No Tail No Fail: Life Cycles of the Zoogonidae (Digenea)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sampling
2.2. Morphological Analysis
2.3. Molecular Analysis
2.4. Visualization
3. Results
3.1. General Observations
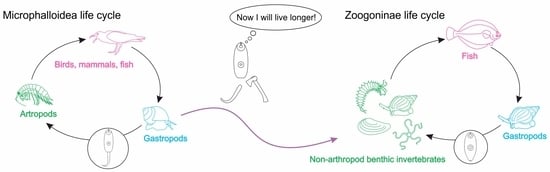
3.2. Molecular Data and Life Cycles Elucidation
3.3. Phylogenetic Position of Pseudozoogonoides subaequiporus
3.4. Infection Data and Morphological Descriptions
3.4.1. Maritae of Zoogonoides viviparus
3.4.2. Metacercariae of Zoogonoides viviparus
3.4.3. Sporocysts, Developing and Infective Cercariae of Zoogonoides viviparus (Figure 3A–C and Figure 4)

3.4.4. Maritae of Pseudozoogonoides subaequiporus
3.4.5. Metacercariae of Pseudozoogonoides subaequiporus (Figure 5)

3.4.6. Sporocysts, Developing and Infective Cercariae of Pseudozoogonoides subaequiporus (Figure 3D–F and Figure 6)


4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pearson, J.C. A phylogeny of life-cycle patterns of the Digenea. Adv. Parasitol. 1972, 10, 153–189. [Google Scholar] [PubMed]
- Cribb, T.H.; Bray, R.A.; Kudlai, P.D.; Littlewood, D.T.J. Life cycle evolution in the Digenea: A new perspective from phylogeny. Adv. Parasitol. 2003, 54, 197–254. [Google Scholar] [PubMed] [Green Version]
- Galaktionov, K.V.; Dovrovolskij, A.A. The Biology and Evolution of Trematodes: An Essay on the Biology, Morphology, Life Cycles, Transmissions, and Evolution of Digenetic Trematodes, 1st ed.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2003; 592p. [Google Scholar]
- Stunkard, H.W. The life history of Cryptocotyle lingua (Creplin), with notes on the physiology of the metacercariae. J. Morphol. 1930, 50, 143–191. [Google Scholar] [CrossRef]
- Cable, R.M. A new marine cercaria from the Woods Hole region and its bearing on the interpretation of larval types in the Fellodistomatidae (Trematoda: Digenea). Biol. Bull. 1954, 106, 15–20. [Google Scholar] [CrossRef]
- Ito, J.; Shimura, S. On a new lepocreadiid cercaria, Cercaria isoninae n. sp. (Trematoda) from a littoral gastropod, Japeuthria ferrea from Kanagawa and Chiba Prefectures, Japan. Jpn. J. Parasitol. 1980, 29, 181–187. [Google Scholar]
- Cable, R.M. Marine cercariae of Puerto Rico. Sci. Surv. Porto Rico Virgin Isl. 1956, 16, 491–577. [Google Scholar]
- Galaktionov, K.V.; Olenev, A.V.; Dobrovolskij, A.A. Two species of cyathocothylate cercariae from the freshwater snail Melanopsis praemorsa. Parazitologiia 1980, 14, 299–307. (In Russian) [Google Scholar]
- Cable, R.M.; Nahhas, F.M. Bivesicula caribbensis sp. n. (Trematoda: Digenea) and its life history. J. Parasitol. 1962, 48, 536–538. [Google Scholar] [CrossRef]
- Matthews, B.F. Cercaria vaullegeardi Pelseneer, 1906 (Digenea: Hemiuridae); the infection mechanism. Parasitology 1981, 83, 587–593. [Google Scholar] [CrossRef]
- Zelmer, D.A.; Esch, G.W. The infection mechanism of the cystophorous cercariae of Halipegus occidualis (Digenea: Hemiuridae). Invertebr. Biol. 1998, 117, 281–287. [Google Scholar] [CrossRef]
- Hunninen, A.V.; Cable, R.M. The life history of Podocotyle atomon (Rudolphi) (Trematoda: Opecoelidae). Trans. Am. Microsc. Soc. 1943, 62, 57–68. [Google Scholar] [CrossRef]
- Køie, M. On the morphology and life-history of Lepidapedon elongatum (Lebour, 1908) Nicoll, 1910 (Trematoda, Lepocreadiidae). Ophelia 1985, 24, 135–153. [Google Scholar] [CrossRef]
- Bartoli, P.; Jousson, O.; Russell-Pinto, F. The life cycle of Monorchis parvus (Digenea: Monorchiidae) demonstrated by developmental and molecular data. J. Parasitol. 2000, 86, 479–489. [Google Scholar] [CrossRef] [PubMed]
- Galaktionov, K.V.; Blasco-Costa, I. Microphallus ochotensis sp. nov. (Digenea, Microphallidae) and relative merits of two-host microphallid life cycles. Parasitol. Res. 2018, 117, 1051–1068. [Google Scholar] [CrossRef] [PubMed]
- Kremnev, G.; Gonchar, A.; Krapivin, V.; Uryadova, A.; Miroliubov, A.; Krupenko, D. Life cycle truncation in Digenea, a case study of Neophasis spp. (Acanthocolpidae). Int. J. Parasitol. Parasites Wildl. 2021, 15, 158–172. [Google Scholar] [CrossRef]
- Bray, R.A.; Justine, J.L. A review of the Zoogonidae (Digenea: Microphalloidea) from fishes of the waters around New Caledonia, with the description of Overstreetia cribbi n. sp. PeerJ 2014, 2, e292. [Google Scholar] [CrossRef] [Green Version]
- Miller Jr, H.M.; Northup, F.E. The seasonal infestation of Nassa obsoleta (Say) with larval trematodes. Biol. Bull. 1926, 50, 490–508. [Google Scholar] [CrossRef]
- Køie, M. On the morphology and life-history of Zoogonoides viviparus (Olsson, 1868) Odhner, 1902 (Trematoda, Zoogonidae). Ophelia 1976, 15, 1–14. [Google Scholar]
- Palombi, A. Il ciclo biologico di Diphterostomum brusinae Stossich (Trematode digenetico: Fam. Zoogonidae Odhner). Considerazioni sui cicli evolutivi dell especie affini e dei trematodi in generale. Pubbl. Stn. Zool. Napoli 1930, 10, 111–149. (In Italian) [Google Scholar]
- Stunkard, H.W. Distomum lasium Leidy, 1891 (syn. Cercariaeum lintoni Miller and Northup, 1926), the larval stage of Zoogonus rubellus (Olsson, 1868) (syn. Z. mirus Looss, 1901). Biol. Bull. 1938, 75, 308–334. [Google Scholar] [CrossRef]
- Stunkard, H.W. The morphology and life history of the digenetic trematode, Zoogonoides laevis Linton, 1940. Biol. Bull. 1943, 85, 227–237. [Google Scholar] [CrossRef]
- Gilardoni, C.; Etchegoin, J.; Cribb, T.; Pina, S.; Rodrigues, P.; Diez, M.E.; Cremonte, F. Cryptic speciation of the zoogonid digenean Diphterostomum flavum n. sp. demonstrated by morphological and molecular data. Parasite 2020, 27, PMC7301638. [Google Scholar] [CrossRef]
- Pina, S.; Tajdari, J.; Russell-Pinto, F.; Rodrigues, P. Morphological and molecular studies on life cycle stages of Diphtherostomum brusinae (Digenea: Zoogonidae) from northern Portugal. J. Helminthol. 2009, 83, 321–331. [Google Scholar] [CrossRef]
- Bray, R.A. Digenean parasites of deep-sea teleosts: A progress report. Int. J. Parasitol. Parasites Wildl. 2020, 12, 251–264. [Google Scholar] [CrossRef] [PubMed]
- Kremnev, G.; Gonchar, A.; Krapivin, V.; Knyazeva, O.; Krupenko, D. First elucidation of the life cycle in the family Brachycladiidae (Digenea), parasites of marine mammals. Int. J. Parasitol. 2020, 50, 997–1009. [Google Scholar] [CrossRef] [PubMed]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, S.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [Green Version]
- Stamatakis, A. RAxML version 8: A tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 2014, 30, 1312–1313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ronquist, F.; Teslenko, M.; Van Der Mark, P.; Ayres, D.L.; Darling, A.; Höhna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Darriba, D.; Taboada, G.L.; Doallo, R.; Posada, D. jModelTest 2: More models, new heuristics and parallel computing. Nat. Methods 2012, 9, 772. [Google Scholar] [CrossRef] [Green Version]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular evolutionary genetics analysis version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Hernández-Mena, D.I.; García-Varela, M.; de León, G.P.P. Filling the gaps in the classification of the Digenea Carus, 1863: Systematic position of the Proterodiplostomidae Dubois, 1936 within the superfamily Diplostomoidea Poirier, 1886, inferred from nuclear and mitochondrial DNA sequences. Syst. Parasitol. 2017, 94, 833–848. [Google Scholar] [CrossRef] [PubMed]
- Tkach, V.; Grabda-Kazubska, B.; Pawlowski, J.; Swiderski, Z. Molecular and morphological evidence for close phylogenetic affinities of the genera Macrodera, Leptophallus, Metaleptophallus and Paralepoderma [Digenea, Plagiorchiata]. Acta Parasitol. 1999, 44, 170–179. [Google Scholar]
- Olson, P.D.; Cribb, T.H.; Tkach, V.V.; Bray, R.A.; Littlewood, D.T.J. Phylogeny and classification of the Digenea (Platyhelminthes: Trematoda). Int. J. Parasitol. 2003, 33, 733–755. [Google Scholar] [CrossRef]
- Morgan, J.A.T.; Blair, D. Nuclear rDNA ITS sequence variation in the trematode genus Echinostoma: An aid to establishing relationships within the 37-collar-spine group. Parasitology 1995, 111, 609–615. [Google Scholar] [CrossRef] [PubMed]
- Lebour, M.V. A trematode larva from Buccinum undatum and notes on trematodes from post-larval fish. J. Mar. Biol. Assoc. UK 1918, 11, 514–518. [Google Scholar] [CrossRef] [Green Version]
- Cutmore, S.C.; Miller, T.L.; Bray, R.A.; Cribb, T.H. A new species of Plectognathotrema Layman, 1930 (Trematoda: Zoogonidae) from an Australian monacanthid, with a molecular assessment of the phylogenetic position of the genus. Syst. Parasitol. 2014, 89, 237–246. [Google Scholar] [CrossRef] [PubMed]
- Sokolov, S.; Gordeev, I.; Lebedeva, D. Redescription of Proctophantastes gillissi (Overstreet et Pritchard, 1977) (Trematoda: Zoogonidae) with discussion on the systematic position of the genus Proctophantastes Odhner, 1911. Acta Parasitol. 2016, 61, 529–536. [Google Scholar] [CrossRef]
- Sokolov, S.; Shchenkov, S.; Gordeev, I.; Ryazanova, T. Description of a metacercaria of a zoogonid trematode Steganoderma cf. eamiqtrema Blend and Racz, 2020 (Microphalloidea: Zoogonidae), with notes on the phylogenetic position of the genus Steganoderma Stafford, 1904, and resurrection of the subfamily Lecithostaphylinae Odhner, 1911. Parasitol. Res. 2021, 120, 1669–1676. [Google Scholar]
- Atopkin, D.M.; Besprozvannykh, V.V.; Ha, N.D.; Nguyen, H.V.; Nguyen, T.V. New trematode species Lecithostaphylus halongi n. sp. (Zoogonidae, Microphalloidea) and Gymnotergestia strongyluri n. sp. (Fellodistomidae, Gymnophalloidea) from beloniform fishes in Vietnam. J. Helminthol. 2022, 96, 529–536. [Google Scholar] [CrossRef]
- Cutmore, S.C.; Bray, R.A.; Cribb, T.H. Two new species of Bacciger Nicoll, 1914 (Trematoda: Faustulidae) in species of Herklotsichthys Whitley (Clupeidae) from Queensland waters. Syst. Parasitol. 2018, 95, 645–654. [Google Scholar] [CrossRef]
- Sokolov, S.G.; Shchenkov, S.V.; Gordeev, I.I. A phylogenetic assessment of Pronoprymna spp. (Digenea: Faustulidae) and Pacific and Antarctic representatives of the genus Steringophorus Odhner, 1905 (Digenea: Fellodistomidae), with description of a new species. J. Nat. Hist. 2021, 55, 867–887. [Google Scholar] [CrossRef]
- Curran, S.S.; Warren, M.B.; Bullard, S.A. Description of a New Species of Bacciger (Digenea: Gymnophalloidea) Infecting the American Gizzard Shad, Dorosoma cepedianum (Lesueur, 1818), and Molecular Characterization of Cercaria rangiae Wardle, 1983, with Molecular Phylogeny of Related Digenea. Comp. Parasitol. 2022, 89, 9–29. [Google Scholar] [CrossRef]
- Bray, R.A.; Gibson, D.I. The Zoogonidae (Digenea) of fishes from the northeast Atlantic. Bull. Br. Mus. Nat. Hist. Zool. 1986, 51, 127–206. [Google Scholar]
- Orrhage, L. Description of the Metacercaria of Zoogonoides viviparus (Olsson, 1868) Odhner, 1902 with Some Remarks on Life Cycles in the Genus Zoogonoides (Trematoda, Digenea, Zoogonidae). Zool. Scr. 1974, 2, 179–182. [Google Scholar] [CrossRef]
- Køie, M. On the histochemistry and ultrastructure of the tegument and associated structures of the cercaria of Zoogonoides viviparus in the first intermediate host. Ophelia 1971, 9, 165–206. [Google Scholar] [CrossRef]
- Zhukov, E.V. Endoparasitic worms of the fishes in the Sea of Japan and South-Kuril shallow-waters. Tr. Zool. Inst. 1960, 28, 3–146. (In Russian) [Google Scholar]
- Scott, J.S. Incidence of trematode parasites of American plaice (Hippoglossoides platessoides) of the Scotian Shelf and Gulf of St. Lawrence in relation to fish length and food. Can. J. Fish Aquat. Sci. 1975, 32, 479–483. [Google Scholar] [CrossRef]
- Scott, J.S. Digenean parasite communities in flatfishes of the Scotian Shelf and southern Gulf of St. Lawrence. Can. J. Zool. 1982, 60, 2804–2811. [Google Scholar] [CrossRef]
- Sirenko, B.I. List of Species of Free-Living Invertebrates of Eurasian Arctic Seas and Adjacent Deep Waters; Russian Academy of Science, Zoological Institute: Saint-Petersburg, Russia, 2001; 134p. [Google Scholar]
- Bray, R.A. A study of the helminth parasites of Anarhichas lupus (Perciformes: Anarhichadidae) in the North Atlantic. J. Fish Biol. 1987, 31, 237–264. [Google Scholar] [CrossRef]
- Falk-Petersen, I.B.; Kanapathippilai, P.; Primicerio, R.; Hansen, T.K. Size, locality and seasonally related feeding preferences of common wolffish (Anarhichas lupus L.) from north-Norwegian waters. Mar. Biol. Res. 2010, 6, 201–212. [Google Scholar] [CrossRef]
- Shulman-Albova, R.E. Fish parasites of the White Sea around the village of Gridina. Part I. Monogenetic and digenetic trematodes. Uchenye Zap. Karelo-Fin. Gos. Univ. 1952, 4, 78–97. (In Russian) [Google Scholar]
- Barnett, L.J.; Miller, T.L.; Cribb, T.H. A review of the currently recognised zoogonid cercariae, including the identification and emergence ecology of Cercaria capricornia XI (Digenea: Zoogonidae) from Nassarius olivaceus (Gastropoda: Nassariidae) in Central Queensland, Australia. Folia Parasitol. 2014, 61, 322–330. [Google Scholar] [CrossRef]
- Pelseneer, P. Trematodes parasites de mollusques marins. Bull. Biol. Fr. Belg. 1906, 40, 161–186. (In French) [Google Scholar]
- Galaktionov, K.V.; Blasco-Costa, I.; Olson, P.D. Life cycles, molecular phylogeny and historical biogeography of the ‘pygmaeus’ microphallids (Digenea: Microphallidae): Widespread parasites of marine and coastal birds in the Holarctic. Parasitology 2012, 139, 1346–1360. [Google Scholar] [CrossRef] [Green Version]
- Galaktionov, K.V.; Malkova, I.I. The glands of trematode cercariae of the family Microphallidae Travassos, 1920. Int. J. Parasitol. 1994, 24, 595–604. [Google Scholar] [CrossRef]
- Shchenkov, S.V.; Denisova, S.A.; Smirnova, A.D.; Shunatova, N.N. Mucoid glands of cercariae. Invert Zool. 2019, 16, 377–392. (In Russian) [Google Scholar] [CrossRef]
- Krupenko, D.; Gonchar, A.; Kremnev, G.; Efeykin, B.; Krapivin, V. New type of xiphidiocercariae (Digenea: Microphalloidea) from South Vietnam. Folia Parasitol. 2020, 67, 1–6. [Google Scholar] [CrossRef]
- Shimura, S.; Ito, J. Two new cercariae, Cercaria brachycaeca n. sp. and Cercaria misakiana n. sp. from top shells, Batillus cornutus and Marmarostoma stenogyrum, with notes of their effects on the hosts. Jpn. J. Parasitol. 1980, 29, 69–76. [Google Scholar]
- Selbach, C.; Poulin, R. Some like it hotter: Trematode transmission under changing temperature conditions. Oecologia 2020, 194, 745–755. [Google Scholar] [CrossRef]
- Ulmer, M.J. Postharmostomum helicis (Leidy, 1847) Robinson 1949, (Trematoda), Its Life History and a Revision of the Subfamily Brachylaeminae: Part I. Trans. Am. Microsc. Soc. 1951, 70, 189–238. [Google Scholar] [CrossRef]
- González-Moreno, O.; Gracenea, M. Life cycle and description of a new species of brachylaimid (Trematoda: Digenea) in Spain. J. Parasitol. 2006, 92, 1305–1312. [Google Scholar] [CrossRef]
- Chernogorenko, M.I.; Komarova, T.I.; Kurandina, D.P. The life cycle of the trematode Plagioporus skrjabini Kgwal, 1951 (Allocreadiata, Opecoelidae). Parazitologiia 1978, 12, 479–486. (In Russian) [Google Scholar]
- Køie, M. On the morphology and life-history of Podocotyle reflexa (Creplin, 1825) Odhner, 1905, and a comparison of its developmental stages with those of P. atomon (Rudolphi, 1802) Odhner, 1905 (Trematoda, Opecoelidae). Ophelia 1981, 20, 17–43. [Google Scholar] [CrossRef]
- Prokofiev, V.V. Influence of temperature and salinity on a life span of cercariae of marine litoral trematodes Cryptocotyle sp. (Heterophyidae), Levinseniella brachysoma and Maritrema subdolum (Microphallidae). Parazitologiia 1999, 33, 520–526. [Google Scholar]
- Shaw, C.R. Observations on Cercariaeum lintoni Miller and Northup and its metacercarial development. Biol. Bull. 1933, 64, 262–275. [Google Scholar] [CrossRef]
- Madhavi, R.; Shameem, U. Cercaria chilkaensis II, a new zoogonid cercaria from the snail, Nassarius orissaensis, from Chilka Lake, India. Proc. Helminthol. Soc. Wash. 1991, 58, 31–34. [Google Scholar]
- Gilardoni, C.; Etchegoin, J.; Diaz, J.I.; Ituarte, C.; Cremonte, F. A survey of larval digeneans in the commonest intertidal snails from Northern Patagonian coast, Argentina. Acta Parasitol. 2011, 56, 163–179. [Google Scholar] [CrossRef] [Green Version]
- Køie, M. On the endoparasites of Buccinum undatum L. with special reference to the trematodes. Ophelia 1969, 6, 251–279. [Google Scholar] [CrossRef]
- Lebour, M.V. A review of the British marine cercariae. Parasitology 1911, 4, 416–456. [Google Scholar] [CrossRef]
- Stunkard, H.W. Specificity and host-relations in the trematode genus Zoogonus. Biol. Bull. 1941, 81, 205–214. [Google Scholar] [CrossRef]
- Prévot, G. Sur deux trématodes larvaires d’Antedon mediterranea Lmk. (Echinoderme): Metacercaria sp. (Monorchiidae Odhner, 1911), et métacercaire de Diphterostomum brusinae Stoss., 1904 (Zoogonidae Odhner, 1911). Ann. Parasitol. Hum. Comp. 1966, 41, 233–242. (In French) [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Francisco, C.J.; Almeida, A.; Castro, A.M.; Pina, S.; Russell-Pinto, F.; Rodrigues, P.; Santos, M.J. Morphological and molecular analysis of metacercariae of Diphtherostomum brusinae (Stossich, 1888) Stossich, 1903 from a new bivalve host Mytilus galloprovincialis. J. Helminthol. 2011, 85, 179–184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martorelli, S.R.; Montes, M.M.; Marcotegui, P.S.; Alda, M.D.P. Primer registro de Diphterostomum brusinae (Digenea, Zoogonidae) parasitando a la corvina Micropogonias furnieri con datos sobre su ciclo biológico. Rev. Argent. Parasitol. 2014, 2, 22–27. (In Spanish) [Google Scholar]
- Uspenskaya, A.V. Parasitic Fauna of Benthic Crustaceans from the Barents Sea; Academia Nauk SSSR, Kolskij Filial: Moskva, Russia, 1963; 128p. (In Russian)
- Kagei, N.; Kon, T. Metacercariae of genus Steganoderma (Trematoda) in a crab, Chionoecetes opilio (O. Fabricius), from Northern-Sea. Jpn. J. Parasitol. 1978, 27, 27–30. [Google Scholar]
- Holton, A.L. Observations on the life history of Deretrema minutum Manter, 1954 (Digenea: Zoogonidae) in freshwater crustacean and fish hosts from Canterbury, New Zealand. N. Z. J. Mar. Freshw. Res. 1983, 17, 373–376. [Google Scholar] [CrossRef] [Green Version]
- Marcogliese, D.J. Evidence for specificity of Steganoderma formosum for its second intermediate host in the Northwest Atlantic. J. Helminthol. 1996, 70, 215–218. [Google Scholar] [CrossRef]
- Abdul-Salam, J.; Sreelatha, B.S. Studies on Cercariae from Kuwait Bay. IX. Description and surface topography of Cercaria kuwaitae IX sp. n. (Digenea: Zoogonidae). J. Helminthol. Soc. Wash. 1998, 65, 141–146. [Google Scholar]
- Briscoe, A.G.; Bray, R.A.; Brabec, J.; Littlewood, D.T.J. The mitochondrial genome and ribosomal operon of Brachycladium goliath (Digenea: Brachycladiidae) recovered from a stranded minke whale. Parasitol. Int. 2016, 65, 271–275. [Google Scholar] [CrossRef]
- Tkach, V.V.; Snyder, S.D. Choanocotyle platti sp. nov. from the northern long-necked turtle, Chelodina rugosa (Pleurodira, Chelidae) in Australia. Acta Parasitol. 2007, 52, 318–324. [Google Scholar] [CrossRef] [Green Version]
- Heneberg, P.; Literák, I. Molecular phylogenetic characterization of Collyriclum faba with reference to its three host-specific ecotypes. Parasitol. Int. 2013, 62, 262–267. [Google Scholar] [CrossRef]
- Unwin, S.; Chantrey, J.; Chatterton, J.; Aldhoun, J.A.; Littlewood, D.T.J. Renal trematode infection due to Paratanaisia bragai in zoo housed Columbiformes and a red bird-of-paradise (Paradisaea rubra). Int. J. Parasitol. Parasites Wildl. 2013, 2, 32–41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shchenkov, S.V.; Denisova, S.A.; Kremnev, G.A.; Dobrovolskij, A.A. Five new morphological types of virgulate and microcotylous xiphidiocercariae based on morphological and molecular phylogenetic analyses. J. Helminthol. 2020, 94, E94. [Google Scholar] [CrossRef]
- Cribb, T.H.; Bray, R.A.; Hall, K.A.; Cutmore, S.C. A review of the genus Antorchis Linton, 1911 (Trematoda: Faustulidae) from Indo-Pacific fishes with the description of a new species. Syst. Parasitol. 2015, 92, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Tkach, V.; Pawlowski, J.; Mariaux, J.; Świderski, Z. Molecular phylogeny of the suborder Plagiorchiata and its position in the system of Digenea. In Interrelationships of the Platyhelminthes; Littlewood, D.T.J., Bray, R.A., Eds.; Taylor and Francis: London, UK, 2001; pp. 186–193. [Google Scholar]
- Bell, J.A.; González-Acuña, D.; Tkach, V.V. First record of Gyrabascus (Digenea, Pleurogenidae) from Dromiciops bozinovici D’Elia et al., 2016 (Marsupialia: Microbiotheriidae) in Chile and its phylogenetic relationships. Comp. Parasitol. 2018, 85, 58–65. [Google Scholar] [CrossRef]
- Kasl, E.L.; Font, W.F.; Criscione, C.D. Resolving evolutionary changes in parasite life cycle complexity: Molecular phylogeny of the trematode genus Alloglossidium indicates more than one origin of precociousness. Mol. Phylogenet. Evol. 2018, 126, 371–381. [Google Scholar] [CrossRef]
- Kudlai, O.; Cribb, T.H.; Cutmore, S.C. A new species of microphallid (Trematoda: Digenea) infecting a novel host family, the Muraenidae, on the northern Great Barrier Reef, Australia. Syst. Parasitol. 2016, 93, 863–876. [Google Scholar] [CrossRef]
- Tkach, V.; Pawlowski, J.; Mariaux, J. Phylogenetic analysis of the suborder Plagiorchiata (Platyhelminthes, Digenea) based on partial lsrDNA sequences. Int. J. Parasitol. 2000, 30, 83–93. [Google Scholar] [CrossRef]
- Tkach, V.V.; Littlewood, D.T.J.; Olson, P.D.; Kinsella, J.M.; Swiderski, Z. Molecular phylogenetic analysis of the Microphalloidea Ward, 1901 (Trematoda: Digenea). Syst. Parasitol. 2003, 56, 1–15. [Google Scholar] [CrossRef]
- Tkach, V.; Grabda-Kazubska, B.; Swiderski, Z. Systematic position and phylogenetic relationships of the family Omphalometridae (Digenea, Plagiorchiida) inferred from partial lsrDNA sequences. Int. J. Parasitol. 2001, 31, 81–85. [Google Scholar] [CrossRef]
- Razo-Mendivil, U.J.; León-Règagnon, V.; de Leon, G.P.P. Monophyly and systematic position of Glypthelmins (Digenea), based on partial lsrDNA sequences and morphological evidence. Org. Divers. Evol. 2006, 6, 308–320. [Google Scholar] [CrossRef] [Green Version]
- Zikmundová, J.; Georgieva, S.; Faltýnková, A.; Soldánová, M.; Kostadinova, A. Species diversity of Plagiorchis Lühe, 1899 (Digenea: Plagiorchiidae) in lymnaeid snails from freshwater ecosystems in central Europe revealed by molecules and morphology. Syst. Parasitol. 2014, 88, 37–54. [Google Scholar] [CrossRef]
- Kanarek, G.; Zaleśny, G.; Czujkowska, A.; Sitko, J.; Harris, P.D. On the systematic position of Collyricloides massanae Vaucher, 1969 (Platyhelminthes: Digenea) with notes on distribution of this trematode species. Parasitol. Res. 2015, 114, 1495–1501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pulis, E.E.; Tkach, V.V.; Newman, R.A. Helminth parasites of the wood frog, Lithobates sylvaticus, in prairie pothole wetlands of the Northern Great Plains. Wetlands 2011, 31, 675–685. [Google Scholar] [CrossRef]
- O’Dwyer, K.; Blasco-Costa, I.; Poulin, R.; Faltýnková, A. Four marine digenean parasites of Austrolittorina spp. (Gastropoda: Littorinidae) in New Zealand: Morphological and molecular data. Syst. Parasitol. 2014, 89, 133–152. [Google Scholar] [CrossRef] [PubMed]
- Cabañas-Granillo, J.; Solórzano-García, B.; Mendoza-Garfias, B.; Pérez-Ponce de León, G. A new species of Lecithostaphylus Odhner, 1911 (Trematoda: Zoogonidae) from the Pacific needlefish, Tylosurus pacificus, off the Pacific coast of Mexico, with a molecular assessment of the phylogenetic position of this genus within the family. Mar. Biodivers. 2020, 50, 83. [Google Scholar] [CrossRef]
- Lockyer, A.E.; Olson, P.D.; Littlewood, D.T.J. Utility of complete large and small subunit rRNA genes in resolving the phylogeny of the Neodermata (Platyhelminthes): Implications and a review of the cercomer theory. Biol. J. Linn. Soc. Lond. 2003, 78, 155–171. [Google Scholar] [CrossRef] [Green Version]
- Tkach, V.V.; Greiman, S.E.; Pulis, E.E.; Brooks, D.R.; Bonilla, C.C. Phylogenetic relationships and systematic position of the enigmatic Urotrema Braun, 1900 (Platyhelminthes: Digenea). Parasitol. Int. 2019, 70, 118–122. [Google Scholar] [CrossRef]



| Host | Number of Dissected Individuals | Zoogonid Infection | ||
|---|---|---|---|---|
| Gastropoda | Buccinidae | Buccinum undatum Linnaeus, 1758 | 2174 | + |
| B. scalariforme Møller, 119 | 19 | + | ||
| Neptunea despecta (Linnaeus, 1758) | 103 | + | ||
| Naticidae | Cryptonatica affinis (Gmelin, 1791) | 270 | - | |
| Euspira pallida (Broderip & GB Sowerby I, 1829) | 24 | - | ||
| Amauropsis islandica (Gmelin, 1791) | 3 | - | ||
| Capulidae | Ariadnaria borealis (Broderip & G. B. Sowerby I, 1829) | 28 | - | |
| Margaritidae | Margarites helicinus (Phipps, 1774) | 17 | - | |
| Muricidae | Boreotrophon clavatus (G. O. Sars, 1878) | 8 | - | |
| Annelida | Nereididae | Nereis pelagica Linnaeus, 1758 | 11 | - |
| Pectinariidae | Cistenides hyperborea Malmgren, 1866 | 9 | - | |
| Nephtyidae | Nephtys sp. | 8 | + | |
| Polynoidae | Lepidonotus squamatus (Linnaeus, 1758) | 5 | - | |
| Orbiniidae | Scoloplos armiger (Müller, 1776) | 4 | - | |
| Trichobranchidae | Terebellides stroemii Sars, 1835 | 1 | - | |
| Lumbrineridae | Scoletoma fragilis (O.F. Müller, 1776) | 1 | - | |
| Bivalvia | Mytilidae | Musculus discors (Linnaeus, 1767) | 31 | - |
| Cardiidae | Serripes groenlandicus (Mohr, 1786) | 66 | + | |
| Ciliatocardium ciliatum (Fabricius, 1780) | 39 | + | ||
| Nuculanidae | Nuculana pernula (O. F. Müller, 1779) | 394 | + | |
| Yoldiidae | Yoldia hyperborea (Gould, 1841) | 78 | + | |
| Portlandia arctica (Gray, 1824) | 6 | - | ||
| Nuculidae | Ennucula tenuis (Montagu, 1808) | 5 | - | |
| Decapoda | Paguridae | Pagurus pubescens Krøyer, 1838 | 12 | - |
| Pandalidae | Pandalus montagui Leach, 1814 | 8 | - | |
| Thoridae | Eualus gaimardii (H. Milne Edwards, 1837) | 4 | - | |
| Crangonidae | Crangon crangon (Linnaeus, 1758) | 2 | - | |
| Sabinea septemcarinata (Sabine, 1824) | 1 | - | ||
| Ophiuroidea | Ophiuridae | Ophiura robusta (Ayres, 1852) | 67 | - |
| Ophiopholidae | Ophiopholis aculeata (Linnaeus, 1767) | 40 | - | |
| ID | Species | Stage | Host Species | GenBank Accession Numbers | |
|---|---|---|---|---|---|
| 28S rDNA | 5.8S rDNA+ITS2 | ||||
| G7.3 | Zoogonoides viviparus | Marita | Limanda limanda | OP956058 | OP956013 |
| G7.2 | Z. viviparus | Marita | Anarhichas lupus | OP956059 | OP956014 |
| A5.36 | Z. viviparus | Marita | L. limanda | OP956060 | OP956015 |
| G7.8 | Putative Z. viviparus | Metacercaria | Nephthys sp. | OP956061 | OP956016 |
| G7.5 | Putative Z. viviparus | Metacercaria | Nuculana pernula | OP956062 | OP956017 |
| G7.4 | Putative Z. viviparus | Metacercaria | Ciliatocardium ciliatum | OP956063 | OP956018 |
| G7.9 | Putative Z. viviparus | Sporocyst with cercariae | Buccinum undatum | OP956064 | OP956019 |
| G7.10 | Putative Z. viviparus | Sporocyst with cercariae | B. undatum | OP956065 | OP956020 |
| A5.37 | Putative Z. viviparus | Sporocyst with cercariae | B. undatum | OP956066 | OP956021 |
| A5.38 | Putative Z. viviparus | Sporocyst with cercariae | B. undatum | OP956067 | OP956022 |
| G7.6 | Pseudozoogonoides subaequiporus | Marita | A. lupus | OP956068 | OP956023 |
| G7.7 | P. subaequiporus | Marita | A. lupus | OP956069 | OP956024 |
| D9.6 | Putative P. subaequiporus | Metacercaria | N. pernula | OP956070 | OP956025 |
| D9.7 | Putative P. subaequiporus | Metacercaria | N. pernula | OP956071 | OP956026 |
| D9.4 | Putative P. subaequiporus | Sporocyst with cercariae and metacercariae | Neptunea despecta | OP956072 | OP956027 |
| D11.3 | Putative P. subaequiporus | Sporocyst with cercariae and metacercariae | B. undatum | OP956073 | OP956028 |
| G7.1 | Lepidophyllum steenstrupi | Marita | A. lupus | OP956074 | OP956029 |
| AG4.12 | Zoogonidae gen. sp. | Sporocyst with germ balls | B. scalariforme | OP956075 | OP956030 |
| Product | Primer | Sequence (5′-3′), Forward (F) and Reverse (R) | Thermocycling Profile | Reference |
|---|---|---|---|---|
| 18S rDNA | 18S1A | F, GGCGATCGAAAAGATTAAGCCATGCA | 94 °C—1 m 52 °C—1 m 72 °C—1 m ×35 | [32] primers and conditions |
| 32 | R, CGAAGTCCTATTCCATTATTC | |||
| 652 | F, GCAGCCGCGGTAATTCCAGCTC | |||
| 28 | R, AGCGACGGGCGGTGTGT | |||
| 28S rDNA | digl2 | F, AAGCATATCACTAAGCGG | 95 °C—30 s 54 °C—30 s 72 °C—2 m ×40 | [33] primers and conditions |
| 1500R | R, GCTATCCTGAGGGAAACTTCG | [34] primers and conditions | ||
| 5.8S+ITS2 | 3S | F, GTACCGGTGGATCACGTGGCTAGTG | 94 °C—30 s 55 °C—30 s 72 °C—1 m ×30 | [35] primers |
| ITS2.2 | R, CCTGGTTAGTTTCTTTTCCTCCGC |
| Zoogonoides viviparus (Maritae) n = 10 | Z. viviparus (Metacercariae) n = 6 | Z. viviparus (Cercariae) n = 11 | Pseudozoogonoides subaequiporus (Maritae) n = 12 | P. subaequiporus (Cercariae) n = 11 | |
|---|---|---|---|---|---|
| Length | 766 (665–831) | 385 (341–446) | 388 (337–451) | 747 (622–929) | 301 (248–385) |
| Width | 246 (206–281) | 115 (101–137) | 139 (125–158) | 295 (237–341) | 118 (98–136) |
| Forebody, length | 273 (227–294) | 172 (151–196) | 179 (153–242) | 255 (219–304) | 128 (96–180) |
| Length:forebody ratio | 1:0.36 (0.31–0.39) | 1:0.45 (0.40–0.50) | 1:0.46 (0.43–0.54) | 1:0.34 (0.29–0.39) | 1:0.42 (0.35–0.48) |
| Oral sucker | 114 (88–137) × 127 (102–144) | 68 (59–81) × 73 (60–99), n = 7 | 73 (63–79) × 73 (65–83) | 129 (107–152) × 130 (113–146) | 58 (50–66) × 57 (50–66) |
| Ventral sucker | 187 (168–222) × 182 (164–202) | 99 (82–114) × 94 (82–132), n = 7 | 103 (93–119) × 87 (71–98) | 138 (127–156) × 137 (121–182) | 60 (54–65) × 60 (55–66) |
| Sucker ratio | 1:1.66 (1.43–1.99) | 1:1.47 (1.33–1.68) n = 7 | 1:1.40 (1.19–1.63) | 1:1.08 (0.97–1.27) | 1:1.05 (0.92–1.24) |
| Stylet | - | - | 16 (12–20) × 5 (4–6) n = 9 | - | 13 (10–16) × 4 (3–5) n = 8 |
| Pharynx | 37 (30–55) × 47 (43–50) | 23 (18–36) × 30 (24–34), n = 7 | 23 (17–36) × 29 (26–32) | 40 (28–50) × 45 (36–53) | 19 (15–21) × 23 (21–25) |
| Esophagus | 84 (71–95) | 52 (36–77) | 61 (46–76) | 78 (44–97) | 43 (26–69) |
| Cirrus-sac | 261 (210–296) × 52 (44–65) | 95 (81–115) × 23 (19–27), n = 4 | 87 (62–102) × 19 (17–23) | 278 (203–325) × 74 (64–89) | 67 (56–85) × 14 (12–15) |
| Ovary | 96 (77–121) × 72 (60–89) | 31 (21–41) × 28 (18–39), n = 5 | 28 (24–30) × 23 (20–27) | 102 (81–150) × 93 (75–144) | 18 (17–20) × 15 (14–17) |
| Testis, anterior | 87 (65–123) × 72 (53–94) | 39 (23–57) × 31 (18–47), n = 5 | 48 (42–57) × 36 (33–40) | 112 (78–144) × 83 (64–103) | 25 (16–30) × 18 (12–23) |
| Testis, posterior | 83 (67–113) × 75 (63–101) | 37 (25–57) × 31 (21–44), n = 5 | 45 (40–50) × 33 (27–37) | 99 (78–138) × 80 (67–112) | 25 (18–30) × 19 (14–23) |
| Eggs | 64 (56–76) × 28 (23–31) | - | - | 70 (61–79) × 30 (27–33), n = 10 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kremnev, G.; Gonchar, A.; Uryadova, A.; Krapivin, V.; Skobkina, O.; Gubler, A.; Krupenko, D. No Tail No Fail: Life Cycles of the Zoogonidae (Digenea). Diversity 2023, 15, 121. https://doi.org/10.3390/d15010121
Kremnev G, Gonchar A, Uryadova A, Krapivin V, Skobkina O, Gubler A, Krupenko D. No Tail No Fail: Life Cycles of the Zoogonidae (Digenea). Diversity. 2023; 15(1):121. https://doi.org/10.3390/d15010121
Chicago/Turabian StyleKremnev, Georgii, Anna Gonchar, Alexandra Uryadova, Vladimir Krapivin, Olga Skobkina, Arseniy Gubler, and Darya Krupenko. 2023. "No Tail No Fail: Life Cycles of the Zoogonidae (Digenea)" Diversity 15, no. 1: 121. https://doi.org/10.3390/d15010121