A Review of the Potential Application of Osteocyte-Related Biomarkers, Fibroblast Growth Factor-23, Sclerostin, and Dickkopf-1 in Predicting Osteoporosis and Fractures
Abstract
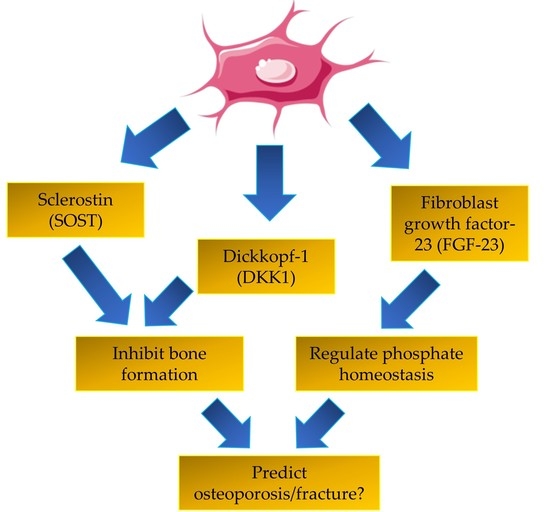
:1. Introduction
2. Fibroblast Growth Factor-23
3. Sclerostin
4. Dickkopf-1
5. Conclusion and Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ASARM | acidic serine–aspartate-rich MEPE-associated motif |
| BMD | bone mineral density |
| BTMs | bone turnover markers |
| CKD | chronic kidney disease |
| DKK1 | dickkopf-1 |
| eGFR | estimated glomerular filtration rate |
| FGF23 | fibroblast growth factor-23 |
| LRP | lipoprotein receptor-related protein |
| MEPE | matrix extracellular phosphoglycoprotein |
| OF | osteoporotic fracture |
| PMO | postmenopausal women with osteoporosis |
| PMNO | postmenopausal women without osteoporosis |
| RANKL | receptor activator of nuclear factor kappa-B ligand |
| SOST | sclerostin |
References
- Ahn, S.H.; Park, S.Y.; Yoo, J.-I.; Chung, Y.-J.; Jeon, Y.K.; Yoon, B.-H.; Kim, H.Y.; Lee, S.H.; Lee, J.; Hong, S. Use of bone turnover markers in clinical practice for the management of osteoporosis in korea: From the survey on the prescription pattern of bone turnover markers. J. Bone Metab. 2019, 26, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Camacho, P.M.; Lopez, N.A. Use of biochemical markers of bone turnover in the management of postmenopausal osteoporosis. Clin. Chem. Lab. Med. 2008, 46, 1345–1357. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, Y.; Datel, O.; Fadoua, H.; Smeh, B.; Mahbouba, J.; Saoussen, Z.; Bejia, I.; Mongi, T.; Mohamed, F.; Naceur, B. The role of biochemical markers of bone turnover in the diagnosis of osteoporosis and predicting fracture risk. Tunis Med. 2014, 92, 304–310. [Google Scholar] [PubMed]
- Rupp, T.; Butscheidt, S.; Vettorazzi, E.; Oheim, R.; Barvencik, F.; Amling, M.; Rolvien, T. High fgf23 levels are associated with impaired trabecular bone microarchitecture in patients with osteoporosis. Osteoporos. Int. 2019, 30, 1655–1662. [Google Scholar] [CrossRef] [PubMed]
- Crandall, C.J.; Vasan, S.; LaCroix, A.; LeBoff, M.S.; Cauley, J.A.; Robbins, J.A.; Jackson, R.D.; Bauer, D.C. Bone turnover markers are not associated with hip fracture risk: A case-control study in the women’s health initiative. J. Bone Miner. Res. 2018, 33, 1199–1208. [Google Scholar] [CrossRef] [PubMed]
- Marques, E.A.; Gudnason, V.; Lang, T.; Sigurdsson, G.; Sigurdsson, S.; Aspelund, T.; Siggeirsdottir, K.; Launer, L.; Eiriksdottir, G.; Harris, T.B. Association of bone turnover markers with volumetric bone loss, periosteal apposition, and fracture risk in older men and women: The ages—Reykjavik longitudinal study. Osteoporos. Int. 2016, 27, 3485–3494. [Google Scholar] [CrossRef] [Green Version]
- Sarkar, S.; Reginster, J.Y.; Crans, G.G.; Diez-Perez, A.; Pinette, K.V.; Delmas, P.D. Relationship between changes in biochemical markers of bone turnover and bmd to predict vertebral fracture risk. J. Bone Miner. Res. 2004, 19, 394–401. [Google Scholar] [CrossRef]
- Dallas, S.; Prideaux, M.; Bonewald, L. The osteocyte: An endocrine cel… and more. Endocr. Rev. 2013, 34, 658–690. [Google Scholar] [CrossRef] [Green Version]
- Qing, H.; Ardeshirpour, L.; Divieti Pajevic, P.; Dusevich, V.; Jähn, K.; Kato, S.; Wysolmerski, J.; Bonewald, L.F. Demonstration of osteocytic perilacunar/canalicular remodeling in mice during lactation. J. Bone Miner. Res. 2012, 27, 1018–1029. [Google Scholar] [CrossRef] [Green Version]
- Bellido, T. Osteocyte-driven bone remodeling. Calcif. Tissue Int. 2014, 94, 25–34. [Google Scholar] [CrossRef] [Green Version]
- Delgado-Calle, J.; Bellido, T. Osteocytes and skeletal pathophysiology. Curr. Mol. Biol. Rep. 2015, 1, 157–167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ke, H.Z.; Richards, W.G.; Li, X.; Ominsky, M.S. Sclerostin and dickkopf-1 as therapeutic targets in bone diseases. Endocr. Rev. 2012, 33, 747–783. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonewald, L.F. The amazing osteocyte. J. Bone Miner. Res. 2011, 26, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, T.; Hayashi, M.; Fukunaga, T.; Kurata, K.; Oh-Hora, M.; Feng, J.Q.; Bonewald, L.F.; Kodama, T.; Wutz, A.; Wagner, E.F. Evidence for osteocyte regulation of bone homeostasis through rankl expression. Nat. Med. 2011, 17, 1231–1234. [Google Scholar] [CrossRef]
- Xiong, J.; Onal, M.; Jilka, R.L.; Weinstein, R.S.; Manolagas, S.C.; O’brien, C.A. Matrix-embedded cells control osteoclast formation. Nat. Med. 2011, 17, 1235–1241. [Google Scholar] [CrossRef] [Green Version]
- Bonewald, L.F.; Wacker, M.J. Fgf23 production by osteocytes. Pediatr. Nephrol. 2013, 28, 563–568. [Google Scholar] [CrossRef]
- Huang, X.; Jiang, Y.; Xia, W. Fgf23 and phosphate wasting disorders. Bone Res. 2013, 1, 120–132. [Google Scholar] [CrossRef] [Green Version]
- Berndt, T.; Kumar, R. Phosphatonins and the regulation of phosphate homeostasis. Annu. Rev. Physiol. 2007, 69, 341–359. [Google Scholar] [CrossRef]
- Rowe, P.S. The chicken or the egg: Phex, fgf23 and siblings unscrambled. Cell. Biochem. Funct. 2012, 30, 355–375. [Google Scholar] [CrossRef] [Green Version]
- Shimada, T.; Hasegawa, H.; Yamazaki, Y.; Muto, T.; Hino, R.; Takeuchi, Y.; Fujita, T.; Nakahara, K.; Fukumoto, S.; Yamashita, T. Fgf-23 is a potent regulator of vitamin d metabolism and phosphate homeostasis. J. Bone Miner. Res. 2004, 19, 429–435. [Google Scholar] [CrossRef] [Green Version]
- Coulson, J.; Bagley, L.; Barnouin, Y.; Bradburn, S.; Butler-Browne, G.; Gapeyeva, H.; Hogrel, J.-Y.; Maden-Wilkinson, T.; Maier, A.B.; Meskers, C. Circulating levels of dickkopf-1, osteoprotegerin and sclerostin are higher in old compared with young men and women and positively associated with whole-body bone mineral density in older adults. Osteoporos. Int. 2017, 28, 2683–2689. [Google Scholar] [CrossRef] [PubMed]
- Isakova, T.; Cai, X.; Lee, J.; Katz, R.; Cauley, J.A.; Fried, L.F.; Hoofnagle, A.N.; Satterfield, S.; Harris, T.B.; Shlipak, M.G. Associations of fgf23 with change in bone mineral density and fracture risk in older individuals. J. Bone Miner. Res. 2016, 31, 742–748. [Google Scholar] [CrossRef] [PubMed]
- Marsell, R.; Mirza, M.; Mallmin, H.; Karlsson, M.; Mellström, D.; Orwoll, E.; Ohlsson, C.; Jonsson, K.B.; Ljunggren, Ö.; Larsson, T. Relation between fibroblast growth factor-23, body weight and bone mineral density in elderly men. Osteoporos. Int. 2009, 20, 1167–1173. [Google Scholar] [CrossRef] [PubMed]
- Imel, E.A.; Liu, Z.; McQueen, A.K.; Acton, D.; Acton, A.; Padgett, L.R.; Peacock, M.; Econs, M.J. Serum fibroblast growth factor 23, serum iron and bone mineral density in premenopausal women. Bone 2016, 86, 98–105. [Google Scholar] [CrossRef] [Green Version]
- Han, W.; Bai, X.-J.; Han, L.-L.; Sun, X.-F.; Chen, X.-M. The relationship between serum fibroblast growth factor 23, klotho, and lumbar spine bone mineral density in northern chinese postmenopausal women. Menopause 2019, 26, 546–553. [Google Scholar] [CrossRef] [PubMed]
- Celik, E.; Guzel, S.; Abali, R.; Guzelant, A.; Celik, E.G.; Kuçukyalcin, V. The relationship between fibroblast growth factor 23 and osteoporosis in postmenopausal women. Minerva Med. 2013, 104, 497–504. [Google Scholar] [PubMed]
- Choksi, P.; Jepsen, K.J.; Clines, G.A. The challenges of diagnosing osteoporosis and the limitations of currently available tools. Clin. Diabetes Endocrinol. 2018, 4, 12. [Google Scholar] [CrossRef] [Green Version]
- Mirza, M.A.; Karlsson, M.K.; Mellström, D.; Orwoll, E.; Ohlsson, C.; Ljunggren, Ö.; Larsson, T.E. Serum fibroblast growth factor-23 (fgf-23) and fracture risk in elderly men. J. Bone Miner. Res. 2011, 26, 857–864. [Google Scholar] [CrossRef]
- Kanda, E.; Yoshida, M.; Sasaki, S. Applicability of fibroblast growth factor 23 for evaluation of risk of vertebral fracture and chronic kidney disease-mineral bone disease in elderly chronic kidney disease patients. BMC Nephrol. 2012, 13, 122. [Google Scholar] [CrossRef] [Green Version]
- Osteoporotic Fractures in Men (MrOS) Study Group; Lane, N.E.; Parimi, N.; Corr, M.; Yao, W.; Cauley, J.A.; Nielson, C.M.; Ix, J.H.; Kado, D.; Orwoll, E. Association of serum fibroblast growth factor 23 (fgf23) and incident fractures in older men: The osteoporotic fractures in men (mros) study. J. Bone Miner. Res. 2013, 28, 2325–2332. [Google Scholar]
- Jovanovich, A.; Bùžková, P.; Chonchol, M.; Robbins, J.; Fink, H.A.; De Boer, I.H.; Kestenbaum, B.; Katz, R.; Carbone, L.; Lee, J. Fibroblast growth factor 23, bone mineral density, and risk of hip fracture among older adults: The cardiovascular health study. J. Clin. Endocrinol. Metab. 2013, 98, 3323–3331. [Google Scholar] [CrossRef]
- Li, X.; Zhang, Y.; Kang, H.; Liu, W.; Liu, P.; Zhang, J.; Harris, S.E.; Wu, D. Sclerostin binds to lrp5/6 and antagonizes canonical wnt signaling. J. Biol. Chem. 2005, 280, 19883–19887. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Florio, M.; Gunasekaran, K.; Stolina, M.; Li, X.; Liu, L.; Tipton, B.; Salimi-Moosavi, H.; Asuncion, F.J.; Li, C.; Sun, B. A bispecific antibody targeting sclerostin and dkk-1 promotes bone mass accrual and fracture repair. Nat. Commun. 2016, 7, 11505. [Google Scholar] [CrossRef] [PubMed]
- Recker, R.R.; Benson, C.T.; Matsumoto, T.; Bolognese, M.A.; Robins, D.A.; Alam, J.; Chiang, A.Y.; Hu, L.; Krege, J.H.; Sowa, H. A randomized, double-blind phase 2 clinical trial of blosozumab, a sclerostin antibody, in postmenopausal women with low bone mineral density. J. Bone Miner. Res. 2015, 30, 216–224. [Google Scholar] [CrossRef] [PubMed]
- Sheng, Z.; Tong, D.; Ou, Y.; Zhang, H.; Zhang, Z.; Li, S.; Zhou, J.; Zhang, J.; Liao, E. Serum sclerostin levels were positively correlated with fat mass and bone mineral density in central south chinese postmenopausal women. Clin. Endocrinol. 2012, 76, 797–801. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Zhang, H.; Wang, C.; Zhang, Z.; Yue, H.; Hu, W.; Gu, J.; Fu, W.; Hu, Y.; Li, M. Associations of serum sclerostin and polymorphisms in the sost gene with bone mineral density and markers of bone metabolism in postmenopausal chinese women. J. Clin. Endocrinol. Metab. 2014, 99, E665–E673. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lim, Y.; Kim, C.H.; Lee, S.-Y.; Kim, H.; Ahn, S.H.; Lee, S.H.; Koh, J.-M.; Rhee, Y.; Baek, K.H.; Min, Y.-K. Decreased plasma levels of sclerostin but not dickkopf-1 are associated with an increased prevalence of osteoporotic fracture and lower bone mineral density in postmenopausal korean women. Calcif. Tissue Int. 2016, 99, 350–359. [Google Scholar] [CrossRef]
- Polyzos, S.; Anastasilakis, A.; Bratengeier, C.; Woloszczuk, W.; Papatheodorou, A.; Terpos, E. Serum sclerostin levels positively correlate with lumbar spinal bone mineral density in postmenopausal women—The six-month effect of risedronate and teriparatide. Osteoporos. Int. 2012, 23, 1171–1176. [Google Scholar] [CrossRef]
- Urano, T.; Shiraki, M.; Ouchi, Y.; Inoue, S. Association of circulating sclerostin levels with fat mass and metabolic disease—Related markers in japanese postmenopausal women. J. Clin. Endocrinol. Metab. 2012, 97, E1473–E1477. [Google Scholar] [CrossRef] [Green Version]
- The Study of Osteoporotic Fractures Research Group; Arasu, A.; Cawthon, P.M.; Lui, L.-Y.; Do, T.P.; Arora, P.S.; Cauley, J.A.; Ensrud, K.E.; Cummings, S.R. Serum sclerostin and risk of hip fracture in older caucasian women. J. Clin. Endocrinol. Metab. 2012, 97, 2027–2032. [Google Scholar] [CrossRef] [Green Version]
- Ardawi, M.S.M.; Rouzi, A.A.; Al-Sibiani, S.A.; Al-Senani, N.S.; Qari, M.H.; Mousa, S.A. High serum sclerostin predicts the occurrence of osteoporotic fractures in postmenopausal women: The center of excellence for osteoporosis research study. J. Bone Miner. Res. 2012, 27, 2592–2602. [Google Scholar] [CrossRef]
- Szulc, P.; Boutroy, S.; Vilayphiou, N.; Schoppet, M.; Rauner, M.; Chapurlat, R.; Hamann, C.; Hofbauer, L.C. Correlates of bone microarchitectural parameters and serum sclerostin levels in men: The strambo study. J. Bone Miner. Res. 2013, 28, 1760–1770. [Google Scholar] [CrossRef] [PubMed]
- Kalem, M.N.; Kalem, Z.; Akgun, N.; Bakırarar, B. The relationship between postmenopausal women’s sclerostin levels and their bone density, age, body mass index, hormonal status, and smoking and consumption of coffee and dairy products. Arch. Gynecol. Obstet. 2017, 295, 785–793. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Xu, X.-J.; Shen, L.; Yang, Y.-P.; Zhu, R.; Shuai, B.; Zhu, X.-W.; Li, C.-G.; Ma, C.; Lv, L. Association of serum dkk-1 levels with β-catenin in patients with postmenopausal osteoporosis. J. Huazhong. Univ. Sci. Technol. Med. Sci. 2015, 35, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Luque-Fernandez, I.; Garcia-Martin, A.; Reyes-Garcia, R.; Morales-Santana, S.; Garcia-Fontana, B.; Munoz-Torres, M. Usefulness of Serum Sclerostin as a Diagnostic Marker of Osteoporosis in a Cohort of Spanish Postmenopausal Women; 16th European Congress of Endocrinology; BioScientifica: North Bristol, UK, 2014. [Google Scholar]
- Suarjana, I.N.; Isbagio, H.; Soewondo, P.; Rachman, I.A.; Sadikin, M.; Prihartono, J.; Malik, S.G.; Soeroso, J. The role of serum expression levels of microrna-21 on bone mineral density in hypostrogenic postmenopausal women with osteoporosis: Study on level of rankl, opg, tgfβ-1, sclerostin, rankl/opg ratio, and physical activity. Acta Med. Indones. 2019, 51, 245–252. [Google Scholar] [PubMed]
- Garnero, P.; Sornay-Rendu, E.; Munoz, F.; Borel, O.; Chapurlat, R. Association of serum sclerostin with bone mineral density, bone turnover, steroid and parathyroid hormones, and fracture risk in postmenopausal women: The ofely study. Osteoporos. Int. 2013, 24, 489–494. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Grisanti, M.; Fan, W.; Asuncion, F.J.; Tan, H.L.; Dwyer, D.; Han, C.Y.; Yu, L.; Lee, J.; Lee, E. Dickkopf-1 regulates bone formation in young growing rodents and upon traumatic injury. J. Bone Miner. Res. 2011, 26, 2610–2621. [Google Scholar] [CrossRef]
- Colditz, J.; Thiele, S.; Baschant, U.; Niehrs, C.; Bonewald, L.F.; Hofbauer, L.C.; Rauner, M. Postnatal skeletal deletion of dickkopf-1 increases bone formation and bone volume in male and female mice, despite increased sclerostin expression. J. Bone Miner. Res. 2018, 33, 1698–1707. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Shi, Y.; Regan, J.; Karuppaiah, K.; Ornitz, D.M.; Long, F. Osx-cre targets multiple cell types besides osteoblast lineage in postnatal mice. PLoS ONE 2014, 9, e85161. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.S.; Lee, H.E.; Park, D.J.; Kim, H.-H.; Kim, W.H.; Park, K.U. Clinical significance of serum and tissue dickkopf-1 levels in patients with gastric cancer. Clin. Chim. Acta 2012, 413, 1753–1760. [Google Scholar] [CrossRef]
- Butler, J.S.; Murray, D.W.; Hurson, C.J.; O’Brien, J.; Doran, P.P.; O’Byrne, J.M. The role of dkk1 in bone mass regulation: Correlating serum dkk1 expression with bone mineral density. J. Orthop. Res. 2011, 29, 414–418. [Google Scholar] [CrossRef]
- Hajialilo, M.; Rameshknia, V.; Ghorbanihaghjo, A.; Rashtchizadeh, N.; Khabbazi, A.; Kolahi, S.; Jafari-Nakhjavani, M.R.; Yousefi, B.; Raeisi, S.; Shahidi, M. Dickkopf-1 levels and serum osteoprotegerin in women with osteoporosis. J. Anal. Res. Clin. Med. 2013, 1, 68–74. [Google Scholar]
- Dovjak, P.; Dorfer, S.; Föger-Samwald, U.; Kudlacek, S.; Marculescu, R.; Pietschmann, P. Serum levels of sclerostin and dickkopf-1: Effects of age, gender and fracture status. Gerontology 2014, 60, 493–501. [Google Scholar] [CrossRef] [PubMed]
- Mödder, U.I.; Hoey, K.A.; Amin, S.; McCready, L.K.; Achenbach, S.J.; Riggs, B.L.; Melton, L.J., III; Khosla, S. Relation of age, gender, and bone mass to circulating sclerostin levels in women and men. J. Bone Miner. Res. 2011, 26, 373–379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Ref | Study Design | Sample Population | Association with BMD | Difference between Groups | Association with OF | Difference between OF and Non-OF | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| FGF23 | SOST | DKK1 | FGF23 | SOST | DKK1 | FGF23 | SOST | DKK1 | FGF23 | SOST | DKK1 | |||
| [4] | Cross-sectional study | 82 German patients with osteoporosis (66 women and 16 men). Mean age was 64 years. | Nil a,d | |||||||||||
| [21] | Cross-sectional study | 272 older men and women (69–81 years) and 171 younger men and women (18–30 years) in five European countries. | Nil | + in old group e | + in old, young, all e | Nil (old vs. young) and male (male vs. female) | ↑ in old (old vs. young) and male (male vs. female) | ↑ in old (old vs. young) | ||||||
| [22] | Prospective cohort study | 2786 elderly population, Caucasian and African American men and women, aged 70–79 years. | Nil d | Nil | ||||||||||
| [24] | Cross-sectional study | 1631 Caucasian and 296 African American women, aged 20–55 years. | Nil a,b | |||||||||||
| [25] | Cross-sectional study | 355 postmenopausal Chinese women (aged 62.92 + 8.78 years). | Nil a | Nil * | ||||||||||
| [26] | Case–control | 28 Turkish postmenopausal osteoporosis (PMO), 32 postmenopausal osteopenia, 30 controls. | BMD ↓ in PMO | ↑ In PMO (compare to osteopenian and controls) | ||||||||||
| [28] | Prospective cohort study | 2868 Swedish older men (75.4 ± 3.2 years). | ↑ OF § | |||||||||||
| [30] | Prospective cohort study | 1772 older men in the US, 90% Caucasian, mean age 73 years. | ↑ in OF † | |||||||||||
| [31] | Prospective cohort study | 2008 women and 1329 men, Caucasian and African American (16%), older population, aged 78 ± 5 years. | + in men a,d | ↓ in men (men vs. women) | Nil (in men or women) | |||||||||
| [35] | Cross-sectional study | 260 postmenopausal Chinese women (50–76 years). | + a,b,d,e | ↓ in PMO (PMO vs. PMNO) | ||||||||||
| [36] | Cross-sectional study | 703 Chinese postmenopausal women (50–94.5 years). | + a,b,d | |||||||||||
| [37] | Case–control study | 103 osteoporotic fracture cases and 103 controls of postmenopausal women (62.8 + 6.1 years in each group). | + a,b,c,d | Nil a,b,c,d | ↑ in OF as SOST ↓ | Nil | ↓ in OF § | Nil | ||||||
| [38] | Interventional study | 49 Greek Postmenopausal women (50–80 years). | + a | |||||||||||
| [39] | Cross-sectional study | 352 postmenopausal Japanese women (65.5 + 9.3 years). | + a | |||||||||||
| [40] | Prospective case–control study | Caucasian women aged at least 65 years old (228 hip fractures, 204 controls). | + d | Nil (case vs. control) | ↑ in OF | |||||||||
| [41] | Prospective cohort study | 707 Arabic postmenopausal women (53–91 years). | + e | ↑ in OF | ||||||||||
| [42] | Cross-sectional study | 1134 French men (20–87 years). | + a,d,e | ↓ in OF | ||||||||||
| [43] | Cross-sectional study | 135 Turkish postmenopausal women (68.1 + 9.5 years). | − a,b | |||||||||||
| [44] | Case–control study | 500 (350 postmenopausal Chinese women with osteoporosis and 150 controls), mean age 58.6 years. | − in PMO a | ↓ in PMO (PMO vs. control) | ↑ in PMO (PMO vs. control) | |||||||||
| [45] | Cross-sectional study | 97 postmenopausal Spanish women (41–83 years). | Nil | ↓ in PMO (PMO vs. PMNO) | Nil | |||||||||
| [46] | Case–control study | 120 Indonesian postmenopausal women (60 with osteoporosis and 60 without), aged between 55–70 years. | Nil in PMO and PMNO | ↑ in PMO (PMO vs. PMNO) | ||||||||||
| [47] | Prospective cohort study | 572 French postmenopausal women (67 + 8.5 years). | + a,d | Nil | ||||||||||
| [52] | Case–control study | 18 patients and 18 controls, Irish men and women (48.2 + 19.8 years). | − a,b | ↑ in OP(OP vs. control) | ||||||||||
| [53] | Case–control study | 88 (44 patients and 44 controls) Iranian postmenopausal women (mean age, 59 years). | − b | ↑ in PMO(PMO vs. control) | ||||||||||
| [54] | Case–control study | 256 Austrian older patients (≥50 years) and 67 young controls (<50 years), men and women. | ↑ in men (men vs. women) | ↓ in men, ↑ older | ↓ in OF ‡ | ↑ in OF ‡ | ||||||||
| [55] | Cross-sectional | 362 women and 318 men, mainly Caucasian (98%), aged 21–97 years. | + a,*** | |||||||||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramli, F.F.; Chin, K.-Y. A Review of the Potential Application of Osteocyte-Related Biomarkers, Fibroblast Growth Factor-23, Sclerostin, and Dickkopf-1 in Predicting Osteoporosis and Fractures. Diagnostics 2020, 10, 145. https://doi.org/10.3390/diagnostics10030145
Ramli FF, Chin K-Y. A Review of the Potential Application of Osteocyte-Related Biomarkers, Fibroblast Growth Factor-23, Sclerostin, and Dickkopf-1 in Predicting Osteoporosis and Fractures. Diagnostics. 2020; 10(3):145. https://doi.org/10.3390/diagnostics10030145
Chicago/Turabian StyleRamli, Fitri Fareez, and Kok-Yong Chin. 2020. "A Review of the Potential Application of Osteocyte-Related Biomarkers, Fibroblast Growth Factor-23, Sclerostin, and Dickkopf-1 in Predicting Osteoporosis and Fractures" Diagnostics 10, no. 3: 145. https://doi.org/10.3390/diagnostics10030145