Grapefruit-Derived Micro and Nanovesicles Show Distinct Metabolome Profiles and Anticancer Activities in the A375 Human Melanoma Cell Line
Abstract
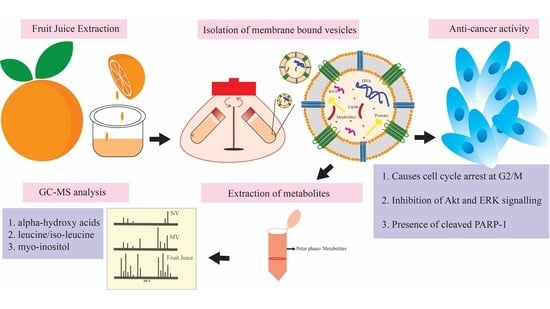
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Vesicle Isolation
2.2. Metabolite Extraction and Derivatization
2.3. GC-MS Analysis and Data Elaboration
2.4. Cell Cultures
2.5. Cell Proliferation and Viability
2.6. Analysis of Cell Cycle by Flow Cytometry
2.7. Western Blotting
2.8. RNA Extraction and qRT-PCR Analyses
3. Results
3.1. Impact of Citrus-Derived Vesicles on Tumour Cell Lines
3.2. Metabolome Profiles of Grapefruit-Derived Micro and Nanovesicles
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Raposo, G.; Stoorvogel, W. Extracellular vesicles: Exosomes, microvesicles, and friends. J. Cell Biol. 2013, 200, 373–383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De la Canal, L.; Pinedo, M. Extracellular vesicles: A missing component in plant cell wall remodeling. J. Exp. Bot. 2018, 69, 4655–4658. [Google Scholar] [CrossRef] [PubMed]
- Woith, E.; Fuhrmann, G.; Melzig, M.F. Extracellular Vesicles-Connecting Kingdoms. Int. J. Mol. Sci. 2019, 20, 5695. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bleackley, M.R.; Dawson, C.S.; Anderson, M.A. Fungal Extracellular Vesicles with a Focus on Proteomic Analysis. Proteomics 2019, 19, 1800232. [Google Scholar] [CrossRef]
- Regente, M.; Pinedo, M.; San Clemente, H.; Balliau, T.; Jamet, E.; de la Canal, L. Plant extracellular vesicles are incorporated by a fungal pathogen and inhibit its growth. J. Exp. Bot. 2017, 68, 5485–5495. [Google Scholar] [CrossRef]
- Baldrich, P.; Rutter, B.D.; Karimi, H.Z.; Podicheti, R.; Meyers, B.C.; Innes, R.W. Plant Extracellular Vesicles Contain Diverse Small RNA Species and Are Enriched in 10- to 17-Nucleotide “Tiny” RNAs. Plant Cell. 2019, 31, 315–324. [Google Scholar] [CrossRef] [Green Version]
- Stanly, C.; Fiume, I.; Capasso, G.; Pocsfalvi, G. Isolation of exosome-like vesicles from plants by ultracentrifugation on sucrose/deuterium oxide (D2O) density cushions. Methods Mol. Biol. 2016, 259–269. [Google Scholar] [CrossRef]
- Mu, J.; Zhuang, X.; Wang, Q.; Jiang, H.; Deng, Z.B.; Wang, B.; Zhang, L.; Kakar, S.; Jun, Y.; Miller, D.; et al. Interspecies communication between plant and mouse gut host cells through edible plant derived exosome-like nanoparticles. Mol. Nutr. Food Res. 2014, 58, 1561–1573. [Google Scholar] [CrossRef]
- Ju, S.; Mu, J.; Dokland, T.; Zhuang, X.; Wang, Q.; Jiang, H.; Xiang, X.; Deng, Z.B.; Wang, B.; Zhang, L.; et al. Grape exosome-like nanoparticles induce intestinal stem cells and protect mice from DSS-induced colitis. Mol. Ther. 2013, 21, 1345–1357. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Zhuang, X.; Mu, J.; Deng, Z.B.; Jiang, H.; Zhang, L.; Xiang, X.; Wang, B.; Yan, J.; Miller, D.; et al. Delivery of therapeutic agents by nanoparticles made of grapefruit-derived lipids. Nat. Commun. 2013, 4, 1867. [Google Scholar] [CrossRef] [Green Version]
- Raimondo, S.; Naselli, F.; Fontana, S.; Monteleone, F.; Lo Dico, A.; Saieva, L.; Zito, G.; Flugy, A.; Manno, M.; Di Bella, M.A.; et al. Citrus limon-derived nanovesicles inhibit cancer cell proliferation and suppress CML xenograft growth by inducing TRAIL-mediated cell death. Oncotarget 2015, 6, 19514–19527. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pocsfalvi, G.; Turiák, L.; Ambrosone, A.; del Gaudio, P.; Puska, G.; Fiume, I.; Silvestre, T.; Vékey, K. Protein biocargo of citrus fruit-derived vesicles reveals heterogeneous transport and extracellular vesicle populations. J. Plant Physiol. 2018, 229, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Zhuang, X.; Deng, Z.B.; Jiang, H.; Mu, J.; Wang, Q.; Xiang, X.; Guo, H.; Zhang, L.; Dryden, G.; et al. Targeted drug delivery to intestinal macrophages by bioactive nanovesicles released from grapefruit. Mol. Ther. 2014, 22, 522–534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- ClinicalTrials.gov Identifier: NCT03493984. Plant Exosomes and Patients Diagnosed With Polycystic Ovary Syndrome (PCOS). Available online: https://clinicaltrials.gov/ct2/show/NCT03493984 (accessed on 27 September 2019).
- ClinicalTrials.gov Identifier: NCT01668849. Edible Plant Exosome Ability to Prevent Oral Mucositis Associated with Chemoradiation Treatment of Head and Neck Cancer. Available online: https://clinicaltrials.gov/ct2/show/NCT01668849 (accessed on 31 August 2020).
- Hadacek, F.; Bachmann, G. Low-molecular-weight metabolite systems chemistry. Front. Environ. Sci. 2015, 3, 12. Available online: https://www.frontiersin.org/article/10.3389/fenvs.2015.00012 (accessed on 4 March 2015). [CrossRef]
- Tebani, A.; Bekri, S. Paving the Way to Precision Nutrition Through Metabolomics. Front. Nutr. 2019, 6, 41. Available online: https://www.frontiersin.org/article/10.3389/fnut.2019.00041 (accessed on 9 April 2019). [CrossRef] [PubMed] [Green Version]
- Berk, Z. Nutritional and health-promoting aspects of citrus consumption. In Citrus Fruit Processing; Academic Press: Cambridge, MA, USA; Elsevier: Amsterdam, The Netherlands, 2016; pp. 261–279. [Google Scholar] [CrossRef]
- Roessner, U.; Wagner, C.; Kopka, J.; Trethewey, R.L.; Willmitzer, N. Simultaneous analysis of metabolites in potato tuber by gas chromatography–mass spectrometry. Plant J. 2000, 23, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Nicoletti, I.; Migliorati, G.; Pagliacci, M.F.; Grignani, C.; Riccardi, C. A rapid and simple method for measuring thymocyte apoptosis by propidium iodide staining and flow cytometry. J. Immunol. Methods. 1991, 139, 271–279. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- King, R.W.; Jackson, P.K.; Kirschner, M.W. Mitosis in transition. Cell 1994, 79, 563–571. [Google Scholar] [CrossRef]
- Lei, C.; Wang, W.; Zhu, Y.; Fang, W.; Tan, W. The decrease of cyclin B2 expression inhibits invasion and metastasis of bladder cancer. Urol. Oncol. Semin. Orig. Investig. 2016, 34, e1–e237. [Google Scholar] [CrossRef]
- El-Deiry, W.S. p21(WAF1) Mediates Cell-Cycle Inhibition, Relevant to Cancer Suppression and Therapy. Cancer Res. 2016, 76, 5189–5191. [Google Scholar] [CrossRef] [Green Version]
- Díaz-Álvarez, M.; Turiel, E. Improved molecularly imprinted polymer grafted to porous polyethylene frits for the solid-phase extraction of thiabendazole from citrus sample extracts. Mol. Impr. 2015, 1–7. [Google Scholar] [CrossRef]
- Karbowniczek, M.; Spittle, C.T.; Morrison, S.; Wu, H.; Henske, E.P. mTOR Is Activated in the Majority of Malignant Melanomas. J. Investig. Dermatol. 2008, 128, 980–987. [Google Scholar] [CrossRef] [Green Version]
- Davies, H.; Bignell, G.C.; Cox, R.; Stephens, P.; Edkins, S.; Clegg, S.; Teague, J.; Woffendin, H.; Garnett, M.W.; Bottomley, J.; et al. Mutations of the BRAF gene in human cancer. Nature 2002, 417, 949–954. [Google Scholar] [CrossRef] [PubMed]
- Slipicevic, A.; Holm, R.; Nguyen, M.P.J.; Bøhler, T.B.; Davidson, P.; Flørenes, V.A. Expression of Activated Akt and PTEN in Malignant Melanomas: Relationship With Clinical Outcome. Am. J. Clin. Pathol. 2005, 124, 528–536. [Google Scholar] [CrossRef]
- Smalley, K.S.M. A pivotal role for ERK in the oncogenic behaviour of malignant melanoma? Int. J. Cancer. 2003, 104, 527–532. [Google Scholar] [CrossRef] [PubMed]
- Hearing, V.J.; Leong, S.P. From Melanocytes to Melanoma: The Progression to Malignancy; Springer: Berlin/Heidelberg, Germany, 2006. [Google Scholar] [CrossRef]
- Lassen, A.; Atefi, M.; Robert, L.; Wong, D.J.; Cerniglia, M.; Comin-Anduix, B.; Ribas, A. Effects of AKT inhibitor therapy in response and resistance to BRAF inhibition in melanoma. Mol. Cancer. 2014, 13, 83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwong, L.N.; Davies, M.A. Navigating the Therapeutic Complexity of PI3K Pathway Inhibition in Melanoma. Clin. Cancer Res. 2013, 19, 5310–5319. [Google Scholar] [CrossRef] [Green Version]
- Savoia, P.; Fava, P.; Casoni, F.; Cremona, O. Targeting the ERK Signaling Pathway in Melanoma. Int. J. Mol. Sci. 2019, 20, 1483. [Google Scholar] [CrossRef] [Green Version]
- Ciołczyk-Wierzbicka, D.; Gil, D.; Laidler, P. Treatment of melanoma with selected inhibitors of signaling kinases effectively reduces proliferation and induces expression of cell cycle inhibitors. Med. Oncol. 2017, 35, 7. [Google Scholar] [CrossRef] [Green Version]
- Roh, J.I.; Lee, H.W. A myo-inositol diet for lung cancer prevention and beyond. J. Thorac. Dis. 2018, 10, S3919–S3921. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Guo, F.; Ouyang, D. A review of the pharmacology and toxicology of aucubin. Fitoterapia 2020, 140, 104443. [Google Scholar] [CrossRef] [PubMed]
- Lv, X.; Zhao, S.; Ning, Z.; Zeng, H.; Shu, Y.; Tao, O.; Xiao, C.; Lu, C.; Liu, Y. Citrus fruits as a treasure trove of active natural metabolites that potentially provide benefits for human health. Chem. Cent. J. 2015, 9, 68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jansen, R.J.; Robinson, D.P.; Stolzenberg-Solomon, R.Z.; Bamlet, W.R.; de Andrade, M.; Oberg, A.L.; Hammer, T.J.; Rabe, K.G.; Anderson, K.E.; Olson, J.E.; et al. Fruit and vegetable consumption is inversely associated with having pancreatic cancer. Cancer Causes Control. 2011, 22, 1613. [Google Scholar] [CrossRef] [PubMed]
- Maserejian, N.E.; Giovannucci, N.; Rosner, B.; Zavras, A.; Joshipura, K. Prospective Study of Fruits and Vegetables and Risk of Oral Premalignant Lesions in Men. Am. J. Epidemiol. 2006, 164, 556–566. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balch, C.M.; Gershenwald, J.E.; Soong, S.-J.; Thompson, J.F.; Atkins, M.B.; Byrd, D.R.; Buzaid, A.C.; Cochran, A.J.; Coit, D.G.; Ding, S.; et al. Final Version of 2009 AJCC Melanoma Staging and Classification. J. Clin. Oncol. 2009, 27, 6199–6206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, K.D.; Siegel, R.L.; Lin, C.C.; Mariotto, A.B.; Kramer, J.L.; Rowland, J.H.; Stein, K.R.; Alteri, D.; Jemal, A. Cancer treatment and survivorship statistics. CA. Cancer J. Clin. 2016, 66, 271–289. [Google Scholar] [CrossRef] [Green Version]
- Curtin, N.J. DNA repair dysregulation from cancer driver to therapeutic target. Nat. Rev. Cancer. 2012, 12, 801–817. [Google Scholar] [CrossRef]
- Viana, L.R.; Tobar, N.; Busanello, E.N.B.; Marques, A.C.; de Oliveira, A.G.; Lima, T.I.; Machado, G.; Castelucci, B.G.; Ramos, C.D.; Brunetto, S.Q.; et al. Leucine-rich diet induces a shift in tumour metabolism from glycolytic towards oxidative phosphorylation, reducing glucose consumption and metastasis in Walker-256 tumour-bearing rats. Sci. Rep. 2019, 9, 15529. [Google Scholar] [CrossRef]
- Chhetri, D.R. Myo-Inositol and Its Derivatives: Their Emerging Role in the Treatment of Human Diseases. Front. Pharmacol. 2019, 10, 1172. [Google Scholar] [CrossRef] [Green Version]
- Gu, M.; Roy, S.; Raina, K.; Agarwal, C.; Agarwal, R. Inositol Hexaphosphate Suppresses Growth and Induces Apoptosis in Prostate Carcinoma Cells in Culture and Nude Mouse Xenograft: PI3K-Akt Pathway as Potential Target. Cancer Res. 2009, 69, 9465–9472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kapral, M.; Wawszczyk, J.; Jesse, K.; Paul-Samojedny, M.; Kuśmierz, D.; Węglarz, L. Inositol Hexaphosphate Inhibits Proliferation and Induces Apoptosis of Colon Cancer Cells by Suppressing the AKT/mTOR Signaling Pathway. Molecules 2017, 22, 1657. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miura, Y.; Furuse, T.; Yagasaki, K. Inhibitory effect of serum from rats administered with coffee on the proliferation and invasion of rat ascites hepatoma cells. Cytotechnology 1997, 25, 221–225. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.H.; Zhang, S.Y.; Yu, Y.G.Q.; Su, Y. (-)-4-O-(4-O-β-D-glucopyranosylcaffeoyl)quinic acid presents antitumor activity in HT-29 human colon cancer in vitro and in vivo. Mol. Cell. Toxicol. 2015, 11, 457–463. [Google Scholar] [CrossRef]
- Kyoung, S.P.; Chang, I.M. Anti-inflammatory activity of aucubin by inhibition of tumor necrosis factor-α production in RAW 264.7 cells. Planta Med. 2004, 70, 778–779. [Google Scholar] [CrossRef]
- Chang, I.M. Antiviral activity of aucubin against hepatitis B virus replication. Phyther. Res. 1997, 11, 189–192. [Google Scholar] [CrossRef]
- Hung, J.Y.; Yang, C.J.; Tsai, Y.M.; Huang, H.W.; Huang, M.S. Antiproliferative Activity Of Aucubin Is Through Cell Cycle Arrest And Apoptosis In Human Non-Small Cell Lung Cancer A549 Cells. Clin. Exp. Pharmacol. Physiol. 2008, 35, 995–1001. [Google Scholar] [CrossRef]
- Shim, K.M.; Choi, S.H.; Jeong, M.J.; Kang, S.S. Effects of Aucubin on the Healing of Oral Wounds. Vivo 2007, 21, 1037–1041. Available online: http://iv.iiarjournals.org/content/21/6/1037.full.pdf+html (accessed on 30 August 2007).
- Benito, P.B.; Lanza, A.M.D.; Sen, A.M.S.; Galindez, J.D.S.; Matellano, L.F.; Gómez, A.S.; Martínez, M.J. Effects of some iridoids from plant origin on arachidonic acid metabolism in cellular systems. Planta Med. 2000, 66, 324–328. [Google Scholar] [CrossRef]
- Newell, M.; Baker, K.; Postovit, L.M.; Field, C.J. Critical Review on the Effect of Docosahexaenoic Acid (DHA) on Cancer Cell Cycle Progression. Int. J. Mol. Sci. 2017, 18, 1784. [Google Scholar] [CrossRef] [Green Version]
- Shaikh, I.I.; Brown, A.A.; Schofield, A.C.; Wahle, K.S.D.; Heys, W. Docosahexaenoic acid enhances the efficacy of docetaxel in prostate cancer cells by modulation of apoptosis: The role of genes associated with the NF-κB pathway. Prostate J. 2008, 68, 1635–1646. [Google Scholar] [CrossRef] [PubMed]
- Iravani, S.; Varma, R.S. Plant-Derived Edible Nanoparticles and miRNAs: Emerging Frontier for Therapeutics and Targeted Drug-Delivery. ACS Sustain. Chem. Eng. 2019, 7, 8055–8069. [Google Scholar] [CrossRef]




| A | ||
| N° | Area Counts × Min | Name |
| 1 | 8.4 × 108 | D-(−)-Fructose, pentakis(trimethylsilyl) ether, methyloxime (syn) |
| 2 | 4.2 × 108 | Citric acid, 4TMS derivative |
| 3 | 4.2 × 108 | d-Glucose, 2,3,4,5,6-pentakis-O-(trimethylsilyl)-, o-methyloxyme, (1Z)- |
| 4 | 3.7 × 108 | Sucrose, 8TMS derivative |
| 5 | 2.7 × 108 | Myo-Inositol, 6TMS derivative |
| 6 | 1.7 × 108 | d-Galactose, 2,3,4,5,6-pentakis-O-(trimethylsilyl)-, o-methyloxyme, (1Z)- |
| 7 | 6.5 × 107 | Lactulose, octakis(trimethylsilyl) ether, methyloxime (isomer 1) |
| 8 | 6.2 × 107 | Quininic acid (5TMS) |
| 9 | 4.8 × 107 | L-Aspartic acid, 2TMS derivative+L-Aspartic acid, 3TMS derivative |
| 10 | 3.1 × 107 | Malic acid, 3TMS derivative |
| 11 | 2.3 × 107 | Serine, 3TMS derivative |
| 12 | 2.1 × 107 | Glycolic acid, 2TMS derivative |
| 13 | 1.9 × 107 | L-Proline, 2TMS derivative |
| 14 | 1.7 × 107 | d-Ribose, 2,3,4,5-tetrakis-O-(trimethylsilyl)-, O-methyloxime |
| 15 | 1.6 × 107 | 4-Aminobutanoic acid, 3TMS derivative |
| 16 | 1.4 × 107 | N-Acetyl glucosamine methoxime, tetrakis(trimethylsilyl) |
| 17 | 1.3 × 107 | D-(+)-Turanose, octakis(trimethylsilyl) ether |
| 18 | 9.9 × 106 | L-Alanine, 2TMS derivative |
| 19 | 9.1 × 106 | L-Glutamic acid, 3TMS derivative |
| 20 | 7.2 × 106 | α-D-Glucopyranoside, methyl 2-(acetylamino)-2-deoxy-3-O-(trimethylsilyl)-, cyclic methylboronate |
| B | ||
| N° | Area Counts × Min | Name |
| 1 | 6.0 × 108 | D-Fructose, 1,3,4,5,6-pentakis-O-(trimethylsilyl)-, O-methyloxime |
| 2 | 4.1 × 108 | Sucrose, 8TMS derivative |
| 3 | 3.7 × 108 | d-Glucose, 2,3,4,5,6-pentakis-O-(trimethylsilyl)-, o-methyloxyme, (1Z)- |
| 4 | 2.9 × 108 | Citric acid, 3TMS derivative+Citric acid, 4TMS derivative |
| 5 | 2.1 × 108 | Myo-Inositol, 6TMS derivative |
| 6 | 1.7 × 108 | d-Galactose, 2,3,4,5,6-pentakis-O-(trimethylsilyl)-, o-methyloxyme, (1Z)- |
| 7 | 1.4 × 108 | Oxalic acid, 2TMS derivative |
| 8 | 6.9 × 107 | D-(+)-Turanose, octakis(trimethylsilyl) ether |
| 9 | 4.0 × 107 | Quininic acid (5TMS) |
| 10 | 3.9 × 107 | β-D-Galactopyranoside, methyl 2,3-bis-O-(trimethylsilyl)-, cyclic methylboronate |
| 11 | 3.4 × 107 | α-D-Glucopyranoside, methyl 2-(acetylamino)-2-deoxy-3-O-(trimethylsilyl)-, cyclic methylboronate |
| 12 | 3.2 × 107 | Lactulose, octakis(trimethylsilyl) ether (isomer 1) |
| 13 | 2.8 × 107 | (2S,3R)-3-[(4E,7E)-Nona-4,7-dienoyl]-N,N-bis(trimethylsilyl)oxirane-2-carboxamide |
| 14 | 2.1 × 107 | N-Acetyl glucosamine methoxime, tetrakis(trimethylsilyl) |
| 15 | 2.0 × 107 | D-Mannitol, 6TMS derivative |
| 16 | 1.6 × 107 | D-(−)-Tagatose, pentakis(trimethylsilyl) ether, methyloxime (anti) |
| 17 | 1.5 × 107 | L-Aspartic acid, 2TMS derivative+L-Aspartic acid, 3TMS derivative |
| 18 | 1.4 × 107 | Aucubin, hexakis(trimethylsilyl) ether |
| 19 | 1.0 × 106 | Malic acid, 3TMS derivative |
| 20 | 8.1 × 106 | Myo-Inositol, pentakis-O-(trimethylsilyl)-, bis(trimethylsilyl) phosphate |
| C | ||
| N° | Area Counts × Min | Name |
| 1 | 1.1 × 108 | Glycolic acid, 2TMS derivative |
| 2 | 3.1 × 107 | (2S,3R)-3-[(4E,7E)-Nona-4,7-dienoyl]-N,N-bis(trimethylsilyl)oxirane-2-carboxamide |
| 3 | 4.7 × 107 | L-Isoleucine, 2TMS derivative |
| 4 | 4.3 × 106 | L-Leucine, TMS derivative+Leucine, 2TMS derivative |
| 5 | 3.9 × 106 | d-Glucose, 2,3,4,5,6-pentakis-O-(trimethylsilyl)-, o-methyloxyme, (1Z)- |
| 6 | 3.6 × 106 | D-(−)-Tagatose, pentakis(trimethylsilyl) ether, methyloxime (anti) |
| 7 | 2.1 × 106 | D-Psicose, pentakis(trimethylsilyl) ether, methyloxime (syn) |
| 8 | 1.3 × 106 | Urea, 2TMS derivative |
| 9 | 1.2 × 106 | D-(+)-Turanose, octakis(trimethylsilyl) ether |
| 10 | 1.1 × 106 | Lactic Acid, 2TMS derivative |
| 11 | 9.4 × 105 | d-Mannose, 2,3,4,5,6-pentakis-O-(trimethylsilyl)-, o-methyloxyme, (1Z)- |
| 12 | 7.6 × 105 | L-(−)-Sorbose, pentakis(trimethylsilyl) ether, methyloxime (anti) |
| 13 | 5.9 × 105 | Citric acid, 4TMS derivative |
| 14 | 4.5 × 105 | Sucrose, 8TMS derivative |
| 15 | 1.5 × 105 | Myo-Inositol, 6TMS derivative |
| 16 | 1.3 × 105 | Palmitic Acid, TMS derivative |
| 17 | 7.5 × 104 | 2-Hydroxycyclohexane-1-carboxylic acid, bis(trimethylsilyl) deriv. |
| 18 | 4.7 × 104 | Doconexent, TMS derivative |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stanly, C.; Alfieri, M.; Ambrosone, A.; Leone, A.; Fiume, I.; Pocsfalvi, G. Grapefruit-Derived Micro and Nanovesicles Show Distinct Metabolome Profiles and Anticancer Activities in the A375 Human Melanoma Cell Line. Cells 2020, 9, 2722. https://doi.org/10.3390/cells9122722
Stanly C, Alfieri M, Ambrosone A, Leone A, Fiume I, Pocsfalvi G. Grapefruit-Derived Micro and Nanovesicles Show Distinct Metabolome Profiles and Anticancer Activities in the A375 Human Melanoma Cell Line. Cells. 2020; 9(12):2722. https://doi.org/10.3390/cells9122722
Chicago/Turabian StyleStanly, Christopher, Mariaevelina Alfieri, Alfredo Ambrosone, Antonietta Leone, Immacolata Fiume, and Gabriella Pocsfalvi. 2020. "Grapefruit-Derived Micro and Nanovesicles Show Distinct Metabolome Profiles and Anticancer Activities in the A375 Human Melanoma Cell Line" Cells 9, no. 12: 2722. https://doi.org/10.3390/cells9122722