A Self-Assembled Hetero-Bimetallic [Ni(II)-Sm(III)] Coordination Polymer Constructed from a Salamo-Like Ligand and 4,4′-Bipyridine: Synthesis, Structural Characterization, and Properties
Abstract
:1. Introduction
2. Experimental
2.1. Materials and Physical Measurements
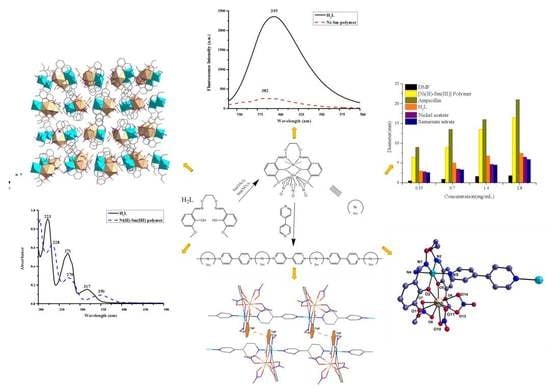
2.2. Preparation of H2L
2.3. Preparation of the Ni(II)-Sm(III) Polymer
2.4. Crystal Structure Determination of the Ni(II)-Sm(III) Polymer
3. Results and Discussion
3.1. PXRD Analysis
3.2. FT-IR Spectra of H2L and the Title Polymer
3.3. Description of the Molecular Structure of the Title Polymer
3.4. Supramolecular Interactions in the Title Polymer
3.5. Molar Conductance and Solubility and Mass Spectrometry Analysis of the Title Polymer
3.6. UV/Vis Absorption Spectra of H2L and the Title Polymer
3.7. Fluorescence Properties of the Title Polymer
3.8. Antimicrobial Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Akine, S.; Varadi, Z.; Nabeshima, T. Synthesis of planar metal complexes and the stacking abilities of naphthaenediol-based acyclic and macrocyclic salen-type ligands. Eur. J. Inorg. Chem. 2013, 35, 5987–5998. [Google Scholar] [CrossRef]
- Wu, H.L.; Pan, G.L.; Bai, Y.C.; Wang, H.; Kong, J.; Shi, F.; Zhang, Y.H.; Wang, X.L. Preparation, structure, DNA-binding properties, and antioxidant activities of a homodinuclear erbium(III) complex with a pentadentate Schiff base ligand. J. Chem. Res. 2014, 38, 211–217. [Google Scholar] [CrossRef]
- Song, X.Q.; Liu, P.P.; Liu, Y.A.; Zhou, J.J.; Wang, X.L. Two dodecanuclear heterometallic [Zn6Ln6] clusters constructed by a multidentate salicylamide salen-like ligand: Synthesis, structure, luminescence and magnetic properties. Dalton Trans. 2016, 45, 8154–8163. [Google Scholar] [CrossRef] [PubMed]
- Chattopadhyay, S.; Drew, M.G.B.; Ghosh, A. Methylene spacer-regulated structural variation in cobalt(II/III) complexes with bridging acetate and salen- or salpn-type Schiff-base ligands. Eur. J. Inorg. Chem. 2008, 10, 1693–1701. [Google Scholar] [CrossRef]
- Wang, F.; Gao, L.; Zhao, Q.; Zhang, Y.; Dong, W.K.; Ding, Y.J. A highly selective fluorescent chemosensor for CN− based on a novel bis(salamo)-type tetraoxime ligand. Spectrochim. Acta A 2018, 190, 111–115. [Google Scholar] [CrossRef] [PubMed]
- Akine, S.; Utsuno, F.; Taniguchi, T.; Nabeshima, T. Dinuclear complexes of the N2O2 oxime chelate ligand with zinc(II)-lanthanide(III) as a selective sensitization system for Sm3+. Eur. J. Inorg. Chem. 2010, 20, 3143–3152. [Google Scholar] [CrossRef]
- Bhowmik, P.; Nayek, H.P.; Corbella, M.; Aliaga-Alcalde, N.; Chattopadhyay, S. Control of molecular architecture by steric factors: Mononuclear vs polynuclear manganese (III) compounds with tetradentate N2O2 donor Schiff bases. Dalton Trans. 2011, 40, 7916–7926. [Google Scholar] [CrossRef]
- Novozhilova, M.V.; Smirnova, E.A.; Polozhentseva, J.A.; Danilova, J.A.; Chepurnaya, I.A.; Karushev, M.P.; Malev, V.V.; Timonov, A.M. Multielectron redox processes in polymeric cobalt complexes with N2O2 Schiff base ligands. Electrochim. Acta 2018, 282, 105–115. [Google Scholar] [CrossRef]
- Pushkarev, A.P.; Balashova, T.V.; Kukinov, A.A.; Arsenyev, M.V.; Yablonskiy, A.N.; Kryzhkov, D.I.; Andreev, B.A.; Rumyantcev, R.V.; Fukin, G.K.; Bochkarev, M.N. Sensitization of NIR luminescence of Yb3+ by Zn2+ chromophores in heterometallic complexes with a bridging Schiff-base ligand. Dalton Trans. 2017, 46, 10408–10417. [Google Scholar] [CrossRef] [Green Version]
- Łḛpicka, K.; Pieta, P.; Francius, G.; Walcarius, A.; Kutner, W. Structure-reactivity requirements with respect to nickel-salen based polymers for enhanced electrochemical stability. Electrochim. Acta 2019, 315, 75–83. [Google Scholar] [CrossRef]
- Liu, X.; Manzurc, C.; Novoa, N.; Celedónc, S.; Carrilloc, D.; Hamon, J.R. Multidentate unsymmetrically substituted Schiff bases and their metal complexes: Synthesis, functional materials properties, and applications to catalysis. Coord. Chem. Rev. 2018, 357, 144–172. [Google Scholar] [CrossRef]
- Finelli, A.; Hérault, N.; Crochet, A.; Fromm, K.M. Threading salen-type Cu- and Ni-complexes into one-dimensional coordination polymers: Solution versus solid state, and the size effect of the alkali metal ion. Cryst. Growth Des. 2018, 18, 1215–1226. [Google Scholar] [CrossRef] [Green Version]
- Akine, S. Novel ion recognition systems based on cyclic and acyclic oligo (salen)-type ligands. J. Incl. Phenom. Macrocycl. Chem. 2012, 72, 25–54. [Google Scholar] [CrossRef]
- Celedón, S.; Dorcet, V.; Roisnel, T.; Singh, A.; Ledoux-Rak, I.; Hamon, J.R.; Carrillo, D.; Manzur, C. Main-chain oligomers from NiII- and CuII-centered unsymmetrical N2O2 Schiff-base complexes: Synthesis and spectral, structural, and second-order nonlinear optical properties. Eur. J. Inorg. Chem. 2014, 29, 4984–4993. [Google Scholar] [CrossRef]
- Kang, Q.P.; Li, X.Y.; Zhao, Q.; Ma, J.C.; Dong, W.K. Structurally characterized homotrinuclear Salamo-type nickel(lI) complexes: Synthesis, solvent effect and fluorescence properties. Appl. Organomet. Chem. 2018, 32, e4379. [Google Scholar] [CrossRef]
- Petrick, M.; Florian, B.; Katja, L.; Guntram, R.; Réne, P. Cooperative Al(Salen)-pyridinium catalysts for the asymmetric synthesis of trans-configured β-Lactones by [2 + 2]-cyclocondensation of acylbromides and aldehydes: Investigation of pyridinium substituent effects. Molecule 2012, 17, 7121–7150. [Google Scholar]
- Wei, Z.L.; Wang, L.; Wang, J.F.; Guo, W.T.; Zhang, Y.; Dong, W.K. Two highly sensitive and efficient salamo-like copper(II) complex probes for recognition of CN−. Spectrochim. Acta A 2020, 228, 117775. [Google Scholar] [CrossRef]
- Wang, L.; Wei, Z.L.; Chen, Z.Z.; Liu, C.; Dong, W.K.; Ding, Y.J. A chemical probe capable for fluorescent and colorimetric detection to Cu2+ and CN− based on coordination and nucleophilic addition mechanism. Microchem. J. 2020, 155, 104801. [Google Scholar] [CrossRef]
- Liu, C.; Wei, Z.L.; Mu, H.R.; Dong, W.K.; Ding, Y.J. A novel unsymmetric bis(salamo)-based chemosensor for detecting Cu2+ and continuous recognition of amino acids. J. Photochem. Photobio. A 2020, 397, 112569. [Google Scholar] [CrossRef]
- Li, X.Y.; Kang, Q.P.; Liu, L.Z.; Ma, J.C.; Dong, W.K. Trinuclear Co(II) and mononuclear Ni(II) Salamo-type bisoxime coordination compounds. Crystals 2018, 8, 43. [Google Scholar] [CrossRef] [Green Version]
- Jaros, S.W.; Guedes da Silva, M.F.C.; Florek, M.; Oliveira, M.C.; Smoleński, P.; Pombeiro, A.J.L.; Kirillov, A.M. Aliphatic dicarboxylate directed assembly of Silver(I) 1,3,5-triaza-7-phosphaadamantane coordination networks: Topological versatility and antimicrobial activity. Cryst. Growth Des. 2014, 14, 5408–5417. [Google Scholar] [CrossRef]
- Horcajada, P.; Serre, C.; Vallet-Regí, M.; Sebban, M.; Taulelle, F.; Férey, G. Metal–organic frameworks as efficient materials for drug delivery. Angew. Chem. Int. Ed. 2006, 45, 5974–5978. [Google Scholar] [CrossRef] [PubMed]
- Jaros, W.S.; Guedes da Silva, M.F.C.; Król, J.M.; Oliveira, M.C.; Smoleński, P.; Pombeiro, A.L.; Kirillov, A.M. Bioactive silver–organic networks assembled from 1,3,5-triaza-7-phosphaadamantane and flexible cyclohexanecarboxylate blocks. Inorg. Chem. 2016, 55, 1486–1496. [Google Scholar] [CrossRef] [PubMed]
- Jaros, W.S.; Smoleński, P.; Guedes da Silva, M.F.C.; Florek, M.; Król, J.; Staroniewicz, Z.; Pombeiro, A.J.L.; Kirillov, A.M. New silver bioMOFs driven by 1,3,5-triaza-7-phosphaadamantane-7-sulfide (PTAS): Synthesis, topological analysis and antimicrobial activity. CrystEngComm 2013, 15, 8060–8064. [Google Scholar] [CrossRef]
- Lu, X.Y.; Ye, J.W.; Zhang, D.K.; Xie, R.X.; Bogale, R.F.; Sun, Y.; Zhao, L.M.; Zhao, Q.; Ning, G.L. Silver carboxylate metal–organic frameworks with highly antibacterial activity and biocompatibility. J. Inorg. Biochem. 2013, 138, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Wyszogrodzka, G.; Marszałek, B.; Gil, B.; Dorożyński, P. Metal-organic frameworks: Mechanisms of antibacterial action and potential applications. Drug Discov. Today 2016, 21, 1009–1018. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, H.S.; Hinestroza, J.P.; Puentes, C.O.; Sierra, C.; Soto, C.Y. Antibacterial activity against Escherichia coli of Cu-BTC (MOF-199) metal-organic framework immobilized onto cellulosic fibers. J. Appl. Polym. Sci. 2014, 131, 40815. [Google Scholar]
- Zhang, L.W.; Li, X.Y.; Kang, Q.P.; Liu, L.Z.; Ma, J.C.; Dong, W.K. Structures and fluorescent and magnetic behaviors of newly synthesized NiII and CuII coordination compounds. Crystals 2018, 8, 173. [Google Scholar] [CrossRef] [Green Version]
- Ren, Z.L.; Hao, J.; Hao, P.; Dong, X.Y.; Bai, Y.; Dong, W.K. Synthesis, crystal structure, luminescence and electrochemical properties of a salamo-type trinuclear cobalt(II) complex. Z. Naturforsch B 2018, 73, 203–210. [Google Scholar] [CrossRef]
- Zhang, L.W.; Liu, L.Z.; Wang, F.; Dong, W.K. Unprecedented fluorescent dinuclear CoII and ZnII coordination compounds with a symmetric bis(salamo)-like tetraoxime. Molecules 2018, 23, 1141. [Google Scholar] [CrossRef] [Green Version]
- Hao, J.; Li, X.Y.; Zhang, Y.; Dong, W.K. A reversible bis(salamo)-based fluorescence sensor for selective detection of Cd2+ in water-containing systems and food samples. Materials 2018, 11, 523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, L.; Liu, C.; Wang, F.; Dong, W.K. Tetra-, penta- and hexa-coordinated transition metal complexes constructedfrom coumarin-containing N2O2 ligand. Crystals 2018, 8, 77. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.Z.; Wang, L.; Yu, M.; Zhao, Q.; Zhang, Y.; Sun, Y.X.; Dong, W.K. A highly sensitive and selective fluorescent “off-on-off” relay chemosensor based on a new bis(salamo)-type tetraoxime for detecting Zn2+ and CN−. Spectrochim. Acta A 2019, 222, 117209. [Google Scholar] [CrossRef]
- Peng, Y.D.; Li, X.Y.; Kang, Q.P.; An, G.X.; Zhang, Y.; Dong, W.K. Synthesis and fluorescence properties of asymmetrical Salamo-type tetranuclear zinc(II) complex. Crystals 2018, 8, 107. [Google Scholar] [CrossRef] [Green Version]
- Dong, X.Y.; Kang, Q.P.; Li, X.Y.; Ma, J.C.; Dong, W.K. Structurally characterized solvent-induced homotrinuclear cobalt(II) N2O2-donor bisoxime-type complexes. Crystals 2018, 8, 139. [Google Scholar] [CrossRef] [Green Version]
- Yu, M.; Zhang, Y.; Pan, Y.Q.; Wang, L. Two novel copper(II) salamo-based complexes: Syntheses, X-ray crystal structures, spectroscopic properties and Hirshfeld surfaces analyses. Inorg. Chim. Acta 2020, 509, 119701. [Google Scholar] [CrossRef]
- Zhang, S.Z.; Chang, J.; Zhang, H.J.; Sun, Y.X.; Wu, Y.; Wang, Y.B. Synthesis, crystal structure and spectral properties of binuclear Ni(II) and cubane-like Cu4(μ3-O)4 cored tetranuclear Cu(II) complexes based on coumarin Schiff base. Chin. J. Inorg. Chem. 2020, 36, 503–514. [Google Scholar]
- Chang, J.; Zhang, S.Z.; Wu, Y.; Zhang, H.J.; Sun, Y.X. Three supramolecular trinuclear nickel(II) complexes based on Salamo-type chelating ligand: Syntheses, crystal structures, solvent effect, Hirshfeld surface analysis and DFT calculation. Transit. Met. Chem. 2020, 45, 279–293. [Google Scholar] [CrossRef]
- Gao, L.; Wang, F.; Zhao, Q.; Zhang, Y.; Dong, W.K. Mononuclear Zn(II) and trinuclear Ni(II) complexes derived from a coumarin-containing N2O2 ligand: Syntheses, crystal structures and fluorescence properties. Polyhedron 2018, 139, 7–16. [Google Scholar] [CrossRef]
- Li, J.; Zhang, H.J.; Chang, J.; Sun, Y.X.; Huang, Y.Q. Solvent-induced unsymmetric Salamo-like trinuclear NiII complexes: Syntheses, crystal structures, fluorescent and magnetic properties. Crystals 2018, 8, 176. [Google Scholar] [CrossRef] [Green Version]
- Chang, J.; Zhang, H.J.; Jia, H.R.; Sun, Y.X. Binuclear nickel(II) and zinc(II) complexes based on 2-amino-3-hydroxy-pyridine Schiff base: Syntheses, supramolecular structures and spectral properties. Chin. J. Inorg. Chem. 2018, 34, 2097–2107. [Google Scholar]
- Jia, H.R.; Chang, J.; Zhang, H.J.; Li, J.; Sun, Y.X. Three polyhydroxyl-bridged defective dicubane tetranuclear MnIII complexes: Synthesis, crystal structures, and spectroscopic properties. Crystals 2018, 8, 272. [Google Scholar] [CrossRef] [Green Version]
- Kang, Q.P.; Li, X.Y.; Wang, L.; Zhang, Y.; Dong, W.K. Containing-PMBP N2O2-donors transition metal(II) complexes: Synthesis, crystal structure, Hirshfeld surface analyses and fluorescence properties. Appl. Organomet. Chem. 2019, 33, e5013. [Google Scholar] [CrossRef]
- Liu, L.Z.; Yu, M.; Li, X.Y.; Kang, Q.P.; Dong, W.K. Syntheses, structures, Hirshfeld analyses and fluorescent properties of two Ni(II) and Zn(II) complexes constructed from a bis(salamo)-like ligand. Chin. J. Inorg. Chem. 2019, 35, 1283–1294. [Google Scholar]
- Kang, Q.P.; Li, X.Y.; Wei, Z.L.; Zhang, Y.; Dong, W.K. Symmetric containing-PMBP N2O2-donors nickel(II) complexes: Syntheses, structures, Hirshfeld analyses and fluorescent properties. Polyhedron 2019, 165, 38–50. [Google Scholar] [CrossRef]
- Peng, Y.D.; Wang, F.; Gao, L.; Dong, W.K. Structurally characterized dinuclear zinc(II) bis(salamo)-type tetraoxime complex possessing square pyramidal and trigonal bipyramidal geometries. J. Chin. Chem. Soc. 2018, 65, 893–899. [Google Scholar] [CrossRef]
- Li, X.Y.; Kang, Q.P.; Liu, C.; Zhang, Y.; Dong, W.K. Structurally characterized homo-trinuclear ZnII and hetero-pentanuclear [ZnII4LnIII] complexes constructed from an octadentate bis(Salamo)-based ligand:Hirshfeld surfaces, fluorescence and catalytic properties. New J. Chem. 2019, 43, 4605–4619. [Google Scholar] [CrossRef]
- Zhao, Q.; An, X.X.; Liu, L.Z.; Dong, W.K. Syntheses, luminescences and Hirshfeld surfaces analyses of structurally characterized homo-trinuclear ZnII and hetero-pentanuclear ZnII-LnIII (Ln = Eu, Nd) bis(salamo)-like complexes. Inorg. Chim. Acta 2019, 490, 6–15. [Google Scholar] [CrossRef]
- Yu, M.; Mu, H.R.; Liu, L.Z.; Li, N.; Bai, Y.; Dong, X.Y. Syntheses, structures and Hirshfeld analyses of trinuclear Ni(II) salamo-type complexes. Chin. J. Inorg. Chem. 2019, 35, 1109–1120. [Google Scholar]
- Geary, W.J. The use of conductivity measurements in organic solvents for the characterization of coordination compounds. Coord. Chem. Rev. 1971, 7, 81–122. [Google Scholar] [CrossRef]
- Sun, Y.X.; Pan, Y.Q.; Xu, X.; Zhang, Y. Unprecedented dinuclear CuII N,O-donor complex: Synthesis, structural characterization, fluorescence property, and Hirshfeld analysis. Crystals 2019, 9, 607. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Yu, M.; Pan, Y.Q.; Zhang, Y.; Xu, L.; Dong, W.K. Three rare heteromultinuclear 3d-4f salamo-like complexes constructed from auxiliary ligand 4,4′-bipy: Syntheses, structural characterizations, fluorescence and antimicrobial properties. Appl. Organomet. Chem. 2020, 34, e5442. [Google Scholar] [CrossRef]
- Pan, Y.Q.; Zhang, Y.; Yu, M.; Zhang, Y.; Wang, L. Newly synthesized homomultinuclear Co(II) and Cu(II) bissalamo-like complexes: Structural characterizations, Hirshfeld analyses, fluorescence and antibacterial properties. Appl. Organomet. Chem. 2020, 34, e5441. [Google Scholar] [CrossRef]
- Liu, C.; An, X.X.; Cui, Y.F.; Xie, K.F.; Dong, W.K. Novel structurally characterized hetero-bimetallic [Zn(II)2M(II)] (M = Ca and Sr) bis(salamo)-type complexes: DFT calculation, Hirshfeld analyses, antimicrobial and fluorescent properties. Appl. Organomet. Chem. 2020, 34, e5272. [Google Scholar] [CrossRef]
- Pan, Y.Q.; Xu, X.; Zhang, Y.; Zhang, Y.; Dong, W.K. A highly sensitive and selective bis(salamo)-type fluorescent chemosensor for identification of Cu2+ and the continuous recognition of S2−, Arginine and Lysine. Spectrochim. Acta A 2020, 229, 117927. [Google Scholar] [CrossRef]
- Chai, L.Q.; Li, Y.X.; Chen, L.C.; Zhang, J.Y.; Huang, J.J. Synthesis, X-ray structure, spectroscopic, electrochemical properties and DFT calculation of a bridged dinuclear copper(II) complex. Inorg. Chim. Acta 2016, 444, 193–201. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, L.Z.; Peng, Y.D.; Li, N.; Dong, W.K. Structurally characterized trinuclear nickel(II) and copper(II) Salamo-type complexes: Syntheses, Hirshfeld analyses and fluorescent properties. Transit. Met. Chem. 2019, 44, 627–639. [Google Scholar] [CrossRef]
- Chai, L.Q.; Tang, L.J.; Chen, L.C.; Huang, J.J. Structural, spectral, electrochemical and DFT studies of two mononuclear manganese(II) and zinc(II) complexes. Polyhedron 2017, 122, 228–240. [Google Scholar] [CrossRef]
- Zhou, L.; Hu, Q.; Chai, L.Q.; Mao, K.H.; Zhang, H.S. X-ray characterization, spectroscopic, DFT calculations and Hirshfeld surface analysis of two 3-D supramolecular mononuclear zinc(II) and trinuclear copper(II) complexes. Polyhedron 2019, 158, 102–116. [Google Scholar] [CrossRef]
- Wang, F.; Liu, L.Z.; Gao, L.; Dong, W.K. Unusual constructions of two Salamo-based copper(II) complexes. Spectrochim. Acta A 2018, 203, 56–64. [Google Scholar] [CrossRef]
- An, X.X.; Zhao, Q.; Mu, H.R.; Dong, W.K. A new half-salamo-based homo-trinuclear nickel(II) complex: Crystal structure, Hirshfeld surface analysis, and fluorescence properties. Crystals 2019, 9, 101. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.W.; Zhang, Y.; Cui, Y.F.; Yu, M.; Dong, W.K. Heterobimetallic [NiIILnIII] (Ln = Sm and Tb) N2O4-donor coordination polymers: Syntheses, crystal structures and fluorescence properties. Inorg. Chim. Acta 2020, 506, 119534. [Google Scholar] [CrossRef]
- Zhang, H.J.; Chang, J.; Jia, H.R.; Sun, Y.X. Syntheses, supramolecular structures and spectroscopic properties of Cu(II) and Ni(II) complexes with Schiff base containing oxime group. Chin. J. Inorg. Chem. 2018, 34, 2261–2270. [Google Scholar]
- An, X.X.; Chen, Z.Z.; Mu, H.R.; Zhao, L. Investigating into crystal structures and supramolecular architectures of four newly synthesized hetero-octanuclear [CuII4-LnIII4] (Ln = Sm, Eu, Tb and Dy) complexes produced by a hexadentate bisoxime chelate ligand. Inorg. Chim. Acta 2020, 511, 119823. [Google Scholar] [CrossRef]
- Colinas, I.R.; Rojas-Andrade, M.D.; Chakraborty, I.; Oliver, S.R.J. Two structurally diverse Zn-based coordination polymers with excellent antibacterial activity. CrystEngComm 2018, 20, 3353–3362. [Google Scholar] [CrossRef]














| Polymer | The [Ni(II)-Sm(III)] Coordination Polymer |
|---|---|
| Empirical formula | C28H26N7NiO15Sm |
| Formula weight | 909.62 |
| T, K | 173(2) |
| Radiation; wavelength (Å) | Mo Kα; 0.71073 |
| Crystal system | monoclinic |
| Space group | P21/c |
| a, Å | 11.3928(4) |
| b, Å | 19.4539(7) |
| c, Å | 19.4229(8) |
| β, deg | 98.689(2) |
| Volume, Å3 | 4255.4(3) |
| Z | 4 |
| Dcalcd, g cm−3 | 1.42 |
| Crystal size, mm3 | 0.18 × 0.20 × 0.22 |
| Absorption coefficient, mm−1 | 1.9 |
| range data collection, deg | 2.400 to 26.000 |
| F(000), e | 1812 |
| hklrange | ±14, ±24, ±24 |
| Refl. collected/unique/Rint | 60159/9380/0.0196 |
| Data/restraints/ref. parameters | 9380/11/471 |
| Final R1/wR2 [I > 2 σ(I)] a,b | 0.0332/0.1039 |
| Final R1 wR2(all data) a,b | 0.0405/0.1063 |
| GoF c | 0.989 |
| Δρmax/min,e Å−3 | 1.02/–1.01 |
| Bond | Lengths | Bond | Lengths | Bond | Lengths |
| Sm1-O1 | 2.653(4) | Sm1-O10 | 2.617(4) | Ni1-N1 | 2.072(4) |
| Sm1-O2 | 2.353(2) | Sm1-O11 | 2.518(4) | Ni1-N2 | 2.030(4) |
| Sm1-O5 | 2.416(2) | Sm1-O13 | 2.510(3) | Ni1-N3 | 2.172(2) |
| Sm1-O6 | 2.6013(19) | Sm1-O14 | 2.481(2) | Ni1-N4 #1 | 2.167(2) |
| Sm1-O7 | 2.529(3) | Ni1-O2 | 2.0370(19) | ||
| Sm1-O8 | 2.463(4) | Ni1-O5 | 2.0399(19) | ||
| Bond | Angles | Bond | Angles | Bond | Angles |
| O1-Sm1-O2 | 61.11(7) | O5-Sm1-O10 | 173.74(9) | O10-Sm1-O13 | 63.93(10) |
| O1-Sm1-O5 | 117.08(7) | O5-Sm1-O11 | 124.32(10) | O10-Sm1-O14 | 102.11(9) |
| O1-Sm1-O6 | 143.61(9) | O5-Sm1-O13 | 114.44(8) | O11-Sm1-O13 | 73.92(12) |
| O1-Sm1-O7 | 71.32(11) | O5-Sm1-O14 | 73.25(7) | O11-Sm1-O14 | 73.63(10) |
| O1-Sm1-O8 | 78.12(11) | O6-Sm1-O7 | 73.41(10) | O13-Sm1-O14 | 51.02(7) |
| O1-Sm1-O10 | 64.15(10) | O6-Sm1-O8 | 73.12(10) | O2-Ni1-O5 | 78.33(8) |
| O1-Sm1-O11 | 72.52(11) | O6-Sm1-O10 | 121.02(9) | O2-Ni1-N1 | 90.31(12) |
| O1-Sm1-O13 | 128.07(9) | O6-Sm1-O11 | 140.94(10) | O2-Ni1-N2 | 167.66(12) |
| O1-Sm1-O14 | 143.78(9) | O6-Sm1-O13 | 69.80(10) | O2-Ni1-N3 | 90.61(12) |
| O2-Sm1-O5 | 65.35(7) | O6-Sm1-O14 | 72.59(7) | O2-Ni1-N4 #1 | 89.60(12) |
| O2-Sm1-O6 | 126.96(7) | O7-Sm1-O8 | 51.43(10) | O5-Ni1-N1 | 168.08(12) |
| O2-Sm1-O7 | 88.84(9) | O7-Sm1-O10 | 108.73(9) | O5-Ni1-N2 | 90.42(12) |
| O2-Sm1-O8 | 131.26(10) | O7-Sm1-O11 | 143.58(12) | O5-Ni1-N3 | 87.88(12) |
| O2-Sm1-O10 | 111.99(10) | O7-Sm1-O13 | 127.65(10) | O5-Ni1-N4 #1 | 95.18(12) |
| O2-Sm1-O11 | 77.41(10) | O7-Sm1-O14 | 142.47(9) | N1-Ni1-N2 | 101.20(15) |
| O2-Sm1-O13 | 143.33(9) | O8-Sm1-O10 | 66.71(12) | N1-Ni1-N3 | 88.64(14) |
| O2-Sm1-O14 | 99.12(7) | O8-Sm1-O11 | 116.21(12) | N1-Ni1-N4 #1 | 88.27(14) |
| O5-Sm1-O6 | 62.10(6) | O8-Sm1-O13 | 82.62(10) | O10-Sm1-O13 | 94.07(14) |
| O5-Sm1-O7 | 77.17(7) | O8-Sm1-O14 | 129.42(10) | O10-Sm1-O14 | 86.35(14) |
| O5-Sm1-O8 | 119.44(10) | O10-Sm1-O11 | 49.60(12) | O11-Sm1-O13 | 176.91(14) |
| D–H···A. | d(D-H) | d(H···A) | d(D···A) | ∠D–H···A | Symmetry Code |
|---|---|---|---|---|---|
| C1-H1B···O10 | 0.98 | 2.39 | 2.897(7) | 111 | |
| C4-H4···O9 | 0.95 | 2.55 | 3.365(5) | 143 | x, 3/2 − y, 1/2 + z |
| C9-H9A···N3 | 0.99 | 2.44 | 3.357(5) | 154 | |
| C9-H9B···O13 | 0.99 | 2.40 | 3.230(5) | 141 | 1 − x, −1/2 + y, 3/2 − z |
| C23-H23···O2 | 0.95 | 2.58 | 3.093(4) | 114 | |
| C24-H24···N1 | 0.95 | 2.61 | 3.104(5) | 113 | −1 + x, y, z |
| C28-H28···O7 | 0.95 | 2.25 | 3.120(5) | 151 | −1 + x, y, z |
| C19-H19···Cg2 | 0.98 | 2.56 | 2.897(7) | 114 | x, y, z |
| Cg6···Cg6 | 4.065(2) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, R.-Y.; An, X.-X.; Wang, J.-F.; Mu, H.-R.; Zhang, Y.-P.; Zhang, Y.; Dong, W.-K. A Self-Assembled Hetero-Bimetallic [Ni(II)-Sm(III)] Coordination Polymer Constructed from a Salamo-Like Ligand and 4,4′-Bipyridine: Synthesis, Structural Characterization, and Properties. Crystals 2020, 10, 579. https://doi.org/10.3390/cryst10070579
Li R-Y, An X-X, Wang J-F, Mu H-R, Zhang Y-P, Zhang Y, Dong W-K. A Self-Assembled Hetero-Bimetallic [Ni(II)-Sm(III)] Coordination Polymer Constructed from a Salamo-Like Ligand and 4,4′-Bipyridine: Synthesis, Structural Characterization, and Properties. Crystals. 2020; 10(7):579. https://doi.org/10.3390/cryst10070579
Chicago/Turabian StyleLi, Ruo-Yan, Xiao-Xin An, Ji-Fa Wang, Hao-Ran Mu, You-Peng Zhang, Yang Zhang, and Wen-Kui Dong. 2020. "A Self-Assembled Hetero-Bimetallic [Ni(II)-Sm(III)] Coordination Polymer Constructed from a Salamo-Like Ligand and 4,4′-Bipyridine: Synthesis, Structural Characterization, and Properties" Crystals 10, no. 7: 579. https://doi.org/10.3390/cryst10070579