Effect of Fluoroalkyl-Substituent in Bistolane-Based Photoluminescent Liquid Crystals on Their Physical Behavior
Abstract
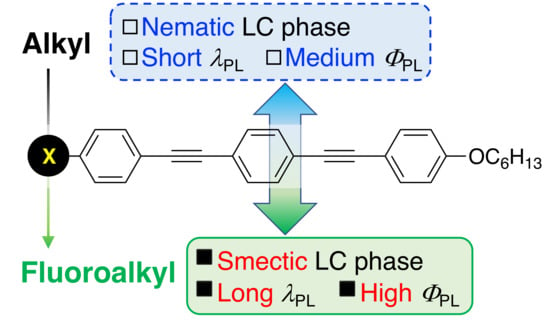
:1. Introduction
2. Materials and Methods
2.1. General
2.2. Materials
2.3. Typical Synthetic Procedure for Preparation of the Bistolanes (as an Example, 2c)
2.3.1. 1-Undecafluorocyclohexyl-4-[2-[4-[2-(4-n-hexyloxyphenyl)ethyn-1-yl]phenyl]ethyn-1-yl]benzene (2c)
2.3.2. 4-[2-[4-[2-(4-n-Hexyloxyphenyl)ethyn-1-yl]phenyl]ethyn-1-yl]toluene (1a)
2.3.3. 1-Cyclohexyl-4-[2-[4-[2-(4-n-hexyloxyphenyl)ethyn-1-yl]phenyl]ethyn-1-yl]benzene (1b)
2.3.4. 4-[2-[4-[2-(4-n-Hexyloxyphenyl)ethyn-1-yl]phenyl]ethyn-1-yl]benzotrifluoride (2a)
2.3.5. 1-(2,3,5,6-Tetrafluorocyclohexyl)-4-[2-[4-[2-(4-n-hexyloxyphenyl)ethyn-1-yl]phenyl]ethyn-1-yl]benzene (2b)
2.4. X-ray Crystallographic Analysis
2.5. Powder X-ray Diffraction (PXRD) Measurements
2.6. Computations
2.7. Thermal Measurements
2.8. Photophysical Measurements
3. Results and Discussion
3.1. Theoretical Assessment
3.2. Synthesis and Crystal Structure Determination
3.3. Phase Transition Behaviors
3.4. Photophysical Behavior
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Caron, S. Where does the fluorine come from? A review on the challenges associated with the synthesis of organofluorine compounds. Org. Process Res. Dev. 2020, 24, 470–480. [Google Scholar] [CrossRef]
- Inoue, M.; Sumii, Y.; Shibata, N. Contribution of organofluorine compounds to pharmaceuticals. ACS Omega 2020, 5, 10633–10640. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, Y.; Tokunaga, E.; Kobayashi, O.; Hirai, K.; Shibata, N. Current contributions of organofluorine compounds to the agrochemical industry. iScience 2020, 23, 101467. [Google Scholar] [CrossRef] [PubMed]
- Kirsch, P. Modern Fluoroorganic Chemistry: Synthesis, Reactivity, Applications, 2nd ed.; Wiley-VCH: Weinheim, Germany, 2013; pp. 7–21. [Google Scholar]
- Behrang, N.; Fischbach, F.; Kipp, M. Mechanism of siponimod: Anti-inflammatory and neuroprotective mode of action. Cells 2019, 8, 24. [Google Scholar] [CrossRef] [Green Version]
- Mei, H.; Han, J.; Fustero, S.; Medio-Simon, M.; Sedgwick, D.M.; Santi, C.; Ruzziconi, R.; Soloshonok, V.A. Fluorine-containing drugs approved by the FDA in 2018. Chem. Eur. J. 2019, 25, 11797–11819. [Google Scholar] [CrossRef]
- Böhm, H.-J.; Banner, D.; Bendels, S.; Kansy, M.; Kuhn, B.; Müller, K.; Obst-Sander, U.; Stahl, M. Fluorine in medicinal chemistry. ChemBioChem 2004, 5, 637–643. [Google Scholar] [CrossRef]
- Lowe, P.T.; O’Hagan, D. A role for fluorine in flavours, fragrances and pheromones. J. Fluor. Chem. 2020, 230, 109420. [Google Scholar] [CrossRef]
- Li, H.; Gao, M.; Chen, Y.; Wang, Y.; Zhu, X.; Yang, G. Discovery of pyrazine-carboxamide-diphenyl-ethers as novel succinate dehydrogenase inhibitors via fragment recombination. J. Agric. Food Chem. 2020, 68, 14001–14008. [Google Scholar] [CrossRef]
- Giornal, F.; Pazenok, S.; Rodefeld, L.; Lui, N.; Vors, J.-P.; Leroux, F.R. Synthesis of diversely fluorinated pyrazoles as novel active agrochemical ingredients. J. Fluor. Chem. 2013, 152, 2–11. [Google Scholar] [CrossRef]
- Fan, Q.; Méndez-Romero, U.; Guo, X.; Wang, E.; Zhang, M.; Li, Y. Fluorinated photovoltaic materials for high-performance organic solar cells. Chem. Asian J. 2019, 14, 3085–3095. [Google Scholar] [CrossRef]
- Funabiki, K.; Saito, Y.; Kikuchi, T.; Yagi, K.; Kubota, Y.; Inuzuka, T.; Miwa, Y.; Yoshida, M.; Sakurada, O.; Kutsumizu, S.J. Aromatic Fluorine-Induced One-Pot Synthesis of Ring-Perfluorinated Trimethine Cyanine Dye and Its Remarkable Fluorescence Properties. J. Org. Chem. 2019, 84, 4372–4380. [Google Scholar] [CrossRef]
- Hird, M. Fluorinated liquid crystals—Properties and applications. Chem. Soc. Rev. 2007, 36, 2070–2095. [Google Scholar] [CrossRef]
- Konno, T. Trifluoromethylated internal alkynes: Versatile building blocks for the preparation of various fluorine-containing molecules. Synlett 2014, 25, 1350–1370. [Google Scholar] [CrossRef]
- Konno, T. Trifluoromethyl (CF3) group insertion methods in stereoselective synthesis. In Stereoselective Synthesis of Drugs and Natural Products; Andrunshko, V., Andrunshko, N., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2013; pp. 769–806. [Google Scholar]
- Yamada, S.; Konno, T. New types of negative dielectric anisotropy liquid crystals: Synthesis of CF2CF2-carbocyclic mesogens and an evaluation of their liquid crystal characteristics. In Chemical Elements (Fluorine, Rhodium and Rubidium): Properties, Synthesis and Applications; Huff, A., Ed.; Nova Science Publishers: New York, NY, USA, 2018; pp. 53–92. [Google Scholar]
- Yamada, S.; Mitsuda, A.; Miyano, K.; Tanaka, T.; Morita, M.; Agou, T.; Kubota, T.; Konno, T. Development of novel solid-state light-emitting materials based on pentafluorinated tolane fluorophores. ACS Omega 2018, 3, 9105–9113. [Google Scholar] [CrossRef]
- Morita, M.; Yamada, S.; Konno, T. Fluorine-induced emission enhancement of tolanes via formation of tight molecular aggregates. New J. Chem. 2020, 44, 6704–6708. [Google Scholar] [CrossRef]
- Yamada, S.; Mitsuda, A.; Adachi, K.; Hara, M.; Konno, T. Development of light-emitting liquid-crystalline polymers with a pentafluorinated bistolane-based luminophore. New J. Chem. 2020, 44, 5684–5691. [Google Scholar] [CrossRef]
- Morita, M.; Yamada, S.; Agou, T.; Kubota, T.; Konno, T. Luminescence tuning of fluorinated bistolanes via electronic or aggregated-structure control. Appl. Sci. 2019, 9, 1905. [Google Scholar] [CrossRef] [Green Version]
- Yamada, S.; Morita, M.; Agou, T.; Kubota, T.; Ichikawa, T.; Konno, T. Thermoresponsive luminescence properties of polyfluorinated bistolane-type light-emitting liquid crystals. Org. Biomol. Chem. 2018, 16, 5609–5617. [Google Scholar] [CrossRef]
- Yamada, S.; Morita, M.; Konno, T. Multi-color photoluminescence induced by electron-density distribution of fluorinated bistolane derivatives. J. Fluorine Chem. 2017, 202, 54–64. [Google Scholar] [CrossRef]
- Yamada, S.; Miyano, K.; Konno, T.; Agou, T.; Kubota, T.; Hosokai, T. Fluorine-containing bistolanes as light-emitting liquid crystalline molecules. Org. Biomol. Chem. 2017, 15, 5949–5958. [Google Scholar] [CrossRef]
- Nishikawa, M.; Kume, S.; Nishihara, H. Stimuli-responsive pyrimidine ring rotation in copper complexes for switching their physical properties. Phys. Chem. Chem. Phys. 2013, 15, 10549–10565. [Google Scholar] [CrossRef]
- Kobayashi, A.; Kato, M. Stimuli-responsive luminescent copper(I) complexes for intelligent emissive devices. Chem. Lett. 2017, 46, 154–162. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELEX. Acta Cryst. 2015, C71, 3–8. [Google Scholar]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision B.01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Zhao, Y.; Truhlar, D.G. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: Two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor. Chem. Acc. 2008, 120, 215–241. [Google Scholar]
- Chan, B.; Gilbert, A.T.B.; Gill, P.M.W.; Radom, L. Performance of density functional theory procedures for the calculation of proton-exchange barriers: Unusual behavior of M06-type functionals. J. Chem. Theory Comput. 2014, 10, 3777–3783. [Google Scholar] [CrossRef]
- Andzelm, J.; Kölmel, C.; Klamt, A. Incorporation of solvent effects into density functional calculations of molecular energies and geometries. J. Chem. Phys. 1995, 103, 9312–9320. [Google Scholar] [CrossRef]
- Barone, V.; Cossi, M. Quantum calculation of molecular energies and energy gradients in solution by a conductor solvent model. J. Phys. Chem. A 1998, 102, 1995–2001. [Google Scholar] [CrossRef]
- Cossi, M.; Rega, N.; Scalmani, G.; Barone, V. Energies, structures, and electronic properties of molecules in solution with the C-PCM solvation model. J. Comput. Chem. 2003, 24, 669–681. [Google Scholar] [CrossRef]
- Grad, J.; Yan, Y.J.; Mukamel, S. Time-dependent self-consistent field approximation for semiclassical dynamics using Gaussian wavepackets in phase space. Chem. Phys. Lett. 1987, 134, 291–295. [Google Scholar] [CrossRef]
- Makri, N. Time-dependent self-consistent field approximation with explicit two-body correlations. Chem. Phys. Lett. 1990, 169, 541–548. [Google Scholar] [CrossRef]
- Durie, A.J.; Slawin, A.M.Z.; Lebl, T.; Kirsch, P.; O’Hagan, D. Fluorocyclohexenes: Synthesis and structure of all-syn-1,2,4,5-tetrafluorocyclohexane. Chem. Commun. 2012, 48, 9643–9645. [Google Scholar] [CrossRef] [PubMed]
- Durie, A.J.; Fujiwara, T.; Cormanich, R.; Bühl, M.; Slawin, A.M.Z.; O’Hagan, D. Synthesis and elaboration of all-cis-1,2,4,5-tetrafluoro-3-phenylcyclohexane: A polar cyclohexane motif. Chem. Eur. J. 2014, 20, 6259–6263. [Google Scholar] [CrossRef] [PubMed]
- Kawahara, S.; Tsutzuki, S.; Uchimaru, T. Theoretical study of the C–F/π interaction: Attractive interaction between fluorinated alkane and an electron-deficient π-system. J. Phys. Chem. A 2004, 108, 6744–6749. [Google Scholar] [CrossRef]
- Li, P.; Maier, J.M.; Vik, E.C.; Yehl, C.J.; Dial, B.E.; Rickher, A.E.; Smith, M.D.; Pellechia, P.J.; Shimizu, K.D. Stabilizing fluorine–π interactions. Angew. Chem. Int. Ed. 2017, 56, 7209–7212. [Google Scholar] [CrossRef]
- Mooibroek, T.J.; Gamez, P.; Reedijk, J. Lone pair–π interactions: A new supramolecular bond? CrystEngComm 2008, 10, 1501–1515. [Google Scholar] [CrossRef]
- Bondi, A. van der Waals volumes and radii. J. Phys. Chem. 1964, 68, 441–451. [Google Scholar] [CrossRef]
- Bauzá, A.; Frontera, A. Electrostatically enhanced F···F interactions through hydrogen bonding, halogen bonding and metal coordination: An ab initio study. Phys. Chem. Chem. Phys. 2016, 18, 20381–20388. [Google Scholar] [CrossRef] [Green Version]
- Beker, R.J.; Colavita, P.E.; Murphy, D.M.; Platts, J.A.; Wallis, J.D. Fluorine-fluorine interactions in the solid state: An experimental and theoretical study. J. Phys. Chem. A 2012, 116, 1435–1444. [Google Scholar] [CrossRef]
- Takezawa, H.; Murase, T.; Resnati, G.; Metrangolo, P.; Fujita, M. Recognition of polyfluorinated compounds through self-aggregation in a cavity. J. Am. Chem. Soc. 2014, 136, 1786–1788. [Google Scholar] [CrossRef]
- Sugisawa, S.; Tabe, Y. Induced smectic phases of stoichiometric liquid crystal mixtures. Soft Matter. 2016, 12, 3103–3109. [Google Scholar] [CrossRef]
- Reichardt, C. Solvatochromic dyes as solvent polarity indicators. Chem. Rev. 1994, 94, 2319–2358. [Google Scholar] [CrossRef]
- Mataga, N.; Kaifu, Y.; Koizumi, M. The solvent effect on fluorescence spectrum, change of solute-solvent interaction during the lifetime of excited solute molecule. Bull. Chem. Soc. Jpn. 1955, 28, 690–691. [Google Scholar] [CrossRef] [Green Version]
- Mataga, N.; Kaifu, Y.; Koizumi, M. Solvent effects upon fluorescence spectra and the dipole moments of excited molecules. Bull. Chem. Soc. Jpn. 1956, 29, 465–470. [Google Scholar] [CrossRef] [Green Version]
- Birks, J.B. Photophysics of Aromatic Molecules; Birks, J.B., Ed.; Wiley: London, UK, 1970. [Google Scholar]
- Thomas, S.W.; Joly, G.D.; Swager, T.M. Chemical sensors based on amplifying fluorescent conjugated polymer. Chem. Rev. 2007, 107, 1339–1386. [Google Scholar] [CrossRef]
- Yaroshchuk, O.; Reznikov, Y. Photoalignment of liquid crystals: Basics and current trends. J. Mater. Chem. 2012, 22, 286–300. [Google Scholar] [CrossRef]
- Kawatsuki, N.; Goto, K.; Yamamoto, T. Photoinduced anisotropy and photoalignment of nematic liquid crystals by a novel polymer liqud crystal with a coumarin-containing side group. Liq. Cryst. 2001, 28, 1171–1176. [Google Scholar] [CrossRef]
- Kawatsuki, N.; Kuwabara, M.; Matsuura, Y.; Sasaki, T.; Ono, H. Photoalignment control of liquid crystals on photo-cross-linkable polymer liquid crystal film. J. Photopolym. Sci. Technol. 2005, 18, 17–22. [Google Scholar]
- Li, X.D.; Zhong, Z.X.; Lee, S.H.; Ghang, G.; Lee, M.H. Liquid crystal photoalignment using soluble photosensitive polyimide. Jpn. J. Appl. Phys. 2006, 45, 906. [Google Scholar] [CrossRef]
- Zhong, Z.X.; Li, X.; Lee, S.H.; Lee, M.H. Liquid crystal photoalignment material based on chloromethylated polyimide. Appl. Phys. Lett. 2004, 85, 2520. [Google Scholar] [CrossRef]







| Compound | µ||/µ⊥ (D) 2 | HOMO/LUMO (eV) | Theoretical Transition Probability (%) | λcalcd (nm) |
|---|---|---|---|---|
| 1a | 0.96/1.79 | −6.88/−1.27 | HOMO→LUMO (92%) | 330 |
| 1b | 0.96/1.79 | −6.87/−1.27 | HOMO→LUMO (92%) | 331 |
| 2a | 6.19/1.49 | −6.99/−1.50 | HOMO→LUMO (87%) | 333 |
| 2b | 2.44/4.17 | −6.93/−1.35 | HOMO→LUMO (91%) | 331 |
| 2c | 6.57/1.54 | −7.00/−1.52 | HOMO→LUMO (87%) | 334 |
| Compound | Solvent [ET(30)] 2 | λabs (nm) [ε, 103 L mol−1 cm−1] | λex (nm) | λPL (nm) | ΦPL 3 |
|---|---|---|---|---|---|
| 1a | CH2Cl2 [40.7] | 328 [80.4], 348 [sh, 55.6] | 330 | 377 | 1.00 |
| Hexane [31.0] | 324 [20.4], 344 [sh, 12.9] | 324 | 353, 368, 380 | 0.30 | |
| CHCl3 [39.1] | 328 [57.2], 348 [sh, 37.3] | 330 | 366, 380 | 0.66 | |
| DMF [43.2] | 328 [33.7], 348 [sh, 23.2] | 330 | 388 | 0.78 | |
| MeCN [45.6] | 324 [38.1], 344 [sh, 26.8] | 324 | 388 | 0.68 | |
| 1b | CH2Cl2 [40.7] | 330 [63.0], 350 [sh, 43.0] | 330 | 386 | 1.00 |
| 2a | CH2Cl2 [40.7] | 331 [50.9], 350 [sh, 37.0] | 330 | 400 | 1.00 |
| Hexane [31.0] | 330 [2.6], 349 [sh, 1.4] | 330 | 361, 377 | 0.20 | |
| CHCl3 [39.1] | 333 [23.4], 350 [sh, 19.0] | 330 | 388 | 0.86 | |
| DMF [43.2] | 333 [23.4], 350 [sh, 19.0] | 330 | 420 | 0.83 | |
| MeCN [45.6] | 325 [5.3], 346 [sh, 3.7] | 325 | 420 | 0.71 | |
| 2b | CH2Cl2 [40.7] | 329 [61.0], 350 [sh, 42.4] | 330 | 386 | 1.00 |
| 2c | CH2Cl2 [40.7] | 332 [56.7], 350 [sh, 41.7] | 330 | 405 | 1.00 |
| Compound | λPL1 | ΦPL 2 |
|---|---|---|
| 1a | 404, 422 | 0.57 |
| 1b | 405, 421 | 0.79 |
| 2a | 418, 434(sh) | 1.00 |
| 2b | 401(sh), 417 | 0.71 |
| 2c | 421, 434(sh) | 0.82 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamada, S.; Wang, Y.; Morita, M.; Zhang, Q.; O’Hagan, D.; Nagata, M.; Agou, T.; Fukumoto, H.; Kubota, T.; Hara, M.; et al. Effect of Fluoroalkyl-Substituent in Bistolane-Based Photoluminescent Liquid Crystals on Their Physical Behavior. Crystals 2021, 11, 450. https://doi.org/10.3390/cryst11040450
Yamada S, Wang Y, Morita M, Zhang Q, O’Hagan D, Nagata M, Agou T, Fukumoto H, Kubota T, Hara M, et al. Effect of Fluoroalkyl-Substituent in Bistolane-Based Photoluminescent Liquid Crystals on Their Physical Behavior. Crystals. 2021; 11(4):450. https://doi.org/10.3390/cryst11040450
Chicago/Turabian StyleYamada, Shigeyuki, Yizhou Wang, Masato Morita, Qingzhi Zhang, David O’Hagan, Masakazu Nagata, Tomohiro Agou, Hiroki Fukumoto, Toshio Kubota, Mitsuo Hara, and et al. 2021. "Effect of Fluoroalkyl-Substituent in Bistolane-Based Photoluminescent Liquid Crystals on Their Physical Behavior" Crystals 11, no. 4: 450. https://doi.org/10.3390/cryst11040450