Utilization of Multiparametric MRI of Prostate in Patients under Consideration for or Already in Active Surveillance: Correlation with Imaging Guided Target Biopsy
Abstract
:1. Introduction
2. Methods
2.1. Patients
2.2. MR Imaging Acquisition
2.3. MR Imaging Interpretation
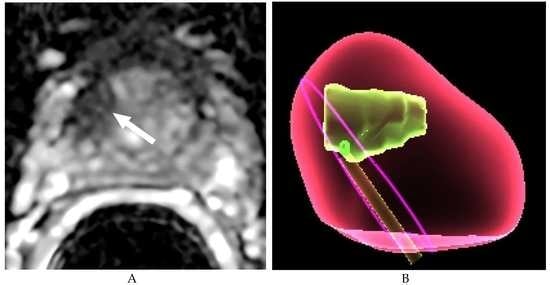
2.4. Imaging Guided Target Prostate Biopsies
2.5. Statistical Analysis
3. Results
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Zhao, Y.Y.; Weir, M.A.; Manno, M.; Cordy, P.; Gomes, T.; Hackam, D.G.; Juurlink, D.N.; Mamdani, M.; Moist, L.; Parikh, C.R.; et al. National institutes of health state-of-the-science conference: Role of active surveillance in the management of men with localized prostate cancer. Ann. Intern. Med. 2012, 156, 591–595. [Google Scholar]
- Bastian, P.J.; Carter, B.H.; Bjartell, A.; Seitz, M.; Stanislaus, P.; Montorsi, F.; Cief, G.S.; Schroder, F. Insignificant prostate cancer and active surveillance: From definition to clinical implications. Eur. Urol. 2009, 55, 1321–1330. [Google Scholar] [CrossRef]
- Porten, S.P.; Whitson, J.M.; Cowan, J.E.; Cooperberg, M.R.; Shinohara, K.; Perez, N.; Greene, K.L.; Meng, M.V.; Carroll, P.R. Changes in prostate cancer grade on serial biopsy in men undergoing active surveillance. J. Clin. Oncol. 2011, 29, 2795–2800. [Google Scholar] [CrossRef]
- Tosoian, J.J.; Trock, B.J.; Landis, P.; Feng, Z.; Epstein, J.I.; Partin, A.W.; Walsh, P.C.; Carter, H.B. Active surveillance program for prostate cancer: An update of the Johns Hopkins experience. J. Clin. Oncol. 2011, 29, 2185–2190. [Google Scholar] [CrossRef] [Green Version]
- Berglund, R.K.; Masterson, T.A.; Vora, K.C.; Eggener, S.E.; Eastham, J.A.; Guillonneau, B.D. Pathological upgrading and up staging with immediate repeat biopsy in patients eligible for active surveillance. J. Urol. 2008, 180, 1964–1968. [Google Scholar] [CrossRef] [Green Version]
- Klotz, L.; Zhang, L.; Lam, A.; Nam, R.; Mamedov, A.; Loblaw, A. Clinical results of long-term follow-up of a large, active surveillance cohort with localized prostate cancer. J. Clin. Oncol. 2010, 28, 126–131. [Google Scholar] [CrossRef] [Green Version]
- Duffield, A.S.; Lee, T.K.; Miyamoto, H.; Carter, H.B.; Epstein, J.I. Radical prostatectomy findings in patients in whom active surveillance of prostate cancer fails. J. Urol. 2009, 182, 2274–2278. [Google Scholar] [CrossRef] [Green Version]
- Lee, M.C.; Dong, F.; Stephenson, A.J.; Jones, J.S.; Magi-Galluzzi, C.; Klein, E.A. The Epstein criteria predict for organ-confined but not insignificant disease and a high likelihood of cure at radical prostatectomy. Eur. Urol. 2010, 58, 90–95. [Google Scholar] [CrossRef]
- Turkbey, B.; Mani, H.; Shah, V.; Rastinehad, A.R.; Bernardo, M.; Pohida, T.; Pang, Y.; Daar, D.; Benjamin, C.; McKinney, Y.L.; et al. Multiparametric 3T prostate magnetic resonance imaging to detect cancer: Histopathological correlation using prostatectomy specimens processed in customized magnetic resonance imaging based molds. J. Urol. 2011, 186, 1818–1824. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hegde, J.V.; Mulkern, R.V.; Panych, L.P.; Fennessy, F.M.; Fedorov, A.; Maier, S.E.; Tempany, C.M. Multiparametric MRI of prostate cancer: An update on state-of-the-art techniques and their performance in detecting and localizing prostate cancer. J. Magn. Reason. Imaging 2013, 37, 1035–1054. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vargas, H.A.; Akin, O.; Franiel, T.; Mazaheri, Y.; Zheng, J.; Moskowitz, C.; Udo, K.; Eastham, J.; Hricak, H. Diffusion-weighted Endorectal MR Imaging at 3 T for Prostate Cancer: Tumor Detection and Assessment of Aggressiveness. Radiology 2011, 259, 775–784. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Langer, D.L.; van der Kwast, T.H.; Evans, A.J.; Sun, L.; Yaffe, M.J.; Trachtenberg, J.; Haider, M.A. Intermixed normal tissue within prostate cancer: Effect on MR imaging measurements of apparent diffusion coefficient and T2—Sparse versus dense cancers. Radiology. 2008, 249, 900. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, M.M.; Rais-Bahrami, S.; Turkbey, B.; George, A.K.; Rothwax, J.; Shakir, N.; Okoro, C.; Raskolnikov, D.; Parnes, H.L.; Linehan, W.M.; et al. Comparison of MR/ultrasound fusion–guided biopsy with ultrasound-guided biopsy for the diagnosis of prostate cancer. JAMA 2015, 313, 390–397. [Google Scholar] [CrossRef] [PubMed]
- Sonn, G.A.; Natarajan, S.; Margolis, D.J.; MacAiran, M.; Lieu, P.; Huang, J.; Dorey, F.J.; Marks, L.S. Targeted biopsy in the detection of prostate cancer using an office based magnetic resonance ultrasound fusion device. J. Urol. 2013, 189, 86–92. [Google Scholar] [CrossRef] [Green Version]
- Haffner, J.; Lemaitre, L.; Puech, P.; Haber, G.P.; Leroy, X.; Jones, J.S.; Villers, A. Role of magnetic resonance imaging before initial biopsy: Comparison of magnetic resonance imaging-targeted and systematic biopsy for significant prostate cancer detection. BJU Int. 2011, 108, E171–E178. [Google Scholar] [CrossRef]
- Stamey, T.A.; Freiha, F.S.; McNeal, J.E.; Redwine, E.A.; Whittemore, A.S.; Schmid, H.P. Localized prostate cancer. Relationship of tumor volume to clinical significance for treatment of prostate cancer. Cancer 1993, 71, 933–938. [Google Scholar] [CrossRef]
- Mohler, J.L.; Armstrong, A.J.; Bahnson, R.R.; D’Amico, A.V. NCCN Clinical Practice Guidelines in Oncology for Prostate Cancer, v.3. 2011. Available online: http://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf (accessed on 25 May 2011).
- Puech, P.; Potiron, E.; Lemaitre, L.; Leroy, C.; Biserte, J.; Villers, A. Dynamic contrast-enhanced MRI evaluation of intraprostatic prostate cancer. Correlation with radical prostatectomy specimens. Urology 2009, 74, 1094–1099. [Google Scholar] [CrossRef]
- Dickinson, L.; Ahmed, H.U.; Allen, C.; Barentsz, J.O.; Carey, B.; Futterer, J.J.; Heijmink, S.W.; Hoskin, P.J.; Kirkham, A.; Padhani, A.R.; et al. Magnetic resonance imaging for the detection, localization, and characterization of prostate cancer: Recommendations from a European consensus meeting. Eur. Urol. 2011, 59, 477–494. [Google Scholar] [CrossRef]
- Barentsz, J.O.; Richenberg, J.; Clements, R.; Choyke, P.; Verma, S.; Villeirs, G.; Rouviere, O.; Logager, V.; Fütterer, J.J. European Society of Urogenital Radiology. ESUR prostate MR guidelines. Eur. Radiol. 2012, 22, 746–757. [Google Scholar] [CrossRef] [Green Version]
- Weinreb, J.C.; Barentsz, J.O.; Choyke, P.L.; Cornud, F.; Haider, M.A.; Macura, K.J.; Margolis, D.; Schnall, M.D.; Shtern, F.; Tempany, C.M.; et al. PI-RADS Prostate Imaging—Reporting and Data System: 2015, Version 2. Eur. Urol. 2016, 69, 16–40. [Google Scholar] [CrossRef]
- Akin, O.; Sala, E.; Moskowitz, C.S.; Kuroiwa, K.; Ishill, N.M.; Pucar, D.; Scardino, P.T.; Hricak, H. Transition zone prostate cancers: Features, detection, localization, and staging at endorectal MR imaging. Radiology 2006, 239, 784–792. [Google Scholar] [CrossRef] [PubMed]
- Haider, M.A.; van der Kwast, T.H.; Tanguay, J.; Evans, A.J.; Hashmi, A.; Lockwood, G.; Trachtenberg, J. Combined T2-weighted and diffusion-weighted MRI for localization of prostate cancer. AJR Am. J. Roentgenol. 2007, 189, 323–328. [Google Scholar] [CrossRef] [PubMed]
- de Rooij, M.; Hamoen, E.H.; Fütterer, J.J.; Barentsz, J.O.; Rovers, M.M. Accuracy of multiparametric MRI for prostate cancer detection: A meta-analysis. Am. J. Roentgenol. 2014, 202, 343–351. [Google Scholar] [CrossRef] [PubMed]
- Hoeks, C.M.; Schouten, M.G.; Bomers, J.G.; Hoogendoorn, S.P.; Hulsbergen-van de Kaa, C.A.; Hambrock, T.; Vergunst, H.; Sedelaar, J.P.; Fütterer, J.J.; Barentsz, J.O. Three-Tesla magnetic resonance-guided prostate biopsy in men with increased prostate-specific antigen and repeated, negative, random, systematic, transrectal ultrasound biopsies: Detection of clinically significant prostate cancers. Eur. Urol. 2012, 65, 902–909. [Google Scholar] [CrossRef]
- Sonn, G.A.; Chang, E.; Natarajan, S.; Margolis, D.J.; Macairan, M.; Lieu, P.; Huang, J.; Dorey, F.J.; Reiter, R.E.; Marks, L.S. Value of targeted prostate biopsy using magnetic resonance-ultrasound fusion in men with prior negative biopsy and elevated prostate-specific antigen. Eur. Urol. 2014, 65, 809–815. [Google Scholar] [CrossRef]
- Pinto, P.A.; Chung, P.H.; Rastinehad, A.R.; Baccala, A.A.; Jr Kruecker, J.; Benjamin, C.J.; Xu, S.; Yan, P.; Kadoury, S.; Chua, C.; et al. Magnetic resonance imaging/ultrasound fusion guided prostate biopsy improves cancer detection following transrectal ultrasound biopsy and correlates with multiparametric magnetic resonance imaging. J. Urol. 2011, 186, 1281–1285. [Google Scholar] [CrossRef] [Green Version]
- Wegelin, O.; van Melick, H.H.E.; Hooft, L.; Bosch, J.L.H.R.; Reitsma, H.B.; Barentsz, J.O.; Somford, D.M. Comparing Three Different Techniques for Magnetic Resonance Imaging-targeted Prostate Biopsies: A Systematic Review of In-bore versus Magnetic Resonance Imaging-transrectal Ultrasound fusion versus Cognitive Registration. Is There a Preferred Technique? Eur. Urol. 2017, 71, 517–531. [Google Scholar] [CrossRef] [PubMed]
- Margel, D.; Yap, S.A.; Lawrentschuk, N.; Klotz, L.; Haider, M.; Hersey, K.; Finelli, A.; Zlotta, A.; Trachtenberg, J.; Fleshner, N. Impact of multiparametric endorectal coil prostate magnetic resonance imaging on disease reclassification among active surveillance candidates: A prospective cohort study. J. Urol. 2012, 187, 1247–1252. [Google Scholar] [CrossRef]
- Barrrett, T.; Haider, M. The Emerging Role of MRI in Prostate Cancer Active Surveillance and Ongoing Challenges. AJR 2017, 208, 131–139. [Google Scholar] [CrossRef]
- Serrao, E.M.; Barrett, T.; Wadhwa, K.; Parashar, D.; Frey, J.; Koo, B.C.; Warren, A.Y.; Doble, A.; Kastner, C.; Gallagher, F.A. Investigating the ability of multiparametric MRI to exclude significant prostate cancer prior to transperineal biopsy. Can. Urol Assoc. J. 2015, 9, E853–E858. [Google Scholar] [CrossRef] [Green Version]
- Frye, T.P.; George, A.K.; Kilchevsky, A.; Maruf, M.; Siddiqui, M.M.; Kongnyuy, M.; Muthigi, A.; Han, H.; Parnes, H.L.; Merino, M.; et al. Magnetic Resonance Imaging-Transrectal Ultrasound Guided Fusion Biopsy to Detect Progression in Patients with Existing Lesions on Active Surveillance for Low and Intermediate Risk Prostate Cancer. J. Urol. 2017, 197, 640–646. [Google Scholar] [CrossRef] [PubMed]
- Hoeks, C.M.; Somford, D.M.; van Oort, I.M.; Vergunst, H.; Oddens, J.R.; Smits, G.A.; Roobol, M.J.; Bul, M.; Hambrock, T.; Witjes, J.A.; et al. Value of 3-T multiparametric magnetic resonance imaging and magnetic resonance guided biopsy for early risk restratification in active surveillance of low-risk prostate cancer: A prospective multicenter cohort study. Invest. Radiol. 2014, 49, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Vargas, H.A.; Akin, O.; Afaq, A.; Goldman, D.; Zheng, J.; Moskowitz, C.S.; Shukla-Dave, A.; Eastham, J.; Scardino, P.; Hricak, H. The Value of Magnetic Resonance Imaging in Predicting Confirmatory Prostate Biopsy Findings in Patients Being Considered for Active Surveillance of Clinically Low-risk Prostate Cancer. J. Urol. 2012, 188, 1732–1738. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Distler, F.A.; Radtke, J.; Bonekamp, D.; Kesch, C.; Schlemmer, H.E.; Wieczorek, K.; Kirchner, M.; Pahernik, S.; Hohenfellner, M.; Hadaschik, B.A. The Value of PSA Density in Combination with PI-RADS for the Accuracy of Prostate Cancer Prediction. J. Urol. 2017, 198, 575–582. [Google Scholar] [CrossRef]




| Variable | Value |
|---|---|
| Patients | 354 |
| Median age | Year; 63.4 (50–79) |
| Median PSA, ng/mL | 6.1 (2.8–9.7) |
| Median prostate volume, cc | 41 (18–159) |
| Median PSA Density, ng/mL/cc | 0.15 (0.07–0.31) |
| Number of prior biopsy (%) | 1 (198) |
| 2 (112) | |
| ≥3 (44) | |
| Number of positive biopsy core | 1 (258, 73%) |
| 2 (96, 27%) | |
| Biopsy Gleason score at TRUS biopsy | 3 + 3 (354, 100%) |
| PI-RADS Score | Imaging-Guided bx of CSRs Upgrade | ||
|---|---|---|---|
| No | Yes | Total | |
| 3 | 23 (82%) | 5 (18%) | 28 |
| 4 | 19 (29%) | 47 (71%) | 66 |
| 5 | 3 (6%) | 49 (94%) | 52 |
| Total | 45 | 101 | 146 |
| Coefficient Estimate | Standard Error | p Value | |
|---|---|---|---|
| Intercept | −8.004 | 2.54 | 0.0016 |
| PI-RADS | 2.245 | 0.64 | 0.00054 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, J.; Fulcher, A.S.; Winks, S.; Turner, M.A.; Behl, W.; Ware, A.L.; Mukhopadhyay, N.D.; Kim, C.; Jackson, C.; Bajaj, H.S.; et al. Utilization of Multiparametric MRI of Prostate in Patients under Consideration for or Already in Active Surveillance: Correlation with Imaging Guided Target Biopsy. Diagnostics 2020, 10, 441. https://doi.org/10.3390/diagnostics10070441
Yu J, Fulcher AS, Winks S, Turner MA, Behl W, Ware AL, Mukhopadhyay ND, Kim C, Jackson C, Bajaj HS, et al. Utilization of Multiparametric MRI of Prostate in Patients under Consideration for or Already in Active Surveillance: Correlation with Imaging Guided Target Biopsy. Diagnostics. 2020; 10(7):441. https://doi.org/10.3390/diagnostics10070441
Chicago/Turabian StyleYu, Jinxing, Ann S. Fulcher, Sarah Winks, Mary A. Turner, William Behl, Anna Lee Ware, Nitai D. Mukhopadhyay, Candice Kim, Christopher Jackson, Harnek S. Bajaj, and et al. 2020. "Utilization of Multiparametric MRI of Prostate in Patients under Consideration for or Already in Active Surveillance: Correlation with Imaging Guided Target Biopsy" Diagnostics 10, no. 7: 441. https://doi.org/10.3390/diagnostics10070441