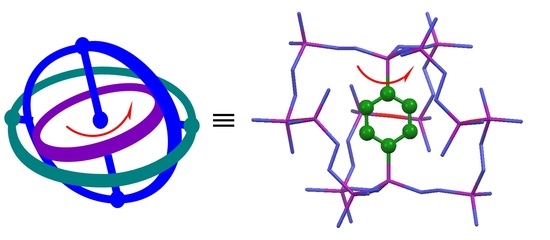
Solid-State 2H NMR Study for Deuterated Phenylene Dynamics in a Crystalline Gyroscope-Like Molecule
Abstract
:1. Introduction
2. Materials and Methods
2.1. General Details
2.2. Synthesis and Characterization of the Deuterated Gyroscope-Like Molecule 1-d4
2.3. Differential Scanning Calorimetry (DSC) Study
2.4. Solid-State 2H NMR Study
3. Results and Discussions
3.1. Outline of the Previous Report on the Dynamics of the Phenylene Moiety in 1 in the Crystalline State
3.2. DSC Analysis of Powdered 1
3.3. Solid-State 2H NMR Spectroscopy of Powdered 1-d4
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Kottas, G.S.; Clarke, L.I.; Horinek, D.; Michl, J. Artificial Molecular Rotors. Chem. Rev. 2005, 105, 1281–1376. [Google Scholar] [CrossRef]
- Erbas-Cakmak, S.; Leigh, D.A.; McTernan, C.T.; Nussbaumer, A.L. Artificial Molecular Machines. Chem. Rev. 2015, 115, 10081–10206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roy, I.; Stoddart, J.F. Amphidynamic Crystals Key to Artificial Molecular Machines. Trends Chem. 2019, 1, 627–629. [Google Scholar] [CrossRef]
- Kaleta, J.; Chen, J.; Bastien, G.; Dračínsky, M.; Mašát, M.; Rogers, C.T.; Feringa, B.L.; Michl, J. Surface inclusion of unidirectional molecular motors in hexagonal tris(o-phenylene) cyclotriphosphazene. J. Am. Chem. Soc. 2017, 139, 10486–10498. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Garibay, M.A. Crystalline molecular machines: Encoding supramolecular dynamics into molecular structure. Proc. Natl. Acad. Sci. USA 2005, 102, 10771–10776. [Google Scholar] [CrossRef] [Green Version]
- Joshi, H.; Kharel, S.; Ehnbom, A.; Skopek, K.; Hess, G.D.; Fiedler, T.; Hampel, F.; Bhuvanesh, N.; Gladysz, J.A. Three-Fold Intramolecular Ring Closing Alkene Metatheses of Square Planar Complexes with cis Phosphorus Donor Ligands P(X(CH2)mCH=CH2)3 (X = −, m = 5–10; X = O, m = 3–5): Syntheses, Structures, and Thermal Properties of Macrocyclic Dibridgehead Diphosphorus Complexes. J. Am. Chem. Soc. 2018, 140, 8463–8478. [Google Scholar]
- Commins, P.; Nuñez, J.E.; Garcia-Garibay, M.A. Synthesis of Bridged Molecular Gyroscopes with Closed Topologies: Triple One-Pot Macrocyclization. J. Org. Chem. 2011, 76, 8355–8363. [Google Scholar] [CrossRef]
- Setaka, W.; Yamaguchi, K. Thermal modulation of birefringence observed in a crystalline molecular gyrotop. Proc. Natl. Acad. Sci. USA 2012, 109, 9271–9275. [Google Scholar] [CrossRef] [Green Version]
- Setaka, W.; Yamaguchi, K. Order-Disorder Transition of Dipolar Rotor in a Crystalline Molecular Gyrotop and Its Optical Change. J. Am. Chem. Soc. 2013, 135, 14560–14563. [Google Scholar] [CrossRef]
- Setaka, W.; Yamaguchi, K. A Molecular Balloon: Expansion of a Molecular Gyrotop Cage Due to Rotation of the Phenylene Rotor. J. Am. Chem. Soc. 2012, 134, 12458–12461. [Google Scholar] [CrossRef] [PubMed]
- Tsurunaga, M.; Inagaki, Y.; Momma, H.; Kwon, E.; Yamaguchi, Y.; Yoza, K.; Setaka, W. Dielectric Relaxation of Powdered Molecular Gyrotops Having a Thiophene Dioxide-diyl as a Dipolar Rotor. Org. Lett. 2018, 20, 6934–6937. [Google Scholar] [CrossRef] [PubMed]
- Setaka, W.; Ohmizu, S.; Kabuto, C.; Kira, M. A Molecular Gyroscope Having Phenylene Rotator Encased in Three-Spoke Silicon-Based Stator. Chem. Lett. 2007, 36, 1076–1077. [Google Scholar] [CrossRef]
- Setaka, W.; Ohmizu, S.; Kira, M. Molecular Gyroscope Having a Halogen-substituted p-Phenylene Rotator and Silaalkane Chain Stators. Chem. Lett. 2010, 39, 468–469. [Google Scholar] [CrossRef]
- Marahatta, A.B.; Kanno, M.; Hoki, K.; Setaka, W.; Irle, S.; Kono, H. Theoretical Investigation of the Structures and Dynamics of Crystalline Molecular Gyroscopes. J. Phys. Chem. C 2012, 116, 24845–24854. [Google Scholar] [CrossRef]
- Setaka, W.; Ohmizu, S.; Kira, M. Kinetic stabilization against the oxidation reaction induced by a silaalkane cage in a thiophene-bridged molecular gyroscope. Chem. Commun. 2014, 50, 1098–1110. [Google Scholar] [CrossRef]
- Sidhu, P.S.; Penner, G.H.; Jeffrey, K.R.; Zhao, B.; Wang, Z.L.; Goh, I. Solid-State NMR Study of Guest Molecule Dynamics in 4-Alkyl-tert-butylbenzene/Thiourea Inclusion Compounds. J. Phys. Chem. B 1997, 101, 9087–9097. [Google Scholar] [CrossRef]
- Pake, G.E. Nuclear Resonance Absorption in Hydrated Crystals: Fine Structure of the Proton Line. J. Chem. Phys. 1948, 16, 327–336. [Google Scholar] [CrossRef]
- Liepuoniute, I.; Jellen, M.J.; Garcia-Garibay, M.A. Correlated motion and mechanical gearing in amphidynamic crystalline molecular machines. Chem. Sci. 2020, 11, 12994–13007. [Google Scholar] [CrossRef]
- Colin-Molina, A.; Jellen, M.J.; García-Quezada, E.; Cifuentes-Quintal, M.E.; Murillo, F.; Barroso, J.; Pérez-Estrada, S.; Toscano, R.A.; Merino, G.; Rodríguez-Molina, B. Origin of the isotropic motion in crystalline molecular rotors with carbazole stators. Chem. Sci. 2019, 10, 4422–4429. [Google Scholar] [CrossRef] [Green Version]
- Hughes, A.R.; Brownbill, N.J.; Lalek, R.C.; Briggs, M.E.; Slater, A.G.; Cooper, A.I.; Frédéric, B. Ultra-Fast Molecular Rotors within Porous Organic Cages. Chem. Eur. J. 2017, 23, 17217–17221. [Google Scholar]
- Macho, V.; Brombacher, L.; Spiess, H.W. The NMR-WEBLAB: An Internet Approach to NMR Lineshape Analysis. Appl. Magn. Reson. 2001, 20, 405–432. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Setaka, W.; Yamaguchi, K.; Kira, M. Solid-State 2H NMR Study for Deuterated Phenylene Dynamics in a Crystalline Gyroscope-Like Molecule. Chemistry 2021, 3, 39-44. https://doi.org/10.3390/chemistry3010004
Setaka W, Yamaguchi K, Kira M. Solid-State 2H NMR Study for Deuterated Phenylene Dynamics in a Crystalline Gyroscope-Like Molecule. Chemistry. 2021; 3(1):39-44. https://doi.org/10.3390/chemistry3010004
Chicago/Turabian StyleSetaka, Wataru, Kentaro Yamaguchi, and Mitsuo Kira. 2021. "Solid-State 2H NMR Study for Deuterated Phenylene Dynamics in a Crystalline Gyroscope-Like Molecule" Chemistry 3, no. 1: 39-44. https://doi.org/10.3390/chemistry3010004