Physicochemical Investigation of Biosynthesis of a Protein Coating on Glass That Promotes Mammalian Cell Growth Using Lactobacillus rhamnosus GG Bacteria
Abstract
:1. Introduction
2. Materials and Methods
2.1. General Instrumentation and Materials
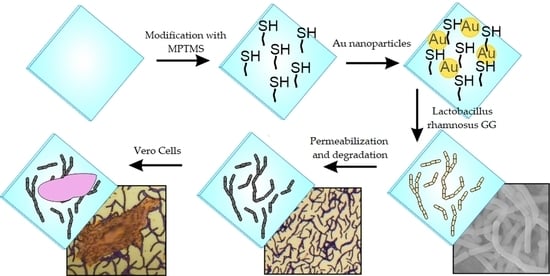
2.2. Coating Glass with Gold Nanoparticles
2.3. Surface Inoculation with Lactobacillus rhamnosus GG Bacteria
2.4. Formation of the Final Coating
2.5. Microscopic Imaging of Surfaces Obtained at Various Stages of the Synthesis
2.5.1. Scanning Electron Microscopy (SEM)
2.5.2. Optical Microscopy and Counting Mammalian Cells
2.6. DLS and Zeta Potential Measurements
2.7. Evaluation of Bacterial Counts by Crystal Violet Staining
2.8. Study of Cell Cultures Growth on the New Coating
3. Results and Discussion
3.1. Formation of the Gold Nanoparticle Layer on Glass
3.2. Lactobacillus rhamnosus GG Bacteria on the Obtained Surface
3.3. Chemical Analysis and Imaging of the Final Coating
3.4. Aplication of Obtained Coatings for Cell Cultures
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Risbridger, G.P. Human Cell Lines as Tools of Our Trade: “Laying It on the (Cell) Line”. Mol. Endocrinol. 2015, 29, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Mirabelli, P.; Coppola, L.; Salvatore, M. Cancer Cell Lines Are Useful Model Systems for Medical Research. Cancers 2019, 11, 1098. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Segeritz, C.-P.; Vallier, L. Basic Science Methods for Clinical Researchers Chapter 9—Cell Culture: Growing Cells as Model Systems In Vitro; Academic Press: Cambridge, MA, USA, 2017; pp. 151–172. ISBN 9780128030776. [Google Scholar]
- Verma, A.; Verma, M.; Singh, A. Animal tissue culture principles and applications. Anim. Biotechnol. 2020, 269–293. [Google Scholar] [CrossRef]
- Lerman, M.J.; Lembong, J.; Muramoto, S.; Gillen, G.; Fisher, J.P. The Evolution of Polystyrene as a Cell Culture Material. Tissue Eng. Part B Rev. 2018, 24, 359–372. [Google Scholar] [CrossRef] [Green Version]
- Cooke, M.J.; Phillips, S.R.; Shah, D.S.; Athey, D.; Lakey, J.H.; Przyborski, S.A. Enhanced cell attachment using a novel cell culture surface presenting functional domains from extracellular matrix proteins. Cytotechnology 2008, 56, 71–79. [Google Scholar] [CrossRef] [Green Version]
- McKeehan, W.L.; Ham, R.G. Stimulation of clonal growth of normal fibroblasts with substrata coated with basic polymers. J. Cell Biol. 1976, 71, 727–734. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joseph, B.; James, J.; Kalarikkal, N.; Thomas, S. Recycling of medical plastics. Adv. Ind. Eng. Polym. Res. 2021, 4, 199–208. [Google Scholar] [CrossRef]
- Vallet-Regi, M.; Ragel, C.V.; Salinas, A.J. Glasses with Medical Applications. Eur. J. Inorg. Chem. 2003, 2003, 1029–1042. [Google Scholar] [CrossRef]
- Iijima, K.; Suzuki, R.; Iizuka, A.; Ueno-Yokohata, H.; Kiyokawa, N.; Hashizume, M. Surface functionalization of tissue culture polystyrene plates with hydroxyapatite under body fluid conditions and its effect on differentiation behaviors of mesenchymal stem cells. Colloids Surf. B Biointerfaces 2016, 147, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Grudzien, J.; Jarosz, M.; Kaminski, K.; Kobasa, M.; Wolski, K.; Koziel, M.; Pisarek, M.; Sulka, G.D. Growth of Lactic Acid Bacteria on Gold-Influence of Surface Roughness and Chemical Composition. Nanomaterials 2020, 10, 2499. [Google Scholar] [CrossRef]
- Jarosz, M.; Grudzien, J.; Kaminski, K.; Gawlak, K.; Wolski, K.; Nowakowska, M.; Sulka, G.D. Novel bioelectrodes based on polysaccharide modified gold surfaces and electrochemically active Lactobacillus rhamnosus GG biofilms. Electrochim. Acta 2019, 296, 999–1008. [Google Scholar] [CrossRef]
- Gu, X.; Xu, Z.; Gu, L.; Xu, H.; Han, F.; Chen, B.; Pan, X. Preparation and antibacterial properties of gold nanoparticles: A review. Environ. Chem. Lett. 2021, 19, 167–187. [Google Scholar] [CrossRef]
- Mohamed, M.M.; Fouad, S.A.; Elshoky, H.A.; Mohammed, G.M.; Salaheldin, T.A. Antibacterial effect of gold nanoparticles against Corynebacterium pseudotuberculosis. Int. J. Vet. Sci. Med. 2017, 5, 23–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roselli, M.; Finamore, A.; Britti, M.; Mengheri, E. Probiotic bacteria Bifidobacterium animalis MB5 and Lactobacillus rhamnosus GG protect intestinal Caco-2 cells from the inflammation-associated response induced by enterotoxigenic Escherichia coli K88. Br. J. Nutr. 2006, 95, 1177–1184. [Google Scholar] [CrossRef] [PubMed]
- Jia, R.; Chen, H.; Chen, H.; Ding, W. Effects of fermentation with Lactobacillus rhamnosus GG on product quality and fatty acids of goat milk yogurt. J. Dairy Sci. 2016, 99, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Canani, R.B.; Sangwan, N.; Stefka, A.T.; Nocerino, R.; Paparo, L.; Aitoro, R.; Calignano, A.; Khan, A.A.; Gilbert, J.A.; Nagler, C.R. Lactobacillus rhamnosus GG-supplemented formula expands butyrate-producing bacterial strains in food allergic infants. ISME. J. 2016, 10, 742–750. [Google Scholar] [CrossRef]
- Sarkar, A.; Lehto, S.M.; Harty, S.; Dinan, T.G.; Cryan, J.F.; Burnet, P.W.J. Psychobiotics and the Manipulation of Bacteria–Gut–Brain Signals. Trends Neurosci. 2016, 39, 763–781. [Google Scholar] [CrossRef] [Green Version]
- Shi, C.W.; Cheng, M.Y.; Yang, X.; Lu, Y.Y.; Yin, H.D.; Zeng, Y.; Wang, R.Y.; Jiang, Y.L.; Yang, W.T.; Wang, J.Z.; et al. Probiotic Lactobacillus rhamnosus GG Promotes Mouse Gut Microbiota Diversity and T Cell Differentiation. Front. Microbiol. 2020, 11, 607735. [Google Scholar] [CrossRef]
- Jazayeri, M.H.; Amani, H.; Pourfatollah, A.A.; Pazoki-Toroudi, H.; Sedighimoghaddam, B. Various methods of gold nanoparticles (GNPs) conjugation to antibodies. Sens. Bio-Sens. Res. 2016, 9, 17–22. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.-J.; Struk, K.N.; Brennan, A.B. Surface Modification of Silicate Glass Using 3-(Mercaptopropyl)trimethoxysilane for Thiol-Ene Polymerization. Langmuir 2011, 27, 13754–13761. [Google Scholar] [CrossRef]
- Darmady, E.M.; Hughes, K.E.A.; Jones, J.D.; Prince, D.; Tuke, W. Sterilization by dry heat. J. Clin. Path. 1961, 14, 38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ammerman, N.C.; Beier-Sexton, M.; Azad, A.F. Growth and Maintenance of Vero Cell Lines. Curr. Protoc. Microbiol. 2008, 11, A-4E. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sène, M.A.; Kiesslich, S.; Djambazian, H.; Ragoussis, J.; Xia, Y.; Kamen, A.A. Haplotype-resolved de novo assembly of the Vero cell line genome. npj Vaccines 2021, 6, 106. [Google Scholar] [CrossRef] [PubMed]
- Link, S.; El-Sayed, M.A. Size and Temperature Dependence of the Plasmon Absorption of Colloidal Gold Nanoparticles. J. Phys. Chem. B 1999, 103, 4212–4217. [Google Scholar] [CrossRef]
- Huang, P.; Li, Z.; Hu, H.; Cui, D. Synthesis and Characterization of Bovine Serum Albumin-Conjugated Copper Sulfide Nanocomposites. J. Nanomater. 2010, 2010, 641545. [Google Scholar] [CrossRef]
- Jokar, S.; Pourjavadia, A.; Adeli, M. Albumin–graphene oxide conjugates; carriers for anticancer drugs. RSC Adv. 2014, 4, 33001–33006. [Google Scholar] [CrossRef]
- Acuña-Nelson, S.M.; Bastías-Montes, J.M.; Cerda-Leal, F.R.; Parra-Flores, J.E.; Aguirre-García, J.S.; Toledo, P.G. Nanocoatings of Bovine Serum Albumin on Glass: Effects of pH and Temperature. J. Nanomater. 2020, 2020, 11. [Google Scholar] [CrossRef]
- Raphel, J.; Karlsson, J.; Galli, S.; Wennerberg, A.; Lindsay, C.; Haugh, M.G.; Pajarinen, J.; Goodman, S.B.; Jimbo, R.; Andersson, M.; et al. Engineered protein coatings to improve the osseointegration of dental and orthopaedic implants. Biomaterials 2016, 83, 269–282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Gaal, R.C.; Vrehen, A.F.; Van Sprang, J.F.; Fransen, P.P.; Van Turnhout, M.C.; Dankers, P.Y. Biomaterial screening of protein coatings and peptide additives: Towards a simple synthetic mimic of a complex natural coating for a bio-artificial kidney. Biomater. Sci. 2021, 9, 2209–2220. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaminski, K.; Syrek, K.; Grudzień, J.; Obloza, M.; Adamczyk, M.; Sulka, G.D. Physicochemical Investigation of Biosynthesis of a Protein Coating on Glass That Promotes Mammalian Cell Growth Using Lactobacillus rhamnosus GG Bacteria. Coatings 2021, 11, 1410. https://doi.org/10.3390/coatings11111410
Kaminski K, Syrek K, Grudzień J, Obloza M, Adamczyk M, Sulka GD. Physicochemical Investigation of Biosynthesis of a Protein Coating on Glass That Promotes Mammalian Cell Growth Using Lactobacillus rhamnosus GG Bacteria. Coatings. 2021; 11(11):1410. https://doi.org/10.3390/coatings11111410
Chicago/Turabian StyleKaminski, Kamil, Karolina Syrek, Joanna Grudzień, Magdalena Obloza, Monika Adamczyk, and Grzegorz D. Sulka. 2021. "Physicochemical Investigation of Biosynthesis of a Protein Coating on Glass That Promotes Mammalian Cell Growth Using Lactobacillus rhamnosus GG Bacteria" Coatings 11, no. 11: 1410. https://doi.org/10.3390/coatings11111410