diagnostics 9, no. 1: 1. https://doi.org/10.3390/diagnostics9010001