Accuracy of Pulse Oximetry in the Presence of Fetal Hemoglobin—A Systematic Review
Abstract
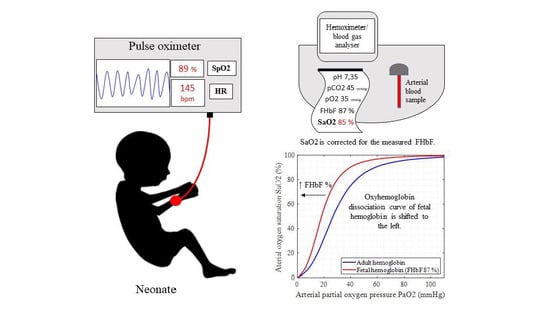
:1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Study Selection
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Madar, J.; Roehr, C.C.; Ainsworth, S.; Ersdal, H.; Morley, C.; Rüdiger, M.; Skåre, C.; Szczapa, T.; Te Pas, A.; Trevisanuto, D.; et al. European Resuscitation Council Guidelines 2021: Newborn resuscitation and support of transition of infants at birth. Resuscitation 2021, 161, 291–326. [Google Scholar] [CrossRef]
- Aziz, K.; Lee, H.C.; Escobedo, M.B.; Hoover, A.V.; Kamath-Rayne, B.D.; Kapadia, V.S.; Magid, D.J.; Niermeyer, S.; Schmölzer, G.M.; Szyld, E.; et al. Part 5: Neonatal Resuscitation 2020 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Pediatrics 2021, 147 (Suppl. 1), e2020038505E. [Google Scholar] [CrossRef] [PubMed]
- Dawson, J.A.; Kamlin, C.O.F.; Vento, M.; Wong, C.; Cole, T.J.; Donath, S.M.; Davis, P.G.; Morley, C.J. Defining the reference range for oxygen saturation for infants after birth. Pediatrics 2010, 125, e1340–e1347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jennis, M.S.; Peabody, J.L. Pulse oximetry: An alternative method for the assessment of oxygenation in newborn infants. J. Pediatr. 1987, 79, 524–528. [Google Scholar]
- Praud, J.P.; Gaultier, C.L.; Carofilis, A.; Lacaille, F.; Dehan, M.; Bridey, F. Accuracy of two wavelength pulse oximetry in neonates and infants. Pediatr. Pulmonol. 1989, 6, 180–182. [Google Scholar] [CrossRef]
- Rajadurai, V.S.; Walker, A.M.; Yu, V.Y.H.; Oates, A. Effect of fetal hemoglobin on the accuracy of pulse oximetry in preterm infants. J. Paediatr. Child Health 1992, 28, 43–46. [Google Scholar] [CrossRef]
- Ramanathan, R.; Durand, M.; Larrazabal, C. Pulse oximetry in very low birth weight infants with acute and chronic lung injury. Pediatrics 1987, 79, 612–617. [Google Scholar]
- Wukitsch, M.W.; Petterson, M.T.; Tobler, D.R.; Pologe, J.A. Pulse oximetry: Analysis of theory, technology, and practice. J. Clin. Monit. 1988, 4, 290–301. [Google Scholar] [CrossRef] [PubMed]
- Nitzan, M.; Romem, A.; Koppel, R. Pulse oximetry: Fundamentals and technology update. Med. Devices 2014, 7, 231–239. [Google Scholar] [CrossRef]
- Louw, A.; Cracco, C.; Cerf, C.; Harf, A.; Duvaldestin, P.; Lemaire, F.; Brochard, L. Accuracy of pulse oximetry in the intensive care unit. Intensive Care Med. 2001, 27, 1606–1613. [Google Scholar] [CrossRef]
- Perkins, G.D.; McAuley, D.F.; Giles, S.; Routledge, H.; Gao, F. Do changes in pulse oximeter oxygen saturation predict equivalent changes in arterial oxygen saturation? Crit. Care 2003, 7, R67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jubran, A.; Tobin, M.J. Reliability of pulse oximetry in titrating supplemental oxygen therapy in ventilator-dependent patients. Chest 1990, 97, 1420–1425. [Google Scholar] [CrossRef] [PubMed]
- Ross, P.; Newth, C.; Khemani, R. Accuracy of pulse oximetry in children. Pediatrics 2014, 133, 22–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wackernagel, D.; Blennow, M.; Hellström, A. Accuracy of pulse oximetry in preterm and term infants is insufficient to de-termine arterial oxygen saturation and tension. Acta Paediatr. 2020, 109, 2251–2257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oski, F.A.; Delivoria-Papadopoulos, M. The shift to the left. Pediatrics 1971, 48, 853–856. [Google Scholar] [PubMed]
- Sankaran, V.G.; Orkin, S.H. The Switch From Fetal to Adult Hemoglobin. Cold Spring Harb. Perspect. Med. 2013, 3, a011643. [Google Scholar] [CrossRef] [Green Version]
- Bunn, H.F.; Briehl, R.W. The interaction of 2,3-diphosphoglycerate with various human hemoglobins. J. Clin. Investig. 1970, 49, 1088–1095. [Google Scholar] [CrossRef] [Green Version]
- Orzalesi, M.M.; Hay, W.W. The regulation of oxygen affinity of fetal blood. I. In vitro experiments and results in normal infants. Pediatrics 1971, 48, 857–864. [Google Scholar]
- Bard, H. Postnatal fetal and adult hemoglobin synthesis in early preterm newborn infants. J. Clin. Investig. 1973, 52, 1789–1795. [Google Scholar] [CrossRef]
- Wilson, K.; Hawken, S.; Murphy, M.S.; Atkinson, K.M.; Potter, B.K.; Sprague, A.; Walker, M.; Chakraborty, P.; Little, J. Postnatal prediction of gestational age using newborn fetal hemoglobin levels. EBioMedicine 2017. [Google Scholar] [CrossRef] [Green Version]
- Cochran-Black, D.L.; Cowan, L.D.; Neas, B.R. The relation between newborn hemoglobin F fractions and risk factors for sudden infant death syndrome. Arch. Pathol. Lab. Med. 2001, 125, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Stutchfield, C.J.; Jain, A.; Odd, D.; Wiliams, C.; Markham, R. Foetal haemoglobin, blood transfusion, and retinopathy of prematurity in very preterm infants: A pilot prospective cohort study. Eye 2017, 31, 1451–1455. [Google Scholar] [CrossRef] [PubMed]
- Inoue, H.; Takabe, F.; Maeno, Y.; Iwasa, M. Identification of fetal hemoglobin in blood stains by high performance liquid chro-matography. Z. Rechtsmed. 1989, 102, 437–444. [Google Scholar] [CrossRef] [PubMed]
- Davis, M.D.; Walsh, B.K.; Sittig, S.E.; Restrepo, R.D. AARC Clinical practice guideline: Blood gas analysis and hemoximetry: 2013. Respir. Care 2013, 58, 1694–1703. [Google Scholar] [CrossRef]
- ABL800 FLEX Reference Manual from Software Version 6.00; Code number: 989-963; Radiometer: Copenhagen, Denmark, 2008.
- Zijlstra, W.G.; Buursma, A.; Meeuwsen-van der Roest, W.P. Absorption spectra of human fetal and adult oxyhemoglobin, de-oxyhemoglobin, carboxyhemoglobin, and methemoglobin. Clin. Chem. 1991, 37, 1633–1638. [Google Scholar] [CrossRef]
- Krzeminski, A. How Is Fetal Hemoglobin Determined and Corrected for in the OSM3, the ABL 510, and the ABL 520? Radiometer: Copenhagen, Denmark, 1992; pp. 1–4. [Google Scholar]
- Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A. Preferred reporting items for systematic review and meta-analysis-protocols (Prisma P) 2015 statement. Syst. Rev. 2015, 4, 1. [Google Scholar] [CrossRef] [Green Version]
- Shiao, S.Y.P.K. Effects of fetal hemoglobin on accurate measurements of oxygen saturation in neonates. J. Perinat. Neonatal Nurs. 2005, 19, 348–361. [Google Scholar] [CrossRef]
- Shiao, S.Y.P.K.; Ou, C.N.; Pierantoni, H. The measurement of accurate fetal hemoglobin and related oxygen saturation by the hemoximeter. Clin. Chim. Acta 2006, 374, 75–80. [Google Scholar] [CrossRef] [Green Version]
- Shiao, S.Y.; Ou, C.N. Validation of oxygen saturation monitoring in neonates. Am. J. Crit. Care 2007, 16, 168–178. [Google Scholar] [CrossRef]
- Durand, M.; Ramanathan, R. Pulse oximetry for continuous oxygen monitoring in sick newborn infants. J. Pediatr. 1986, 109, 1052–1056. [Google Scholar] [CrossRef]
- Wimberley, P.D.; Helledie, N.R.; Friis-Hansen, B.; Fogh-Andersen, N.; Olesen, H. Pulse oximetry versus transcutaneous pO2 in sick newborn infants. Scand. J. Clin. Lab. Investig. 1987, 188, 19–25. [Google Scholar] [CrossRef]
- Nitzan, I.; Hammerman, C.; Mimouni, F.B.; Bin-Nun, A. Packed red blood cells transfusions in neonates: Effect on FiO2 and PaO2/SaO2 ratio and implications for neonatal saturation targeting. J. Perinatol. 2018, 38, 693–695. [Google Scholar] [CrossRef]
- Cornelissen, P.J.H.; van Woensel, C.L.M.; van Oel, W.C.; de Jong, P.A. Correction factors for hemoglobin derivatives in fetal blood, as measured with the IL 282 Co-oximeter. Clin. Chem. 1983, 29, 1555–1556. [Google Scholar] [CrossRef]
- Émond, D.; Lachance, C.; Gagnon, J.; Bard, H. Arterial partial pressure of oxygen required to achieve 90% saturation of hae-moglobin in very low birth weight newborns. Pediatrics 1993, 91, 602–604. [Google Scholar]
- Bohnhorst, B.; Peter, C.S.; Poets, C.F. Detection of hyperoxaemia in neonates: Data from three new pulse oximeters. Arch. Dis. Child. Fetal Neonatal Ed. 2002, 87, F217–F219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Support Study Group of the Eunice Kennedy Shriver NICHD Neonatal Research Network; Carlo, W.A.; Finer, N.N.; Walsh, M.C.; Rich, W.; Gantz, M.G.; Laptook, A.R.; Yoder, B.A.; Faix, R.G.; Das, A.; et al. Target ranges of oxygen saturation in extremely pre-term infants. N. Engl. J. Med. 2010, 362, 1959–1969. [Google Scholar]
- Vaucher, Y.E.; Peralta-Carcelen, M.; Finer, N.N.; Carlo, W.A.; Gantz, M.G.; Walsh, M.C.; Laptook, A.R.; Yoder, B.A.; Faix, R.G.; Das, A.; et al. Neurodevelopmental outcomes in the early CPAP and pulse oximetry trial. N. Engl. J. Med. 2012, 367, 2495–2504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmidt, B.; Whyte, R.K.; Asztalos, E.V.; Moddemann, D.; Poets, C.; Rabi, Y.; Solimano, A.; Roberts, R.S.; the Canadian Oxygen Trial (COT) Group. Effects of targeting higher vs lower arterial oxygen saturations on death or disability in extremely preterm infants: A randomized clinical trial. JAMA 2013, 309, 2111–2120. [Google Scholar] [CrossRef]
- Group BIUKC; Group BIAC; Group BINZC; Stenson, B.J.; Tarnow-Mordi, W.O.; Darlow, B.A.; Simes, J.; Juszczak, E.; Askie, L.; Battin, M.; et al. Oxygen saturation and outcomes in preterm infants. N. Engl. J. Med. 2013, 368, 2094–2104. [Google Scholar]
- Darlow, B.A.; Marschner, S.L.; Donoghoe, M.; Battin, M.R.; Broadbent, R.S.; Elder, M.J.; Hewson, M.P.; Meyer, M.P.; Ghadge, A.; Graham, P.; et al. Randomized controlled trial of oxygen saturation targets in very pre-term infants: Two year outcomes. J. Pediatr. 2014, 165, 30–35. [Google Scholar] [CrossRef] [PubMed]
- The BOOST-II Australia and United Kingdom Collaborative Groups; Tarnow-Mordi, W.O.; Stenson, B.J.; Kirby, A.; Juszczak, E.; Donoghoe, M.; Deshpande, S.; Morley, C.; King, A.; Doyle, L.W.; et al. Outcomes of two trials of oxygen-saturation targets in preterm infants. N. Engl. J. Med. 2016, 374, 749–760. [Google Scholar] [PubMed]
- Khadawardi, E.; Al Hazzani, F. Oxygen saturation and outcomes in preterm infants: The BOOST II United Kingdom, Australia, and New Zealand Collaborative Groups. J. Clin. Neonatol. 2013, 2, 73–75. [Google Scholar] [CrossRef] [Green Version]
- Lakshminrusimha, S.; Manja, V.; Mathew, B.; Suresh, G.K. Oxygen targeting in preterm infants: A physiologic interpretation. J. Perinatol. 2015, 35, 8–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poets, C.F. Noninvasive monitoring and assessment of oxygenation in infants. Clin. Perinatol. 2019, 46, 417–433. [Google Scholar] [CrossRef] [PubMed]


| Ref | 1st Author, Year | Number of Patients/ HbF Blood Samples | Blood Sample Type | HbF Measurement Method | Gestation Distribution (Weeks) | Time of Sample Collection and Non-Invasive Monitoring | Blood Oxygenation Parameters | Blood Gas Analyzer /Hemoximeter | Pulse Oximeter (Company Name) | Additional Bedside Oxygenation Monitoring Device (Company Name) | Relevant Results |
|---|---|---|---|---|---|---|---|---|---|---|---|
| [32]. | Durand, 1986 | 75/140 | Arterial | Alkali denaturation method | 24–42 | 1–14 days + 30–153 days after birth | paO2, SaO2 | Radiometer BMS3 Mark II / Co-oximeter IL 282 | Nellcor N-100 (Hayward, CA, USA) | tc-pO2 Oxygen electrode (Novametrix, Wallingford, CT, USA) | HbF values of 4.3% to 95% did not influence the accuracy of pulse oximeter readings. |
| [7]. | Ramanathan, 1987 | 68/132 | Arterial | Alkali denaturation method | 25–31 | 1–6 days + 20–80 days after birth | paO2, SaO2 | Radiometer BMS3 Mark II / Co-oximeter IL 282 | Nellcor N-100 (Hayward, CA, USA) | tc-pO2 Oxygen electrode (Novametrix, Wallingford, CT, USA) | HbF values of 4.3% to 92.2% did not influence the accuracy of pulse oximeter readings. |
| [33]. | Wimberley, 1987 | 18/18 | Arterial | Alkali denaturation method | 25–34 | Within 5 days after birth | paO2, SaO2 | ABL300/ Hemoximeter OSM3 | Ohmeda Biox 3700 | tc-pO2 Radiometer TCM3 | FHbF ranged from 44–97%. The variations in the levels of HbF, pH, pCO2 and 2,3-DPG resulted in a variable paO2-SaO2 relation. |
| [4]. | Jennis, 1987 | 26/49 | Arterial | Electrophoresis | 24–40 | 1–49 days after birth | SaO2 | Co-oximeter IL-282 | Nellcor N-100 (Hayward, CA, USA) | NA | FHbF > 50% generated a 2.8% to 3.6% error (underestimation) in SpO2 reading. |
| [5]. | Praud, 1989 | 71/52 | Arterial | Electrophoresis and alkali denaturation method | 25–40 | 1–14 days after birth + 4.5–38 weeks after birth | SaO2 | Hemoximeter OSM2 | Nellcor N-100 (Hayward, CA, USA) | NA | For FHbF < 50% and SaO2 ≤ 95%, SpO2 was overestimated. |
| Ref | 1st Author, Year | Number of Patients/ HbF Blood Samples | Blood Sample Type | HbF Measurement Method | Gestation Distribution (Weeks) | Time of Sample Collection and Non-Invasive Monitoring | Blood Oxygenation Parameters | Blood Gas Analyzer/Hemoximeter | Pulse Oximeter (Company Name) | Additional Bedside Oxygenation Monitoring Device (Company Name) | Relevant Results |
|---|---|---|---|---|---|---|---|---|---|---|---|
| [6]. | Rajadurai, 1992 | 22/64 | Arterial | Visible absorption spectroscopy (hemoximeter) | 25–36 | 1 h–73 days after birth | Functional SaO2 * | ABL30 Analyzer/ Hemoximeter OSM3 | Nellcor N-100 (Hayward, CA, USA) | NA | Pulse oximeter saturations were unaffected by FHbF values which ranged from 0 to 100%. |
| [29]. | Shiao, 2005 | 20/210 | Arterial and venous | Visible absorption spectroscopy (hemoximeter) | 24–34 | First 5 days after birth | paO2, SaO2, SvO2, HbO2 | Hemoximeter OSM3 | Nellcor NPB 290 (Pleasanton, CA, USA) | NA | Bias of SpO2 vs HbO2 was +1.6% (2SD 5.6) and SpO2 vs SaO2 −0.6% (2SD 5.9). There was no statistical analysis of HbF contribution to the bias. |
| [30]. | Shiao, 2006 | 39/188 | Arterial and venous | Visible absorption spectroscopy (hemoximeter) + HPLC | 25–38 | First 5 days after birth | paO2, SaO2, SvO2, HbO2 | Hemoximeter OSM3 | Nellcor NPB 290 (Tyco Healthcare, Mansfield, MA, USA) | NA | Lower HbF levels after the transfusion resulted in lower SpO2 for the same paO2 range of 50–75 mmHg. There was no statistical analysis of HbF contribution to the SpO2-SaO2 bias. |
| [31]. | Shiao, 2007 | 78/771 | Arterial and venous | Visible absorption spectroscopy (hemoximeter) | 25–38 | First 5 days after birth (every 6–8 h) | paO2, SaO2, HbO2 | Hemoximeter OSM3 | Nellcor (NPB 290, Pleasanton, CA, USA) | SaO2m, SvO2m *** Oximetric 3-wavelength monitors (Abbott, Chicago, IL, USA) | Bias of SpO2 vs HbO2 in arterial blood samples was 2.5% (SD 3.1). There was no statistical analysis of HbF contribution to the SpO2-SaO2 bias. |
| [34]. | Nitzan, 2018 | 14/28 | Arterial | Visible absorption spectroscopy (hemoximeter) | 24–33 | Within 12 h before and after the blood transfusion (first 5 days after birth) | paO2, SaO2 | ABL 90 FLEX | Nellcor (Covidien-Medtronic, Mansfield, MA, USA) | NA | HbF declined significantly after transfusion and FiO2 increased by > 12% to keep SpO2 within the same range. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pritišanac, E.; Urlesberger, B.; Schwaberger, B.; Pichler, G. Accuracy of Pulse Oximetry in the Presence of Fetal Hemoglobin—A Systematic Review. Children 2021, 8, 361. https://doi.org/10.3390/children8050361
Pritišanac E, Urlesberger B, Schwaberger B, Pichler G. Accuracy of Pulse Oximetry in the Presence of Fetal Hemoglobin—A Systematic Review. Children. 2021; 8(5):361. https://doi.org/10.3390/children8050361
Chicago/Turabian StylePritišanac, Ena, Berndt Urlesberger, Bernhard Schwaberger, and Gerhard Pichler. 2021. "Accuracy of Pulse Oximetry in the Presence of Fetal Hemoglobin—A Systematic Review" Children 8, no. 5: 361. https://doi.org/10.3390/children8050361