Total-Evidence Framework Reveals Complex Morphological Evolution in Nightbirds (Strisores)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Terminology and Clade Definitions
2.2. Phylogenetic Analysis
3. Results
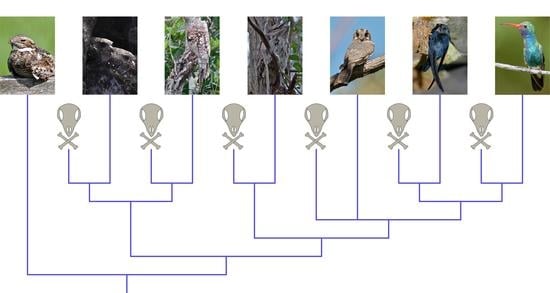
3.1. Phylogenetic Topologies
3.2. Morphological Synapomorphies
4. Discussion
4.1. Conflict Between Molecular and Morphological Data
4.2. Effects of Alternative Total-Evidence Approaches
4.3. Affinities of Hassiavis
4.4. Affinities of Fluvioviridavis
4.5. Morphological Evolution of Strisoreans
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Taxon | Phylogenetic Definition |
|---|---|
| Caprimulgimorphae Cracraft, 2013 [98] | The most inclusive clade including Caprimulgus europaeus, Steatornis caripensis, Nyctibius grandis, Podargus strigoides, Aegotheles cristatus, and Apus apus but not Phoenicopterus ruber, Columba oenas, Otis tarda, Musophaga violacea, Opisthocomus hoazin, Grus grus, Charadrius hiaticula, Phaethon aethereus, Procellaria aequinoctialis, or Vultur gryphus |
| Notes: Although Mayr [2] had already recruited the name Strisores for this grouping, Cracraft [98] coined Caprimulgimorphae to provide a name with a suitable suffix for a superordinal clade. (Caprimulgiformes sensu lato, as used by alternative taxonomic schemes such as del Hoyo et al. [47] and Gill and Donsker [99], also corresponds to this clade. A less inclusive concept of Caprimulgiformes, as adopted here, has been used by others such as Chesser et al. [100] and Piacentini et al. [101].) Given that Mayr was the first to explicitly propose a name for this clade based on rigorous phylogenetic analysis, we favour the use of Strisores here. However, our proposed phylogenetic taxonomy assigns slightly different definitions to each name, allowing both to remain in use while preserving their intended taxonomic content. We suggest a branch-based definition for Caprimulgimorphae, following precedent set by most previous defined clades that contain the suffix “-morpha”, including those that encompass parts of the avian stem-group, such as Maniraptoromorpha [102] and Dinosauromorpha [103]. As defined here, Caprimulgimorphae corresponds to the total group of Strisores and would therefore also include stem-strisoreans should any be identified in future studies. The precise interrelationships of neoavian birds remain unsettled, thus the external specifiers here include representatives from all major groups consistently recovered as monophyletic by recent studies [3,44,75]: Mirandornithes (Phoenicopterus ruber), Columbimorphae (Columba oenas), Otidimorphae (Otis tarda and Musophaga violacea), Opisthocomiformes (Opisthocomus hoazin), Gruiformes (Grus grus), Charadriiformes (Charadrius hiaticula), Phaethontimorphae (Phaethon aethereus), Aequornithes (Procellaria aequinoctialis), and Telluraves (Vultur gryphus). | |
| Strisores Baird, 1858 [104] | The least inclusive clade including Caprimulgus europaeus, Steatornis caripensis, Nyctibius grandis, Podargus strigoides, Aegotheles cristatus, and Apus apus |
| Notes: See notes for Caprimulgimorphae. As defined here, Strisores is a crown group that includes the last common ancestor of extant strisoreans and its descendants. | |
| Podargocypseli Mayr, 2010 [2] | The most inclusive clade including Caprimulgus europaeus, Nyctibius grandis, Podargus strigoides, Aegotheles cristatus, and Apus apus but not Steatornis caripensis |
| Notes: Mayr [2] coined this name in accordance with a specific phylogenetic hypothesis, that podargids, caprimulgids, nyctibiids, and daedalornitheans form a clade exclusive of steatornithids. As such, our proposed definition reflects this specific topology. Under studies that do not recover this grouping as a clade (such as the present study), this name would not be formally recognized. | |
| Cypselomorphae Huxley, 1867 [105] | The most inclusive clade including Caprimulgus europaeus, Nyctibius grandis, Aegotheles cristatus, and Apus apus but not Steatornis caripensis or Podargus strigoides |
| Notes: Mayr [53] recruited this name in accordance with a specific phylogenetic hypothesis, that caprimulgids, nyctibiids, and daedalornitheans form a clade exclusive of podargids and steatornithids. As such, our proposed definition reflects this specific topology. The present study does not recover this grouping as a clade. | |
| Caprimulgi Ridgway, 1881 [106] | The most inclusive clade including Caprimulgus europaeus and Nyctibius grandis but not Steatornis caripensis, Podargus strigoides, Aegotheles cristatus, or Apus apus |
| Notes: Mayr [2] recruited this name in accordance with a specific phylogenetic hypothesis, that caprimulgids and nyctibiids form a clade exclusive of other strisoreans. As such, our proposed definition reflects this specific topology. The present study does not recover this grouping as a clade. | |
| Caprimulgiformes Ridgway, 1881 [106] | The most inclusive clade including Caprimulgus europaeus but not Steatornis caripensis, Nyctibius grandis, Podargus strigoides, Aegotheles cristatus, or Apus apus |
| Notes: Under the phylogenetic taxonomy proposed here, ordinal clades (with the suffix “-iformes”) have been given branch-based definitions, whereas familial clades (with the suffix “-idae”) have been given node-based definitions pertaining to crown groups. This usage has precedent in previous literature on fossil birds (e.g.: the use of Coliiformes vs. Coliidae by Ksepka and Clarke [107]). Furthermore, in cases where only one family has been assigned to an order (e.g.: Caprimulgidae is the only familial clade under Caprimulgiformes sensu stricto), this allows well-established ordinal and familial clades to both retain utility under unranked phylogenetic classifications. Caprimulgiformes is used by some taxonomic authorities to include all members of Strisores (see notes for Caprimulgimorphae); under such taxonomic schemes, the clade delineated here would simply be called “total-group Caprimulgidae”. | |
| Caprimulgidae Vigors, 1825 [108] | The least inclusive clade including Eurostopodus mystacalis, Lyncornis macrotis, and Caprimulgus europaeus |
| Notes: See notes for Caprimulgiformes. Choice of internal specifiers based on the results of Braun and Huddleston [6], Han et al. [49], Sigurðsson and Cracraft [50], and White et al. [51]. | |
| Vanescaves new taxon | The most inclusive clade including Steatornis caripensis, Nyctibius grandis, Podargus strigoides, Aegotheles cristatus, and Apus apus but not Caprimulgus europaeus |
| Etymology: From Latin vanesco meaning “vanish” and avis meaning “bird”, referring to the relictual geographic distribution of many of its constituent clades, and doubles as a reference to the poem “A Route of Evanescence” by Emily Dickinson, which features a hummingbird as its main subject. Notes: This name is proposed to reflect a specific phylogenetic hypothesis, that steatornithids, nyctibiids, podargids, and daedalornitheans form a clade exclusive of caprimulgids. This group is robustly supported by the present study as well as by Prum et al. [3]. However, this name would not be recognized under studies that do not recover this clade (e.g.: [2,9]). | |
| Steatornithiformes Mayr, 2010 [2] | The most inclusive clade including Steatornis caripensis but not Caprimulgus europaeus, Nyctibius grandis, Podargus strigoides, Aegotheles cristatus, or Apus apus |
| Notes: See notes for Caprimulgiformes. Under ranked taxonomic schemes that use Caprimulgiformes sensu lato, the clade delineated here would simply be called “total-group Steatornithidae”, though Steatornithes of Sharpe [109] may be feasibly recruited. Steatornithiformes is sometimes attributed to Sharpe (e.g.: [101]), possibly because he coined Steatornithes under the rank of suborder. However, the International Code of Zoological Nomenclature (ICZN) [110] does not regulate taxon names ranked above the family group (as defined in ICZN Article 35.1). As such, the Principle of Coordination (ICZN Article 36) does not apply to this case, and the use of Steatornithes did not automatically establish the name Steatornithiformes. Thus, to our knowledge Mayr was the first to formally use Steatornithiformes, as Mayr himself correctly indicated. | |
| Steatornithidae Bonaparte, 1842 [111] | The least inclusive clade including all individuals of the species Steatornis caripensis |
| Notes: See notes for Caprimulgiformes. Monotypic. | |
| Nyctibiiformes Yuri et al., 2013 [112] | The most inclusive clade including Nyctibius grandis but not Caprimulgus europaeus, Steatornis caripensis, Podargus strigoides, Aegotheles cristatus, or Apus apus |
| Notes: See notes for Caprimulgiformes. Under ranked taxonomic schemes that use Caprimulgiformes sensu lato, the clade delineated here would simply be called “total-group Nyctibiidae”. | |
| Nyctibiidae Chenu and des Murs, 1851 [113] | The least inclusive clade including Phyllaemulor bracteatus, Nyctibius grandis, and Nyctibius griseus |
| Notes: See notes for Caprimulgiformes. Choice of internal specifiers based on the results of Braun and Huddleston [6] and White et al. [114]. | |
| Podargiformes Mathews, 1918 [115] | The most inclusive clade including Podargus strigoides but not Caprimulgus europaeus, Steatornis caripensis, Nyctibius grandis, Aegotheles cristatus, or Apus apus |
| Notes: See notes for Caprimulgiformes. Under ranked taxonomic schemes that use Caprimulgiformes sensu lato, the clade delineated here would simply be called “total-group Podargidae”, though Podargi of Sharpe [109] may be feasibly recruited. | |
| Podargidae Bonaparte, 1838 [116] | The least inclusive clade including Podargus strigoides, Rigidipenna inexpectata, and Batrachostomus auritus |
| Notes: See notes for Caprimulgiformes. Internal specifiers include all three extant genera. | |
| Apodimorphae Sibley et al., 1988 [117] | The most inclusive clade including Aegotheles cristatus and Apus apus but not Caprimulgus europaeus, Steatornis caripensis, Nyctibius grandis, or Podargus strigoides |
| Notes: Mayr [2] recruited this name to refer to the clade uniting aegothelids and apodiforms to the exclusion of other strisoreans. Although Sangster [11] had already coined the name Daedalornithes for this group, he explicitly defined Daedalornithes as a crown-group clade, allowing Apodimorphae to be retained for total-group Daedalornithes. | |
| Daedalornithes Sangster, 2005 [11] | The least inclusive clade including Aegotheles cristatus and Apus apus |
| Notes: Defined by Sangster [11]. Cracraft [98] recruited Trochiloidea for this group. Given that Sangster was the first to explicitly propose a name for the clade, we favour the use of Daedalornithes here. Both total- and crown-group Daedalornithes have been assigned names under our proposed phylogenetic taxonomy, so we instead recruit Trochiloidea for total-group Trochilidae. | |
| Aegotheliformes Worthy et al., 2007 [85] | The most inclusive clade including Aegotheles cristatus but not Caprimulgus europaeus, Steatornis caripensis, Nyctibius grandis, Podargus strigoides, or Apus apus |
| Notes: See notes for Caprimulgiformes. This taxon is sometimes attributed to Simonetta [118] (e.g.: [85]), but Mayr [2] pointed out that it should be attributed to Worthy et al. | |
| Aegothelidae Bonaparte, 1853 [119] | The least inclusive clade including Aegotheles cristatus, Aegotheles savesi, and Aegotheles insignis |
| Notes: See notes for Caprimulgiformes. Choice of internal specifiers based on the results of Dumbacher et al. [120]. | |
| Apodiformes Peters, 1940 [121] | The most inclusive clade including Apus apus, Hemiprocne longipennis, and Trochilus polytmus but not Caprimulgus europaeus, Steatornis caripensis, Nyctibius grandis, Podargus strigoides, or Aegotheles cristatus |
| Notes: See notes for Caprimulgiformes. Under ranked taxonomic schemes that use Caprimulgiformes sensu lato, the clade delineated here is currently unnamed. | |
| Trochiloidea Vigors, 1825 [108] | The most inclusive clade including Trochilus polytmus but not Apus apus or Hemiprocne longipennis |
| Notes: See notes for Daedalornithes. | |
| Trochilidae Vigors, 1825 [108] | The least inclusive clade including Trochilus polytmus, Topaza pella, Phaethornis superciliosus, Polytmus guainumbi, and Lesbia nuna |
| Notes: See notes for Caprimulgiformes. Choice of internal specifiers based on the results of McGuire et al. [122]. | |
| Apodi Peters, 1940 [121] | The least inclusive clade including Apus apus and Hemiprocne longipennis |
| Notes: This name has consistently been used in reference to the group uniting Hemiprocnidae and Apodidae [2,57,121]. As such, this is reflected in our proposed definition. | |
| Hemiprocnidae Oberholser, 1906 [123] | The least inclusive clade including Hemiprocne longipennis, Hemiprocne coronata, Hemiprocne comata, and Hemiprocne mystacea |
| Notes: See notes for Caprimulgiformes. Internal specifiers include all four extant species. | |
| Apodidae Olphe-Galliard, 1887 [124] | The least inclusive clade including Apus apus, Cypseloides fumigatus, Streptoprocne zonaris, Collocalia esculenta, and Chaetura pelagica |
| Notes: See notes for Caprimulgiformes. Internal specifiers based on the results of Price et al. [125], Thomassen et al. [126], and Päckert et al. [127]. | |
Appendix B









References
- Hackett, S.J.; Kimball, R.T.; Reddy, S.; Bowie, R.C.K.; Braun, E.L.; Braun, M.J.; Chojnowski, J.L.; Cox, W.A.; Han, K.-L.; Harshman, J.; et al. A phylogenomic study of birds reveals their evolutionary history. Science 2008, 320, 1763–1768. [Google Scholar] [CrossRef] [PubMed]
- Mayr, G. Phylogenetic relationships of the paraphyletic ‘caprimulgiform’ birds (nightjars and allies). J. Zool. Syst. Evol. Res. 2010, 48, 126–137. [Google Scholar] [CrossRef]
- Prum, R.O.; Berv, J.S.; Dornburg, A.; Field, D.J.; Townsend, J.P.; Lemmon, E.M.; Lemmon, A.R. A comprehensive phylogeny of birds (Aves) using targeted next-generation DNA sequencing. Nature 2015, 526, 569–573. [Google Scholar] [CrossRef] [PubMed]
- Mayr, G. Osteological evidence for paraphyly of the avian order Caprimulgiformes (nightjars and allies). J. Ornithol. 2002, 143, 82–97. [Google Scholar] [CrossRef]
- Ericson, P.G.P.; Anderson, C.L.; Britton, T.; Elzanowski, A.; Johansson, U.S.; Källersjö, M.; Ohlson, J.I.; Parsons, T.J.; Zuccon, D.; Mayr, G. Diversification of Neoaves: Integration of molecular sequence data and fossils. Biol. Lett. 2006, 2, 543–547. [Google Scholar] [CrossRef] [PubMed]
- Braun, M.J.; Huddleston, C.J. A molecular phylogenetic survey of caprimulgiform nightbirds illustrates the utility of non-coding sequences. Mol. Phylogenet. Evol. 2009, 53, 948–960. [Google Scholar] [CrossRef] [PubMed]
- Kimball, R.T.; Wang, N.; Heimer-McGinn, V.; Ferguson, C.; Braun, E.L. Identifying localized biases in large datasets: A case study using the avian tree of life. Mol. Phylogenet. Evol. 2013, 69, 1021–1032. [Google Scholar] [CrossRef]
- Ksepka, D.T.; Clarke, J.A.; Nesbitt, S.J.; Kulp, F.B.; Grande, L. Fossil evidence of wing shape in a stem relative of swifts and hummingbirds (Aves, Pan-Apodiformes). Proc. R. Soc. B Biol. Sci. 2013, 280, 20130580. [Google Scholar] [CrossRef] [Green Version]
- Reddy, S.; Kimball, R.T.; Pandey, A.; Hosner, P.A.; Braun, M.J.; Hackett, S.J.; Han, K.-L.; Harshman, J.; Huddleston, C.J.; Kingston, S.; et al. Why do phylogenomic data sets yield conflicting trees? Data type influences the avian tree of life more than taxon sampling. Syst. Biol. 2017, 66, 857–879. [Google Scholar] [CrossRef]
- White, N.D.; Braun, M.J. Extracting phylogenetic signal from phylogenomic data: Higher-level relationships of the nightbirds (Strisores). Mol. Phylogenet. Evol. 2019. accepted. [Google Scholar]
- Sangster, G. A name for the clade formed by owlet-nightjars, swifts and hummingbirds (Aves). Zootaxa 2005, 799, 1–6. [Google Scholar] [CrossRef]
- Smithwick, F.M.; Brayley, O.; Field, D.J.; Mayr, G.; Vinther, J. Palaeocolour reconstructions support ancestral nocturnality of strisorian birds. (manuscript in preparation).
- Tagliacollo, V.A.; Bernt, M.J.; Craig, J.M.; Oliveira, C.; Albert, J.S. Model-based total evidence phylogeny of Neotropical electric knifefishes (Teleostei, Gymnotiformes). Mol. Phylogenet. Evol. 2016, 95, 20–33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grande, T.C.; Borden, W.C.; Wilson, M.V.H.; Scarpitta, L. Phylogenetic relationships among fishes in the order Zeiformes based on molecular and morphological data. Copeia 2018, 106, 20–48. [Google Scholar] [CrossRef]
- Martin, R.P.; Olson, E.E.; Girard, M.G.; Smith, W.L.; Davis, M.P. Light in the darkness: New perspective on lanternfish relationships and classification using genomic and morphological data. Mol. Phylogenet. Evol. 2018, 121, 71–85. [Google Scholar] [CrossRef] [PubMed]
- Bagley, J.C.; Mayden, R.L.; Harris, P.M. Phylogeny and divergence times of suckers (Cypriniformes: Catostomidae) inferred from Bayesian total-evidence analyses of molecules, morphology, and fossils. PeerJ 2018, 6, e5168. [Google Scholar] [CrossRef] [PubMed]
- O’Leary, M.A.; Bloch, J.I.; Flynn, J.J.; Gaudin, T.J.; Giallombardo, A.; Giannini, N.P.; Goldberg, S.L.; Kraatz, B.P.; Luo, Z.-X.; Meng, J.; et al. The placental mammal ancestor and the post-K-Pg radiation of placentals. Science 2013, 339, 662–667. [Google Scholar] [CrossRef]
- Halliday, T.J.D.; Upchurch, P.; Goswami, A. Resolving the relationships of Paleocene placental mammals: Paleocene mammal phylogeny. Biol. Rev. 2017, 92, 521–550. [Google Scholar] [CrossRef]
- Reeder, T.W.; Townsend, T.M.; Mulcahy, D.G.; Noonan, B.P.; Wood, P.L.; Sites, J.W.; Wiens, J.J. Integrated analyses resolve conflicts over squamate reptile phylogeny and reveal unexpected placements for fossil taxa. PLoS ONE 2015, 10, e0118199. [Google Scholar] [CrossRef]
- Gauthier, J.; Kluge, A.G.; Rowe, T. Amniote phylogeny and the importance of fossils. Cladistics 1988, 4, 105–209. [Google Scholar] [CrossRef]
- Donoghue, M.J.; Doyle, J.A.; Gauthier, J.; Kluge, A.G.; Rowe, T. The importance of fossils in phylogeny reconstruction. Annu. Rev. Ecol. Syst. 1989, 20, 431–460. [Google Scholar] [CrossRef]
- Mayr, G. The higher-level phylogeny of birds—When morphology, molecules, and fossils coincide. Oryctos 2008, 7, 67–73. [Google Scholar]
- Mayr, G. The origins of crown group birds: Molecules and fossils. Palaeontology 2014, 57, 231–242. [Google Scholar] [CrossRef]
- Hsiang, A.Y.; Field, D.J.; Webster, T.H.; Behlke, A.D.; Davis, M.B.; Racicot, R.A.; Gauthier, J.A. The origin of snakes: Revealing the ecology, behavior, and evolutionary history of early snakes using genomics, phenomics, and the fossil record. BMC Evol. Biol. 2015, 15, 87. [Google Scholar] [CrossRef] [PubMed]
- Asher, R.J.; Smith, M.R.; Rankin, A.; Emry, R.J. Congruence, fossils and the evolutionary tree of rodents and lagomorphs. R. Soc. Open Sci. 2019, 6, 190387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miyashita, T.; Coates, M.I.; Farrar, R.; Larson, P.; Manning, P.L.; Wogelius, R.A.; Edwards, N.P.; Anné, J.; Bergmann, U.; Palmer, A.R.; et al. Hagfish from the Cretaceous Tethys Sea and a reconciliation of the morphological–molecular conflict in early vertebrate phylogeny. Proc. Natl. Acad. Sci. USA 2019, 116, 2146–2151. [Google Scholar] [CrossRef] [PubMed]
- Parham, J.F.; Donoghue, P.C.J.; Bell, C.J.; Calway, T.D.; Head, J.J.; Holroyd, P.A.; Inoue, J.G.; Irmis, R.B.; Joyce, W.G.; Ksepka, D.T.; et al. Best practices for justifying fossil calibrations. Syst. Biol. 2012, 61, 346–359. [Google Scholar] [CrossRef]
- Giribet, G. Morphology should not be forgotten in the era of genomics—A phylogenetic perspective. Zool. Anz. 2015, 256, 96–103. [Google Scholar] [CrossRef]
- Lee, M.S.Y.; Palci, A. Morphological phylogenetics in the genomic age. Curr. Biol. 2015, 25, R922–R929. [Google Scholar] [CrossRef]
- Mayr, G. Paleogene Fossil Birds; Springer: Berlin, Germany, 2009. [Google Scholar]
- Mayr, G. Avian Evolution: The Fossil Record of Birds and Its Paleobiological Significance; Wiley-Blackwell: Chichester, UK, 2017. [Google Scholar]
- Olson, S.L. An early Eocene oilbird from the Green River Formation of Wyoming (Caprimulgiformes: Steatornithidae). Doc. Lab. Géol. 1987, 99, 57–69. [Google Scholar]
- Mourer-Chauviré, C. New data concerning the familial position of the genus Euronyctibius (Aves, Caprimulgiformes) from the Paleogene of the Phosphorites du Quercy, France. Paleontol. J. 2013, 47, 1315–1322. [Google Scholar] [CrossRef]
- Mayr, G. The Palaeogene Old World potoo Paraprefica Mayr, 1999 (Aves, Nyctibiidae): Its osteology and affinities to the New World Preficinae Olson, 1987. J. Syst. Palaeontol. 2005, 3, 359–370. [Google Scholar] [CrossRef]
- Mayr, G. Caprimulgiform birds from the Middle Eocene of Messel (Hessen, Germany). J. Vertebr. Paleontol. 1999, 19, 521–532. [Google Scholar] [CrossRef]
- Nesbitt, S.J.; Ksepka, D.T.; Clarke, J.A. Podargiform affinities of the enigmatic Fluvioviridavis platyrhamphus and the early diversification of Strisores (“Caprimulgiformes” + Apodiformes). PLoS ONE 2011, 6, e26350. [Google Scholar] [CrossRef] [PubMed]
- Mayr, G. Eocene fossils and the early evolution of frogmouths (Podargiformes): Further specimens of Masillapodargus and a comparison with Fluvioviridavis. Palaeobiodivers. Palaeoenviron. 2015, 95, 587–596. [Google Scholar] [CrossRef]
- Mayr, G. Reappraisal of Eocypselus—A stem group apodiform from the early Eocene of Northern Europe. Palaeobiodivers. Palaeoenviron. 2010, 90, 395–403. [Google Scholar] [CrossRef]
- Mayr, G. Skeletal morphology of the middle Eocene swift Scaniacypselus and the evolutionary history of true swifts (Apodidae). J. Ornithol. 2015, 156, 441–450. [Google Scholar] [CrossRef]
- Mayr, G. Phylogeny of early Tertiary swifts and hummingbirds (Aves: Apodiformes). Auk 2003, 120, 145–151. [Google Scholar] [CrossRef]
- Mayr, G. Old World fossil record of modern-type hummingbirds. Science 2004, 304, 861–864. [Google Scholar] [CrossRef]
- Bochenski, Z.; Bochenski, Z.M. An Old World hummingbird from the Oligocene: A new fossil from Polish Carpathians. J. Ornithol. 2008, 149, 211–216. [Google Scholar] [CrossRef]
- Louchart, A.; Tourment, N.; Carrier, J.; Roux, T.; Mourer-Chauviré, C. Hummingbird with modern feathering: An exceptionally well-preserved Oligocene fossil from southern France. Naturwissenschaften 2008, 95, 171–175. [Google Scholar] [CrossRef] [PubMed]
- Jarvis, E.D.; Mirarab, S.; Aberer, A.J.; Li, B.; Houde, P.; Li, C.; Ho, S.Y.W.; Faircloth, B.C.; Nabholz, B.; Howard, J.T.; et al. Whole-genome analyses resolve early branches in the tree of life of modern birds. Science 2014, 346, 1320–1331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harris, J.D.; Lamanna, M.C.; You, H.; Ji, S.; Ji, Q. A second enantiornithean (Aves: Ornithothoraces) wing from the Early Cretaceous Xiagou Formation near Changma, Gansu Province, People’s Republic of China. Can. J. Earth Sci. 2006, 43, 547–554. [Google Scholar] [CrossRef]
- Bostwick, K.S.; Brady, M.J. Phylogenetic analysis of wing feather taxis in birds: Macroevolutionary patterns of genetic drift? Auk 2002, 119, 943–954. [Google Scholar] [CrossRef]
- Del Hoyo, J.; Elliott, A.; Sargatal, J.; Christie, D.A.; de Juana, E. Handbook of the Birds of the World Alive. Available online: https://www.hbw.com/ (accessed on 3 August 2019).
- Cleere, N. Nightjars, Potoos, Frogmouths, Oilbird, and Owlet-nightjars of the World; Princeton University Press: Princeton, NJ, USA, 2010. [Google Scholar]
- Han, K.-L.; Robbins, M.B.; Braun, M.J. A multi-gene estimate of phylogeny in the nightjars and nighthawks (Caprimulgidae). Mol. Phylogenet. Evol. 2010, 55, 443–453. [Google Scholar] [CrossRef] [PubMed]
- Sigurðsson, S.; Cracraft, J. Deciphering the diversity and history of New World nightjars (Aves: Caprimulgidae) using molecular phylogenetics: Phylogeny of New World Nightjars. Zool. J. Linn. Soc. 2014, 170, 506–545. [Google Scholar] [CrossRef]
- White, N.D.; Barrowclough, G.F.; Groth, J.G.; Braun, M.J. A multi-gene estimate of higher-level phylogenetic relationships among nightjars (Aves: Caprimulgidae). Ornitol. Neotrop. 2016, 27, 223–236. [Google Scholar]
- Mayr, G. Ein Archaeotrogon (Aves: Archaeotrogonidae) aus dem Mittel-Eozän der Grube Messel (Hessen, Deutschland)? J. Ornithol. 1998, 139, 121–129. [Google Scholar] [CrossRef]
- Mayr, G. New specimens of Hassiavis laticauda (Aves: Cypselomorphae) and Quasisyndactylus longibrachis (Aves: Alcediniformes) from the Middle Eocene of Messel, Germany. Cour. Forschungsinstitut Senckenburg 2004, 252, 23–28. [Google Scholar]
- Ksepka, D.T.; Clarke, J.A. Phylogenetically vetted and stratigraphically constrained fossil calibrations within Aves. Palaeontol. Electron. 2015, 18. [Google Scholar] [CrossRef]
- Mayr, G. A new cypselomorph bird from the middle Eocene of Germany and the early diversification of avian aerial insectivores. Condor 2005, 107, 342–352. [Google Scholar] [CrossRef]
- Mayr, G.; Daniels, M. A new short-legged landbird from the early Eocene of Wyoming and contemporaneous European sites. Acta Palaeontol. Pol. 2001, 46, 393–402. [Google Scholar]
- Harrison, C.J.O. A revision of the fossil swifts (Vertebrata, Aves, suborder Apodi), with descriptions of three new genera and two new species. Meded. Werkgr. Voor Tert. Kwartaire Geol. 1984, 21, 157–177. [Google Scholar]
- Dyke, G.J.; Waterhouse, D.M.; Kristoffersen, A.V. Three new fossil landbirds from the early Paleogene of Denmark. Bull. Geol. Soc. Den. 2004, 51, 77–85. [Google Scholar]
- Peters, D.S. Ein neuer Segler aus der Grube Messel und seine Bedeutung für den Status der Aegialornithidae (Aves: Apodiformes). Senckenberg. Lethaea 1985, 66, 143–164. [Google Scholar]
- Mayr, G.; Peters, D.S. On the systematic position of the Middle Eocene swift Aegialornis szarskii Peters 1985 with description of a new swift-like bird from Messel (Aves, Apodiformes). Neues Jahrb. Geol. Paläontol. 1999, 1999, 312–320. [Google Scholar]
- Mourer-Chauviré, C. Les Aegialornithidae (Aves: Apodiformes) des Phosphorites du Quercy. Comparaison avec la forme de Messel. Cour. Forschungsinstitut Senckenberg 1988, 107, 369–381. [Google Scholar]
- Mayr, G. A new Eocene swift-like bird with a peculiar feathering. Ibis 2003, 145, 382–391. [Google Scholar] [CrossRef]
- Karhu, A.A. A new genus and species of the family Jungornithidae (Apodiformes) from the late Eocene of the Northern Caucasus, with comments on the ancestry of hummingbirds. Smithson. Contrib. Paleobiol. 1999, 89, 207–216. [Google Scholar]
- Karhu, A.A. Novoye semeystvo strizheobraznykh iz paleogena Yevropy. Paleontol. J. 1988, 3, 78–88. [Google Scholar]
- Mayr, G. New specimens of the early Oligocene Old World hummingbird Eurotrochilus inexpectatus. J. Ornithol. 2007, 148, 105–111. [Google Scholar] [CrossRef]
- Mayr, G.; Micklich, N. New specimens of the avian taxa Eurotrochilus (Trochilidae) and Palaeotodus (Todidae) from the early Oligocene of Germany. Paläontol. Z. 2010, 84, 387–395. [Google Scholar] [CrossRef]
- Huelsenbeck, J.P.; Ronquist, F. MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics 2001, 17, 754–755. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ronquist, F.; Huelsenbeck, J.P. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 2003, 19, 1572–1574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, M.A.; Pfeiffer, W.; Schartz, T. Creating the CIPRES Science Gateway for inference of large phylogenetic trees. In Proceedings of the Gateway Computing Environments Workshop (GCE); IEEE Press: Piscataway, NJ, USA, 2010; pp. 1–8. [Google Scholar] [Green Version]
- Lewis, P.O. A likelihood approach to estimating phylogeny from discrete morphological character data. Syst. Biol. 2001, 50, 913–925. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z. Estimating the pattern of nucleotide substitution. J. Mol. Evol. 1994, 39, 105–111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rambaut, A.; Drummond, A.J.; Xie, D.; Baele, G.; Suchard, M.A. Posterior summarization in Bayesian phylogenetics using Tracer 1.7. Syst. Biol. 2018, 67, 901–904. [Google Scholar] [CrossRef] [PubMed]
- Goloboff, P.A.; Catalano, S.A. TNT version 1.5, including a full implementation of phylogenetic morphometrics. Cladistics 2016, 32, 221–238. [Google Scholar] [CrossRef]
- O’Reilly, J.E.; Puttick, M.N.; Parry, L.; Tanner, A.R.; Tarver, J.E.; Fleming, J.; Pisani, D.; Donoghue, P.C.J. Bayesian methods outperform parsimony but at the expense of precision in the estimation of phylogeny from discrete morphological data. Biol. Lett. 2016, 12, 20160081. [Google Scholar] [CrossRef]
- Kimball, R.T.; Oliveros, C.H.; Wang, N.; White, N.D.; Barker, F.K.; Field, D.J.; Ksepka, D.T.; Chesser, R.T.; Moyle, R.G.; Braun, M.J.; et al. A phylogenomic supertree of birds. Diversity 2019, 11, 109. [Google Scholar] [CrossRef]
- Ksepka, D.T.; Stidham, T.A.; Williamson, T.E. Early Paleocene landbird supports rapid phylogenetic and morphological diversification of crown birds after the K–Pg mass extinction. Proc. Natl. Acad. Sci. USA 2017, 114, 8047–8052. [Google Scholar] [CrossRef] [PubMed]
- Berv, J.S.; Field, D.J. Genomic signature of an avian Lilliput Effect across the K-Pg extinction. Syst. Biol. 2018, 67, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Field, D.J.; Berv, J.S.; Hsiang, A.Y.; Lanfear, R.; Landis, M.J.; Dornburg, A. Timing the extant avian radiation: The rise of modern birds, and the importance of modeling molecular rate variation. Peer J. Prepr. 2019, 7, e27521v1. [Google Scholar]
- Worthy, T.H.; Degrange, F.J.; Handley, W.D.; Lee, M.S.Y. The evolution of giant flightless birds and novel phylogenetic relationships for extinct fowl (Aves, Galloanseres). R. Soc. Open Sci. 2017, 4, 170975. [Google Scholar] [CrossRef] [PubMed]
- Cracraft, J. Toward a phylogenetic classification of the recent birds of the world (class Aves). Auk 1981, 98, 681–714. [Google Scholar]
- Cracraft, J. The major clades of birds. In The Phylogeny and Classification of the Tetrapods Volume 1: Amphibians, Reptiles, Birds; Benton, M.J., Ed.; Oxford University Press: Oxford, UK, 1988; pp. 339–361. [Google Scholar]
- Mayr, G. Avian higher-level phylogeny: Well-supported clades and what we can learn from a phylogenetic analysis of 2954 morphological characters. J. Zool. Syst. Evol. Res. 2008, 46, 63–72. [Google Scholar] [CrossRef]
- Laing, A.M.; Doyle, S.; Gold, M.E.L.; Nesbitt, S.J.; O’Leary, M.A.; Turner, A.H.; Wilberg, E.W.; Poole, K.E. Giant taxon-character matrices: The future of morphological systematics. Cladistics 2018, 34, 333–335. [Google Scholar] [CrossRef]
- Rich, P.V.; McEvey, A. A new owlet-nightjar from the early to mid-Miocene of eastern New South Wales. Mem. Natl. Mus. Vic. 1977, 38, 247–253. [Google Scholar] [CrossRef]
- Worthy, T.H.; Tennyson, A.J.D.; Jones, C.; McNamara, J.A.; Douglas, B.J. Miocene waterfowl and other birds from central Otago, New Zealand. J. Syst. Palaeontol. 2007, 5, 1–39. [Google Scholar] [CrossRef] [Green Version]
- Mourer-Chauviré, C. Les oiseaux fossiles des phosphorites du quercy (éocène supérieur a oligocène supérieur): Implications paléobiogéographiques. Geobios 1982, 15, 413–426. [Google Scholar] [CrossRef]
- Mourer-Chauviré, C. Les relations entre les avifaunes du Tertiaire inferieur d’Europe et d’Amerique du Sud. Bull. Soc. Géol. Fr. 1999, 170, 85–90. [Google Scholar]
- Mayr, G. Two-phase extinction of “Southern Hemispheric” birds in the Cenozoic of Europe and the origin of the Neotropic avifauna. Palaeobiodivers. Palaeoenviron. 2011, 91, 325–333. [Google Scholar] [CrossRef]
- Mayr, G. Avian higher level biogeography: Southern Hemispheric origins or Southern Hemispheric relicts? J. Biogeogr. 2017, 44, 956–958. [Google Scholar] [CrossRef] [Green Version]
- Field, D.J.; Hsiang, A.Y. A North American stem turaco, and the complex biogeographic history of modern birds. BMC Evol. Biol. 2018, 18, 102. [Google Scholar] [CrossRef] [PubMed]
- Saupe, E.E.; Farnsworth, A.; Lunt, D.J.; Sagoo, N.; Pham, K.V.; Field, D.J. Climatic shifts drove major contractions in avian latitudinal distributions throughout the Cenozoic. Proc. Natl. Acad. Sci. USA 2019, 116, 12895–12900. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mourer-Chauviré, C. The Archaeotrogonidae of the Eocene and Oligocene Phosphorites du Quercy (France). Contrib. Sci. 1980, 330, 17–31. [Google Scholar]
- Mayr, G. A postcranial skeleton of Palaeopsittacus Harrison, 1982 (Aves incertae sedis) from the middle Eocene of Messel (Germany). Oryctos 2002, 4, 75–82. [Google Scholar]
- Martin, G.; Rojas, L.M.; Ramírez, Y.; McNeil, R. The eyes of oilbirds (Steatornis caripensis): Pushing at the limits of sensitivity. Naturwissenschaften 2004, 91, 26–29. [Google Scholar] [CrossRef]
- Rojas, L.M.; Ramírez, Y.; McNeil, R.; Mitchell, M.; Marín, G. Retinal morphology and electrophysiology of two Caprimulgiformes birds: The cave-living and nocturnal Oilbird (Steatornis caripensis), and the crepuscularly and nocturnally foraging Common Pauraque (Nyctidromus albicollis). Brain. Behav. Evol. 2004, 64, 19–33. [Google Scholar] [CrossRef]
- Snow, D.W. The natural history of the Oilbird, Steatornis caripensis, in Trinidad, W.I. part 2: Population, breeding ecology and food. Zoologica 1962, 47, 199–221. [Google Scholar]
- Serventy, D.L. Feeding methods of Podargus: With remarks on the possible causes of its aberrant habits. Emu Austral Ornithol. 1936, 36, 74–90. [Google Scholar] [CrossRef]
- Cracraft, J. Avian higher-level relationships and classification: Nonpasseriforms. In The Howard and Moore Complete Checklist of Birds of the World; Dickinson, E.C., Remsen, J.V., Eds.; Aves Press: Eastbourne, UK, 2013; pp. xxi–xliii. [Google Scholar]
- Gill, F.; Donsker, D. IOC World Bird List (v9.2). Available online: https://www.worldbirdnames.org (accessed on 3 August 2019).
- Chesser, R.T.; Burns, K.J.; Cicero, C.; Dunn, J.L.; Kratter, A.W.; Lovette, I.J.; Rasmussen, P.J.; Remsen, J.V.; Stotz, D.F.; Winger, B.M.; et al. AOU Checklist of North and Middle American Birds. Available online: http://checklist.aou.org/taxa (accessed on 3 August 2019).
- De Q. Piacentini, V.; Aleixo, A.; Agne, C.E.; Maurício, G.N.; Pacheco, J.F.; Bravo, G.A.; Brito, G.R.R.; Naka, L.N.; Olmos, F.; Posso, S.; et al. Annotated checklist of the birds of Brazil by the Brazilian Ornithological Records Committee. Braz. J. Ornithol. 2015, 23, 91–298. [Google Scholar]
- Cau, A. The assembly of the avian body plan: A 160-million-year long process. Boll. Soc. Paleontol. Ital. 2018, 57, 1–25. [Google Scholar]
- Nesbitt, S.J. The early evolution of archosaurs: Relationships and the origin of major clades. Bull. Am. Mus. Nat. Hist. 2011, 352, 1–292. [Google Scholar] [CrossRef]
- Baird, S.F. Catalogue of North American Birds, Chiefly in the Museum of the Smithsonian Institution; Smithsonian Institution: Washington, DC, USA, 1858. [Google Scholar]
- Huxley, T.H. On the classification of birds: And on the taxonomic value of the modifications of certain of the cranial bones observable in that class. Proc. Zool. Soc. Lond. 1867, 35, 415–472. [Google Scholar]
- Ridgway, R. Nomenclature of North American Birds Chiefly Contained in the United States National Museum; United States Government Publishing Office: Washington, DC, USA, 1881.
- Ksepka, D.T.; Clarke, J.A. Affinities of Palaeospiza bella and the phylogeny and biogeography of mousebirds (Coliiformes). Auk 2009, 126, 245–259. [Google Scholar] [CrossRef]
- Vigors, N.A. Observations on the natural affinities that connect the orders and families of birds. Trans. Linn. Soc. 1825, 14, 395–517. [Google Scholar] [CrossRef]
- Sharpe, R.B. A Review of Recent Attempts to Classify Birds; Office of the Congress: Budapest, Hungary, 1891.
- ICZN. International Code of Zoological Nomenclature; The International Trust for Zoological Nomenclature: London, UK, 1999. [Google Scholar]
- Bonaparte, C.L. Catalogo metodico degli uccelli Europei (continuazione). Nuovi Ann. Sci. Nat. 1842, 8, 134–149. [Google Scholar]
- Yuri, T.; Kimball, R.; Harshman, J.; Bowie, R.; Braun, M.; Chojnowski, J.; Han, K.-L.; Hackett, S.; Huddleston, C.; Moore, W.; et al. Parsimony and model-based analyses of indels in avian nuclear genes reveal congruent and incongruent phylogenetic signals. Biology 2013, 2, 419–444. [Google Scholar] [CrossRef]
- Chenu, J.C.; des Murs, M.A.P. Encyclopédie D’histoire Naturelle; Maresq: Paris, France, 1851. [Google Scholar]
- White, N.D.; Mitter, C.; Braun, M.J. Ultraconserved elements resolve the phylogeny of potoos (Aves: Nyctibiidae). J. Avian Biol. 2017, 48, 872–880. [Google Scholar] [CrossRef]
- Mathews, G.M. The Birds of Australia; Witherby & Co.: London, UK, 1918. [Google Scholar]
- Bonaparte, C.L. Synopsis vertebratorum systematis. Nuovi Ann. Sci. Nat. Bologna 1838, 1, 105–133. [Google Scholar]
- Sibley, C.G.; Ahlquist, J.E.; Monroe, B.L. A classification of the living birds of the world based on DNA-DNA hybridization studies. Auk 1988, 105, 409–423. [Google Scholar]
- Simonetta, A.M. Cinesi e morfologia del cranio negli Ucelli non passeriformi. Studio su varie tendenze evolative. Part II—Striges, Caprimulgiformes ed Apodiformes. Arch. Zool. Ital. 1967, 52, 1–35. [Google Scholar]
- Bonaparte, C.L. Classification ornithologique par séries. Comptes Rendus Acad. Sci. 1853, 37, 641–647. [Google Scholar]
- Dumbacher, J. Phylogeny of the owlet-nightjars (Aves: Aegothelidae) based on mitochondrial DNA sequence. Mol. Phylogenet. Evol. 2003, 29, 540–549. [Google Scholar] [CrossRef]
- Peters, J.L. Check-List of Birds of the World; Harvard University Press: Cambridge, MA, USA, 1940. [Google Scholar]
- McGuire, J.A.; Witt, C.C.; Remsen, J.V.; Corl, A.; Rabosky, D.L.; Altshuler, D.L.; Dudley, R. Molecular phylogenetics and the diversification of hummingbirds. Curr. Biol. 2014, 24, 910–916. [Google Scholar] [CrossRef]
- Oberholser, H.C. The status of the generic name Hemiprocne Nitzsch. Proc. Biol. Soc. Wash. 1906, 19, 67–69. [Google Scholar]
- Olphe-Galliard, L. Contributions à la Faune Ornithologique de l’Europe Occidentale; L. Lasserre: Bayonne, France, 1887. [Google Scholar]
- Price, J.J.; Johnson, K.P.; Bush, S.E.; Clayton, D.H. Phylogenetic relationships of the Papuan Swiftlet Aerodramus papuensis and implications for the evolution of avian echolocation. Ibis 2005, 147, 790–796. [Google Scholar] [CrossRef]
- Thomassen, H.A.; den Tex, R.-J.; de Bakker, M.A.G.; Povel, G.D.E. Phylogenetic relationships amongst swifts and swiftlets: A multi locus approach. Mol. Phylogenet. Evol. 2005, 37, 264–277. [Google Scholar] [CrossRef]
- Päckert, M.; Martens, J.; Wink, M.; Feigl, A.; Tietze, D.T. Molecular phylogeny of Old World swifts (Aves: Apodiformes, Apodidae, Apus and Tachymarptis) based on mitochondrial and nuclear markers. Mol. Phylogenet. Evol. 2012, 63, 606–616. [Google Scholar] [CrossRef]






| Taxon | Age | Locality | Described Material | Recently Hypothesized Phylogenetic Affinities |
|---|---|---|---|---|
| Hassiavis laticauda Mayr, 1998 [52] | Eocene (Lutetian) | Messel Formation, Germany | HLMD Me 9047a+b (nearly complete skeleton), SMF-ME 601 (partial skeleton), SMF-ME 814a+b (forelimb), SMF-ME 1772a+b (nearly complete skeleton) [52], SMF-ME 3545 (complete skeleton) [53] | “Cypselomorphae” incertae sedis (possibly closely related to Archaeotrogon) [53] |
| Protocypselomorphus manfredkelleri Mayr, 2005 [55] | Eocene (Lutetian) | Messel Formation, Germany | SMF-ME 11043 (nearly complete skeleton) [55] | Stem-“cypselomorph” [8,55] |
| Prefica nivea Olson, 1987 [32] | Eocene (Ypresian) | Green River Formation, United States | USNM 336278 (nearly complete skeleton) [32] | Stem-steatornithid [8,32,34,36] |
| Paraprefica kelleri Mayr, 1999 [35] | Eocene (Lutetian) | Messel Formation, Germany | SMF-ME 3376 (partial skeleton), SMF-ME 1635a+b (complete skeleton), SMF-ME 1926 (hindlimb), SMF-ME 2553 (hindlimbs), SMF-ME 3377a+b (partial skeleton), SMNK.PAL.938 (skull and fragmentary limbs) [35], SMF-ME 3727a+b (complete skeleton), SMF-ME 3578 (nearly complete skeleton), SMF-ME 1760 (forelimb) [34] | Stem-nyctibiid [8,34,36] |
| Fluvioviridavis platyrhamphus Mayr and Daniels, 2001 [56] | Eocene (Ypresian) | Green River Formation, United States | SMNK.PAL.2368a+b (nearly complete skeleton) [56], FMNH PA 607 (partial skeleton) [36] 1 | Stem-podargid [8,36], stem-“podargocypselian” [37] |
| Masillapodargus longipes Mayr 1999 [35] | Eocene (Lutetian) | Messel Formation, Germany | SMNK.PAL.1083 (nearly complete skeleton), SMF-ME 1415a+b (partial skeleton), SMNK.PAL.552a+b (skull), SMNK.PAL.557 (skull), SMNK.PAL.2356a+b (skull), SMF-ME 3404a+b (skull), SMF-ME 3405a+b (skull), SMF-ME 3406 (skull) [35], HLMD Me 13359a+b (skull), HLMD Me 7627a+b (partial skeleton), SMF-ME 3658 (skull), SMF-ME 3659 (skull) [37] | Stem-podargid [8,35,36,37] |
| Eocypselus vincenti Harrison, 1984 [57] | Eocene (Ypresian) | London Clay Formation, United Kingdom; Fur Formation, Denmark [58] | NHM A 5429 (partial forelimbs and pectoral girdle) [38,57], MGUH 26729 (partial skeleton), MGUH 26730 (partial skeleton) [58], MGUH 29278 (nearly complete imprint of skeleton) [38] | Stem-apodiform [8,38,40] |
| Eocypselus rowei Ksepka et al., 2013 [8] | Eocene (Ypresian) | Green River Formation, United States | WDC-CGR-109 (complete skeleton) [8] | Stem-apodiform [8] |
| Scaniacypselus wardi Harrison, 1984 [57] | Eocene (Ypresian) | Røsnæs Clay Formation, Denmark | NMHUK A 5430 (partial forelimb) [39,57] | Stem-apodid [8,39,40,55] |
| Scaniacypselus szarskii (Peters, 1985 [59]) | Eocene (Lutetian) | Messel Formation, Germany | SMNK Me 301 (nearly complete skeleton, SMF-ME 606a+b (partial skeleton), SMF-ME 599 (partial skeleton), HLMD Me 7598a+b (partial skeleton) [59], SMF-ME 3576A (complete skeleton) [30,60], SMF-ME 11345a+b (partial skeleton), SMF-ME 340a+b (complete skeleton), SMF-ME 11413a+b (partial skeleton) [39] | Stem-apodid [8,39,40,55,61] |
| Parargornis messelensis Mayr, 2003 [62] | Eocene (Lutetian) | Messel Formation, Germany | HLMD Be 163+193 (complete skeleton) [62] | Stem-trochilid [8,40] |
| Argornis caucasicus Karhu, 1999 [63] | Eocene (Bartonian) [39] | Gorny Luch, Russia | PIN 4425-18 (partial skeleton) [63] | Stem-trochilid [8,40] |
| Jungornis tesselatus Karhu, 1988 [64] | Oligocene (Rupelian) | Maykop Formation, Russia | PIN 1413/208 (partial forelimb and pectoral girdle) [64] | Stem-trochilid [8,40] |
| Eurotrochilus inexpectatus Mayr, 2004 [41] | Oligocene (Rupelian) | Wiesloch-Frauenweiler, Germany | SMNS 80739/4+ SMNK.PAL.5591 (partial skeleton), SMNS 80739/3a+b (partial skeleton) [41], SMNK.PAL.4410a+b (partial skeleton) [65], SMNK.PAL.6599 (partial skeleton) [66] | Stem-trochilid [8,41] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, A.; White, N.D.; Benson, R.B.J.; Braun, M.J.; Field, D.J. Total-Evidence Framework Reveals Complex Morphological Evolution in Nightbirds (Strisores). Diversity 2019, 11, 143. https://doi.org/10.3390/d11090143
Chen A, White ND, Benson RBJ, Braun MJ, Field DJ. Total-Evidence Framework Reveals Complex Morphological Evolution in Nightbirds (Strisores). Diversity. 2019; 11(9):143. https://doi.org/10.3390/d11090143
Chicago/Turabian StyleChen, Albert, Noor D. White, Roger B.J. Benson, Michael J. Braun, and Daniel J. Field. 2019. "Total-Evidence Framework Reveals Complex Morphological Evolution in Nightbirds (Strisores)" Diversity 11, no. 9: 143. https://doi.org/10.3390/d11090143