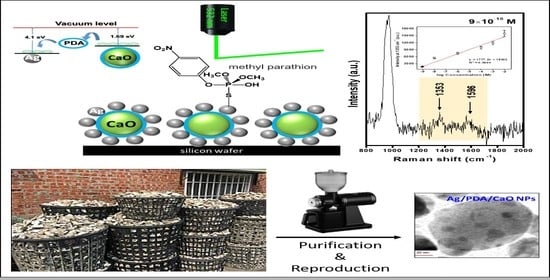
A Novel SERS Substrate Based on Discarded Oyster Shells for Rapid Detection of Organophosphorus Pesticide
Abstract
:1. Introduction
2. Materials and Methods
2.1. Transmission Electron Microscopy (TEM)
2.2. X-ray Diffraction (XRD)
2.3. X-ray Photoelectron Spectroscopy (XPS)
2.4. Raman Spectroscopy
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A

References
- Han, X.X.; Rodriguez, R.S.; Haynes, C.L.; Ozaki, Y.; Zhao, B. Surface-enhanced Raman spectroscopy. Nat. Rev. Methods Primers 2022, 1, 87. [Google Scholar] [CrossRef]
- Mikac, L.; Kovacevic, E.; Ukic, S.; Raic, M.; Jurkin, T.; Maric, I.; Gotic, M.; Ivanda, M. Detection of multi-class pesticide residues with surface-enhanced Raman spectroscopy. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2021, 252, 119478. [Google Scholar] [CrossRef] [PubMed]
- Ho, C.S.; Jean, N.; Hogan, C.A.; Blackmon, L.; Jeffrey, S.S.; Holodniy, M.; Banaei, N.; Saleh, A.A.E.; Ermon, S.; Dionne, J. Rapid identification of pathogenic bacteria using Raman spectroscopy and deep learning. Nat. Commun. 2019, 10, 4927. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.-Y.; Wu, I.H.; Tsai, C.-Y.; Kirankumar, R.; Hsieh, S. Detecting the release of plastic particles in packaged drinking water under simulated light irradiation using surface-enhanced Raman spectroscopy. Anal. Chim. Acta 2022, 1198, 339516. [Google Scholar] [CrossRef]
- Lin, P.-Y.; He, G.; Chen, J.; Dwivedi, A.K.; Hsieh, S. Monitoring the photoinduced surface catalytic coupling reaction and environmental exhaust fumes with an Ag/PDA/CuO modified 3D glass microfiber platform. J. Ind. Eng. Chem. 2020, 82, 424–432. [Google Scholar] [CrossRef]
- Koo, K.M.; Wang, J.; Richards, R.S.; Farrell, A.; Yaxley, J.W.; Samaratunga, H.; Teloken, P.E.; Roberts, M.J.; Coughlin, G.D.; Lavin, M.F.; et al. Design and Clinical Verification of Surface-Enhanced Raman Spectroscopy Diagnostic Technology for Individual Cancer Risk Prediction. ACS Nano 2018, 12, 8362–8371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rajput, V.; Gupta, R.K.; Prakash, J. Engineering metal oxide semiconductor nanostructures for enhanced charge transfer: Fundamentals and emerging SERS applications. J. Mater. Chem. C 2022, 10, 73–95. [Google Scholar] [CrossRef]
- Cui, Y.; Nilius, N.; Freund, H.-J.; Prada, S.; Giordano, L.; Pacchioni, G. Controlling the charge state of single Mo dopants in a CaO film. Phys. Rev. B 2013, 88, 205421. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Hutter, T.; Finnemore, A.S.; Huang, F.M.; Baumberg, J.J.; Elliott, S.R.; Steiner, U.; Mahajan, S. Metal Oxide Nanoparticle Mediated Enhanced Raman Scattering and Its Use in Direct Monitoring of Interfacial Chemical Reactions. Nano Lett. 2012, 12, 4242–4246. [Google Scholar] [CrossRef] [Green Version]
- Rhodes, C.; Franzen, S.; Maria, J.-P.; Losego, M.; Leonard, D.N.; Laughlin, B.; Duscher, G.; Weibel, S. Surface plasmon resonance in conducting metal oxides. J. Appl. Phys. 2006, 100, 054905. [Google Scholar] [CrossRef] [Green Version]
- Ates, M.; Arslan, Z.; Demir, V.; Daniels, J.; Farah, I.O. Accumulation and toxicity of CuO and ZnO nanoparticles through waterborne and dietary exposure of goldfish (Carassius auratus). Environ. Toxicol. 2015, 30, 119–128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hua, J.; Vijver, M.G.; Ahmad, F.; Richardson, M.K.; Peijnenburg, W.J.G.M. Toxicity of different-sized copper nano- and submicron particles and their shed copper ions to zebrafish embryos. Environ. Toxicol. Chem. 2014, 33, 1774–1782. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.; Wang, S.; Zhou, L.; Sun, L. The Potential Liver, Brain, and Embryo Toxicity of Titanium Dioxide Nanoparticles on Mice. Nanoscale Res. Lett. 2017, 12, 478. [Google Scholar] [CrossRef] [PubMed]
- Rizk, M.Z.; Ali, S.A.; Hamed, M.A.; El-Rigal, N.S.; Aly, H.F.; Salah, H.H. Toxicity of titanium dioxide nanoparticles: Effect of dose and time on biochemical disturbance, oxidative stress and genotoxicity in mice. Biomed. Pharmacother. 2017, 90, 466–472. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Thiagarajan, V.; Chandrasekaran, N.; Ravindran, B.; Mukherjee, A. Nanoplastics enhance the toxic effects of titanium dioxide nanoparticle in freshwater algae Scenedesmus obliquus. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2022, 256, 109305. [Google Scholar] [CrossRef]
- Hsieh, S.; Lin, P.-Y.; Chu, L.-Y. Improved Performance of Solution-Phase Surface-Enhanced Raman Scattering at Ag/CuO Nanocomposite Surfaces. J. Phys. Chem. C 2014, 118, 12500–12505. [Google Scholar] [CrossRef]
- Huang, Z.-H.; Peng, S.-W.; Hsieh, S.-L.; Kirankumar, R.; Huang, P.-F.; Chang, T.-M.; Dwivedi, A.K.; Chen, N.-F.; Wu, H.-M.; Hsieh, S. Polydopamine Ultrathin Film Growth on Mica via In-Situ Polymerization of Dopamine with Applications for Silver-Based Antimicrobial Coatings. Materials 2021, 14, 671. [Google Scholar] [CrossRef]
- Cheng, M.; Li, C.; Li, W.; Liu, Y. Trace Cd(2+) Ions Detection on the Flower-Like Ag@CuO Substrate. Nanomaterials 2020, 10, 1664. [Google Scholar] [CrossRef]
- Ye, F.; Ju, S.; Liu, Y.; Jiang, Y.; Chen, H.; Ge, L.; Yan, C.; Yuan, A. Ag-CuO Nanocomposites: Surface-Enhanced Raman Scattering Substrate and Photocatalytic Performance. Cryst. Res. Technol. 2019, 54, 1800257. [Google Scholar] [CrossRef]
- Alvarenga, R.A.F.d.; Galindro, B.M.; Helpa, C.d.F.; Soares, S.R. The recycling of oyster shells: An environmental analysis using Life Cycle Assessment. J. Environ. Manag. 2012, 106, 102–109. [Google Scholar] [CrossRef]
- Smith, R.A.; Wright, E.R. Elemental composition of oyster shell. Tex. J. Sci. 1962, 14, 222–224. [Google Scholar]
- Ryu, J.H.; Messersmith, P.B.; Lee, H. Polydopamine Surface Chemistry: A Decade of Discovery. ACS Appl. Mater. Interfaces 2018, 10, 7523–7540. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Dellatore, S.M.; Miller, W.M.; Messersmith, P.B. Mussel-Inspired Surface Chemistry for Multifunctional Coatings. Science 2007, 318, 426–430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prathap, A.; Shaijumon, M.M.; Sureshan, K.M. CaO nanocrystals grown over SiO2 microtubes for efficient CO2 capture: Organogel sets the platform. Chem. Commun. 2016, 52, 1342–1345. [Google Scholar] [CrossRef] [PubMed]
- Gusev, A.; Sadovnikov, S. Acanthite–argentite transformation in nanocrystalline silver sulfide and the Ag2S/Ag nanoheterostructure. Semiconductors 2016, 50, 682–687. [Google Scholar] [CrossRef]
- Park, H.-H.; Zhang, X.; Choi, Y.-J.; Park, H.-H.; Hill, R.H. Synthesis of Ag Nanostructures by Photochemical Reduction Using Citrate-Capped Pt Seeds. J. Nanomater. 2011, 2011, 265287. [Google Scholar] [CrossRef]
- Taherpour, A.A.; Rizehbandi, M.; Jahanian, F.; Naghibi, E.; Mahdizadeh, N.-A. Theoretical study of electron transfer process between fullerenes and neurotransmitters; acetylcholine, dopamine, serotonin and epinephrine in nanostructures [neurotransmitters].C n complexes. J. Chem. Biol. 2016, 9, 19–29. [Google Scholar] [CrossRef] [Green Version]
- Feng, J.-J.; Zhang, P.-P.; Wang, A.-J.; Liao, Q.-C.; Xi, J.-L.; Chen, J.-R. One-step synthesis of monodisperse polydopamine-coated silver core-shell nanostructures for enhanced photocatalysis. New J. Chem. 2012, 36, 148–154. [Google Scholar] [CrossRef]
- Akin, M.S.; Yilmaz, M.; Babur, E.; Ozdemir, B.; Erdogan, H.; Tamer, U.; Demirel, G. Large area uniform deposition of silver nanoparticles through bio-inspired polydopamine coating on silicon nanowire arrays for practical SERS applications. J. Mater. Chem. B 2014, 2, 4894–4900. [Google Scholar] [CrossRef] [Green Version]
- Hopkins, B.J.; Vick, F.A. Thermionic and Related Properties of Calcium Oxide. Br. J. Appl. Phys. 1958, 9, 257–264. [Google Scholar] [CrossRef]
- Hsieh, C.-W.; Lin, P.-Y.; Hsieh, S. Improved surface-enhanced Raman scattering of insulin fibril templated colloidal gold nanoparticles on silicon. J. NanoPhotonics 2012, 6, 063501. [Google Scholar] [CrossRef]
- Le Ru, E.C.; Blackie, E.; Meyer, M.; Etchegoin, P.G. Surface Enhanced Raman Scattering Enhancement Factors: A Comprehensive Study. J. Phys. Chem. C 2007, 111, 13794–13803. [Google Scholar] [CrossRef]
- Sun, L.; Song, Y.; Wang, L.; Guo, C.; Sun, Y.; Liu, Z.; Li, Z. Ethanol-Induced Formation of Silver Nanoparticle Aggregates for Highly Active SERS Substrates and Application in DNA Detection. J. Phys. Chem. C 2008, 112, 1415–1422. [Google Scholar] [CrossRef]
- Cha, M.G.; Kim, H.M.; Kang, Y.L.; Lee, M.; Kang, H.; Kim, J.; Pham, X.H.; Kim, T.H.; Hahm, E.; Lee, Y.S.; et al. Thin silica shell coated Ag assembled nanostructures for expanding generality of SERS analytes. PLoS ONE 2017, 12, e0178651. [Google Scholar] [CrossRef] [Green Version]
- Huang, J.; Ma, D.; Chen, F.; Chen, D.; Bai, M.; Xu, K.; Zhao, Y. Green in Situ Synthesis of Clean 3D Chestnutlike Ag/WO3−x Nanostructures for Highly Efficient, Recyclable and Sensitive SERS Sensing. ACS Appl. Mater. Interfaces 2017, 9, 7436–7446. [Google Scholar] [CrossRef]
- Yang, L.; Bao, Z.; Wu, Y.; Liu, J. Clean and reproducible SERS substrates for high sensitive detection by solid phase synthesis and fabrication of Ag-coated Fe3O4 microspheres. J. Raman Spectrosc. 2012, 43, 848–856. [Google Scholar] [CrossRef]
- Pal, J.; Ganguly, M.; Dutta, S.; Mondal, C.; Negishi, Y.; Pal, T. Hierarchical Au-CuO nanocomposite from redox transformation reaction for surface enhanced Raman scattering and clock reaction. CrystEngComm 2014, 16, 883–893. [Google Scholar] [CrossRef]
- Hu, J.-W.; Li, J.-F.; Ren, B.; Wu, D.-Y.; Sun, S.-G.; Tian, Z.-Q. Palladium-Coated Gold Nanoparticles with a Controlled Shell Thickness Used as Surface-Enhanced Raman Scattering Substrate. J. Phys. Chem. C 2007, 111, 1105–1112. [Google Scholar] [CrossRef]
- Liu, J.; Meng, G.; Li, Z.; Huang, Z.; Li, X. Ag-NP@Ge-nanotaper/Si-micropillar ordered arrays as ultrasensitive and uniform surface enhanced Raman scattering substrates. Nanoscale 2015, 7, 18218–18224. [Google Scholar] [CrossRef]
- Zhu, C.; Meng, G.; Zheng, P.; Huang, Q.; Li, Z.; Hu, X.; Wang, X.; Huang, Z.; Li, F.; Wu, N. A Hierarchically Ordered Array of Silver-Nanorod Bundles for Surface-Enhanced Raman Scattering Detection of Phenolic Pollutants. Adv. Mater. 2016, 28, 4871–4876. [Google Scholar] [CrossRef]
- Hu, X.; Zheng, P.; Meng, G.; Huang, Q.; Zhu, C.; Han, F.; Huang, Z.; Li, Z.; Wang, Z.; Wu, N. An ordered array of hierarchical spheres for surface-enhanced Raman scattering detection of traces of pesticide. Nanotechnology 2016, 27, 384001. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Wu, L.; Lu, Z.; Li, Q.; Yin, W.; Ding, F.; Han, H. Gecko-Inspired Nanotentacle Surface-Enhanced Raman Spectroscopy Substrate for Sampling and Reliable Detection of Pesticide Residues in Fruits and Vegetables. Anal. Chem. 2017, 89, 2424–2431. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhu, C.; Hu, X.; Xu, Q.; Zhao, H.; Meng, G.; Lei, Y. Highly sensitive surface-enhanced Raman scattering detection of organic pesticides based on Ag-nanoplate decorated graphene-sheets. Appl. Surf. Sci. 2019, 486, 405–410. [Google Scholar] [CrossRef]
- Ma, Y.; Du, Y.; Chen, Y.; Gu, C.; Jiang, T.; Wei, G.; Zhou, J. Intrinsic Raman signal of polymer matrix induced quantitative multiphase SERS analysis based on stretched PDMS film with anchored Ag nanoparticles/Au nanowires. Chem. Eng. J. 2020, 381, 122710. [Google Scholar] [CrossRef]
- Liang, Y.; Wang, H.; Xu, Y.; Pan, H.; Guo, K.; Zhang, Y.; Chen, Y.; Liu, D.; Zhang, Y.; Yao, C.; et al. A novel molecularly imprinted polymer composite based on polyaniline nanoparticles as sensitive sensors for parathion detection in the field. Food Control 2022, 133, 108638. [Google Scholar] [CrossRef]
- Wu, H.; Chen, S.; Wang, Y.; Tan, J.; Feng, Y.; Hou, L.; Wang, H. Spiny gold nanoparticles colloids as substrate for sensing of methyl parathion based on surfaced-enhanced Raman scattering. Mater. Lett. 2022, 313, 131687. [Google Scholar] [CrossRef]






| Materials | Method | Chemicals | Detection Limit (nM) | References |
|---|---|---|---|---|
| AgNPs-Ge/Si | Raman | Methyl Parathion | 100 | [39] |
| Au-Ag alloy | Raman | Methyl Parathion | 5 | [40] |
| Au/Ag-nanosphere/Al2O3-layer/Ag-nanoparticles | Raman | Methyl Parathion | 1 | [41] |
| Ag NPs on 3D PDMS nanotentacle array | Raman | Methyl Parathion | 78 | [42] |
| Silver nanoparticles/graphene | Raman | Methyl Parathion | 600 | [43] |
| Ag/AuNWs/PDMS | Raman | Methyl Parathion | 3.8 | [44] |
| polyaniline nanoparticles | Raman | Methyl Parathion | 11.3 | [45] |
| triangular gold nanoparticles | Raman | Methyl Parathion | 500 | [46] |
| Ag/PDA/CaO | Raman | 4-ATP | 10 | This work |
| Methyl Parathion | 0.9 | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chu, C.-Y.; Lin, P.-Y.; Li, J.-S.; Kirankumar, R.; Tsai, C.-Y.; Chen, N.-F.; Wen, Z.-H.; Hsieh, S. A Novel SERS Substrate Based on Discarded Oyster Shells for Rapid Detection of Organophosphorus Pesticide. Coatings 2022, 12, 506. https://doi.org/10.3390/coatings12040506
Chu C-Y, Lin P-Y, Li J-S, Kirankumar R, Tsai C-Y, Chen N-F, Wen Z-H, Hsieh S. A Novel SERS Substrate Based on Discarded Oyster Shells for Rapid Detection of Organophosphorus Pesticide. Coatings. 2022; 12(4):506. https://doi.org/10.3390/coatings12040506
Chicago/Turabian StyleChu, Chi-Yu, Pei-Ying Lin, Jun-Sian Li, Rajendranath Kirankumar, Chen-Yu Tsai, Nan-Fu Chen, Zhi-Hong Wen, and Shuchen Hsieh. 2022. "A Novel SERS Substrate Based on Discarded Oyster Shells for Rapid Detection of Organophosphorus Pesticide" Coatings 12, no. 4: 506. https://doi.org/10.3390/coatings12040506