Boosting the Intra-Articular Efficacy of Low Dose Corticosteroid through a Biopolymeric Matrix: An In Vivo Model of Osteoarthritis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material Formulations
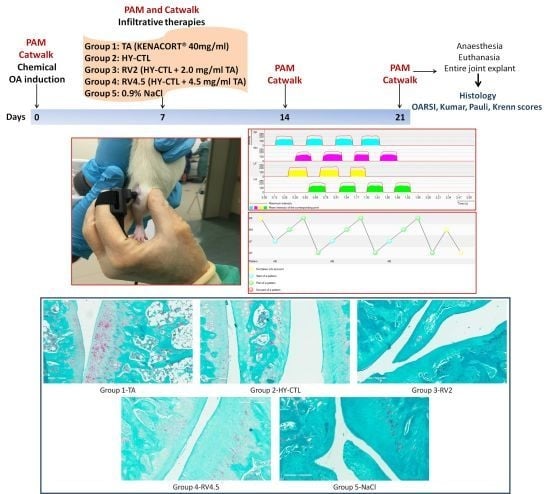
2.2. Experimental Design and OA Induction
- -
- Group 1: TA (Kenacort® 40 mg/mL).
- -
- Group 2: HY-CTL.
- -
- Group 3: RV2 (HY-CTL + 2.0 mg/mL TA).
- -
- Group 4: RV4.5 (HY-CTL + 4.5 mg/mL TA).
- -
- Group 5: NaCl (0.9% sterile saline solution).
2.3. Local Pain Sensitivity and Gait Measurements
- -
- Paw area: the total floor area contacted by the paw during the stance phase.
- -
- Stand: the duration in seconds of contact of a paw with the glass plate.
- -
- Swing: the duration in seconds of no contact of a paw with the glass plate.
- -
- Single stance: the duration in seconds of contact with the glass plate with only one paw.
2.4. Histology
2.5. Immunohistochemistry
2.6. Statistical Analysis
3. Results
3.1. Clinical Observations
3.2. Local Pain Sensitivity and Gait Measurements
3.3. Histology
3.4. Immunohistochemistry
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Neogi, T.; Zhang, Y. Epidemiology of Osteoarthritis. Rheum. Dis. Clin. N. Am. 2013, 39, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Hunter, D.J.; Bienna-Zeinstra, S. Osteoarthritis. Lancet 2019, 393, 1745–1759. [Google Scholar] [CrossRef]
- Delbarre, A.; Amor, B.; Bardoulat, I.; Tetafort, A.; Pelletier-Fleury, N. Do intra-articular hyaluronic acid injections delay total knee replacement in patients with osteoarthritis—A Cox model analysis. PLoS ONE 2017, 12, e0187227. [Google Scholar] [CrossRef] [Green Version]
- Hawker, G.A. Osteoarthritis Is a Serious Disease. Clin. Exp. Rheumatol. 2019, 37 (Suppl. 120), 3–6. [Google Scholar]
- Yusuf, E.; Kortekaas, M.C.; Watt, I.; Huizinga, T.W.J.; Kloppenburg, M. Do Knee Abnormalities Visualised on MRI Explain Knee Pain in Knee Osteoarthritis? A Systematic Review. Ann. Rheum. Dis. 2011, 70, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Shu, C.C.; Zaki, S.; Ravi, V.; Schiavinato, A.; Smith, M.M.; Little, C.B. The relationship between synovial inflammation, structural pathology, and pain in post-traumatic osteoarthritis: Differential effect of stem cell and hyaluronan treatment. Arthritis Res. Ther. 2020, 22, 29. [Google Scholar] [CrossRef] [Green Version]
- Habib, G.S.; Saliba, W.; Nashashibi, M. Local Effects of Intra-Articular Corticosteroids. Clin. Rheumatol. 2010, 29, 347–356. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Nuki, G.; Moskowitz, R.W.; Abramson, S.; Altman, R.D.; Arden, N.K.; Bierma-Zeinstra, S.; Brandt, K.D.; Croft, P.; Doherty, M.; et al. OARSI Recommendations for the Management of Hip and Knee Osteoarthritis: Part III: Changes in Evidence Following Systematic Cumulative Update of Research Published through January 2009. Osteoarthr. Cartil. 2010, 18, 476–499. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iannitti, T.; Bingöl, A.Ö.; Rottigni, V.; Palmieri, B. A new highly viscoelastic hyaluronic acid gel: Rheological properties, biocompatibility and clinical investigation in esthetic and restorative surgery. Int. J. Pharm. 2013, 456, 583–592. [Google Scholar] [CrossRef]
- Raza, K.; Gerlag, D.M. Preclinical inflammatory rheumatic diseases: An overview and relevant nomenclature. Rheum. Dis. Clin. N. Am. 2014, 40, 569–580. [Google Scholar] [CrossRef]
- Dubin, A. Managing Osteoarthritis and Other Chronic Musculoskeletal Pain Disorders. Med. Clin. N. Am. 2016, 100, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, C.; Lefèvre-Colau, M.M.; Poiraudeau, S.; Rannou, F. Evidence and recommendations for use of intra-articular injections for knee osteoarthritis. Ann. Phys. Rehabil. Med. 2016, 59, 184–189. [Google Scholar] [CrossRef] [PubMed]
- Cooper, C.; Rannou, F.; Richette, P.; Bruyère, O.; Al-Daghri, N.; Altman, R.D.; Brandi, M.L.; Collaud Basset, S.; Herrero-Beaumont, G.; Migliore, A.; et al. Use of Intraarticular Hyaluronic Acid in the Management of Knee Osteoarthritis in Clinical Practice. Arthritis Care Res. (Hoboken) 2017, 69, 1287–1296. [Google Scholar] [CrossRef] [PubMed]
- Roskos, S.E. Intra-articular Corticosteroid for Treating Osteoarthritis of the Knee. Am. Fam. Physician 2005, 72, 1222–1223. [Google Scholar]
- Ko, J.Y.; Choi, Y.J.; Jeong, G.J.; Im, G.I. Sulforaphane-PLGA microspheres for the intra-articular treatment of osteoarthritis. Biomaterials 2013, 34, 5359–5368. [Google Scholar] [CrossRef]
- Bannuru, R.R.; Natov, N.S.; Obadan, I.E.; Price, L.L.; Schmid, C.H.; McAlindon, T.E. Therapeutic trajectory of hyaluronic acid versus corticosteroids in the treatment of knee osteoarthritis: A systematic review and meta-analysis. Arthritis Rheum. 2009, 61, 1704–1711. [Google Scholar] [CrossRef]
- McAlindon, T.E.; LaValley, M.P.; Harvey, W.F.; Price, L.L.; Driban, L.B.; Zhang, M.; Ward, R.J. Effect of Intra-articular Triamcinolone vs Saline on Knee Cartilage Volume and Pain in Patients With Knee Osteoarthritis: A Randomized Clinical Trial. JAMA 2017, 317, 1967–1975. [Google Scholar] [CrossRef]
- Salamanna, F.; Giavaresi, G.; Parrilli, A.; Martini, L.; Nicoli Aldini, N.; Abatangelo, G.; Frizziero, A.; Fini, M. Effects of intra-articular hyaluronic acid associated to Chitlac (arty-duo®) in a rat knee osteoarthritis model. J. Orthop. Res. 2019, 37, 867–876. [Google Scholar] [CrossRef]
- Toegel, S.; Bieder, D.; André, S.; Altmann, F.; Walzer, S.M.; Kaltner, H.; Hofstaetter, J.G.; Windhager, R.; Gabius, H.J. Glycophenotyping of osteoarthritic cartilage and chondrocytes by RT-qPCR, mass spectrometry, histochemistry with plant/human lectins and lectin localization with a glycoprotein. Arthritis Res. Ther. 2013, 15, R147. [Google Scholar] [CrossRef] [Green Version]
- Salamanna, F.; Veronesi, F.; Frizziero, A.; Fini, M. Role and translational implication of galectins in arthritis pathophysiology and treatment: A systematic literature review. J. Cell. Physiol. 2019, 234, 1588–1605. [Google Scholar] [CrossRef]
- Donati, I.; Stredanska, S.; Silvestrini, G.; Vetere, A.; Marcon, P.; Marsich, E.; Mozetic, P.; Gamini, A.; Paoletti, S.; Vittur, F. The aggregation of pig articular chondrocyte and synthesis of extracellular matrix by a lactose-modified chitosan. Biomaterials 2005, 26, 987–998. [Google Scholar] [CrossRef] [PubMed]
- Tarricone, E.; Elia, R.; Mattiuzzo, E.; Faggian, A.; Pozzuoli, A.; Ruggieri, P.; Brun, P. The Viability and Anti-Inflammatory Effects of Hyaluronic Acid-Chitlac-Tracimolone Acetonide-β-Cyclodextrin Complex on Human Chondrocytes. Cartilage 2020, 28, 1947603520908658. [Google Scholar] [CrossRef] [PubMed]
- Kilkenny, C.; Browne, W.J.; Cuthill, I.C.; Emerson, M.; Altman, D.G. Improving bioscience research reporting: The ARRIVE guidelines for reporting animal research. J. Pharmacol. Pharmacother. 2010, 1, 94–99. [Google Scholar] [CrossRef] [Green Version]
- Barton, N.J.; Strickland, I.T.; Bond, S.M.; Brash, H.M.; Bate, S.T.; Wilson, A.W.; Chessell, I.P.; Reeve, A.J.; McQueen, D.S. Pressure application measurement (PAM): A novel behavioural technique for measuring hypersensitivity in a rat model of joint pain. J. Neurosci. Methods 2007, 163, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Pritzker, K.P.; Gay, S.; Jimenez, S.A.; Ostergaard, K.; Pelletier, J.P.; Revell, P.A.; Salter, D.; van den Berg, W.B. Osteoarthritis Cartilage Histopathology: Grading and Staging. Osteoarthr. Cartil. 2006, 14, 13–29. [Google Scholar] [CrossRef] [Green Version]
- Kumar, A.; Bendele, A.M.; Blanks, R.C.; Bodick, N. Sustained efficacy of a single intra-articular dose of FX006 in a rat model of repeated localized knee arthritis. Osteoarthr. Cartil. 2015, 23, 151–160. [Google Scholar] [CrossRef] [Green Version]
- Lewis, J.S.; Hembree, W.C.; Furman, B.D.; Tippets, L.; Cattel, D.; Huebner, J.L.; Little, D.; DeFrate, L.E.; Kraus, V.B.; Guilak, F.; et al. Acute joint pathology and synovial inflammation is associated with increased intra-articular fracture severity in the mouse knee. Osteoarthr. Cartil. 2011, 19, 864–873. [Google Scholar] [CrossRef] [Green Version]
- Pauli, C.; Grogan, S.P.; Patil, S.; Otsuki, S.; Hasegawa, A.; Koziol, J.; Lotz, M.K.; D’Lima, D.D. Macroscopic and histopathologic analysis of human knee menisci in aging and osteoarthritis. Osteoarthr. Cartil. 2011, 19, 1132–1141. [Google Scholar] [CrossRef] [Green Version]
- Christensen, R.H.B. Ordinal-Regression Models for Ordinal Data. R package version 2019. Available online: http://www2.uaem.mx/r-mirror/web/packages/ordinal/ (accessed on 1 March 2020).
- Bates, D.; Maechler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Kuznetsova, A.; Brockhoff, P.B.; Christensen, R.H.B. lmerTest Package: Tests in Linear Mixed Effects Models. J. Stat. Softw. 2017, 82, 1–26. [Google Scholar] [CrossRef] [Green Version]
- Length, R.; Singmann, H.; Love, J.; Buerkner, P.; Herve, M. Emmeans: Estimated Marginal Means, aka Least-Squares Means. 2019. Available online: https://github.com/rvlenth/emmeans (accessed on 1 March 2020).
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar]
- Grissom, R.J.; Kim, J.J. Effect size for factorial designs. Effect sizes for research. In Univariate and Multivariate Applications, 2nd ed.; Taylor & Francis Group LLC: New York, NY, USA, 2012. [Google Scholar]
- Huang, Z.; Ding, C.; Li, T.; Yu, P.C.S. Current Status and Future Prospects for Disease Modification in Osteoarthritis. Rheumatology (Oxford) 2018, 57, 108–123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghouri, A.; Conaghan, P.G. Prospects for Therapies in Osteoarthritis. Calcif. Tissue Int. 2020. Online ahead of print. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Brien, M.; Philpott, H.T.; McDougall, J.J. Understanding osteoarthritis pain through animal models. Clin. Exp. Rheumatol. 2017, 35 (Suppl. 107), 47–52. [Google Scholar] [PubMed]
- Guzman, R.E.; Evans, M.G.; Bove, S.; Morenko, B.; Kilgore, K. Mono-iodoacetate-induced histologic changes in subchondral bone and articular cartilage of rat femorotibial joints: An animal model of osteoarthritis. Toxicol. Pathol. 2003, 31, 619–624. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, B.Y.; Dunnigan, K.; Pires-Fernandes, M.; Allen, K.D. Unique Spatiotemporal and Dynamic Gait Compensations in the Rat Monoiodoacetate Injection and Medial Meniscus Transection Models of Knee Osteoarthritis. Osteoarthr. Cartil. 2017, 25, 750–758. [Google Scholar] [CrossRef] [PubMed]
- Fernihough, J.; Gentry, C.; Malcangio, M.; Fox, M.; Rediske, J.; Pellas, T.; Kidd, B.; Bevan, S.; Winter, J. Pain Related Behaviour in Two Models of Osteoarthritis in the Rat Knee. Pain 2004, 112, 83–93. [Google Scholar] [CrossRef]
- Kuyinu, E.L.; Narayanan, G.; Nair, L.S.; Laurencin, C.T. Animal models of osteoarthritis: Classification, update, and measurement of outcomes. J. Orthop. Surg. Res. 2016, 11, 19. [Google Scholar] [CrossRef] [Green Version]
- Udo, M.; Muneta, T.; Tsuji, K.; Ozeki, N.; Nakagawa, Y.; Ohara, T.; Saito, R.; Yanagisawa, K.; Koga, H.; Sekiya, I. Monoiodoacetic acid induces arthritis and synovitis in rats in a dose- and time-dependent manner: Proposed model-specific scoring systems. Osteoarthr. Cartil. 2016, 24, 1284–1291. [Google Scholar] [CrossRef] [Green Version]
- Siu, W.S.; Shum, W.T.; Cheng, W.; Wong, C.W.; Shiu, H.T.; Ko, C.H.; Leung, P.C.; Lam, C.W.K.; Wong, C.K. Topical application of Chinese herbal medicine DAEP relieves the osteoarthritic knee pain in rats. Chin. Med. 2019, 14, 55. [Google Scholar] [CrossRef] [Green Version]
- Ferland, C.E.; Laverty, S.; Beaudry, F.; Vachon, P. Gait analysis and pain response of two rodent models of osteoarthritis. Pharmacol. Biochem. Behav. 2011, 97, 603–610. [Google Scholar] [CrossRef]
- Lakes, E.H.; Allen, K.D. Gait analysis methods for rodent models of arthritic disorders: Reviews and recommendations. Osteoarthr. Cartil. 2016, 24, 1837–1849. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miyamoto, S.; Nakamura, J.; Ohtori, S.; Orita, S.; Nakajima, T.; Omae, T.; Hagiwara, S.; Takazawa, M.; Suzuki, M.; Suzuki, T.; et al. Pain-related Behavior and the Characteristics of Dorsal-Root Ganglia in a Rat Model of Hip Osteoarthritis Induced by Mono-Iodoacetate. J. Orthop. Res. 2017, 35, 1424–1430. [Google Scholar] [CrossRef] [PubMed]
- Miyagi, M.; Ishikawa, T.; Kamoda, H.; Suzuki, M.; Inoue, G.; Sakuma, Y.; Oikawa, Y.; Orita, S.; Uchida, K.; Takahashi, K.; et al. Efficacy of nerve growth factor antibody in a knee osteoarthritis pain model in mice. BMC Musculoskelet. Disord. 2017, 18, 428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferreira-Gomes, J.; Adães, S.; Mendonça, M.; Castro-Lopes, J.M. Analgesic effects of lidocaine, morphine and diclofenac on movement-induced nociception, as assessed by the Knee-Bend and CatWalk tests in a rat model of osteoarthritis. Pharmacol. Biochem. Behav. 2012, 101, 617–624. [Google Scholar] [CrossRef]
- Gupta, P.K.; Chullikana, A.; Rengasamy, M.; Shetty, N.; Pandey, V.; Agarwal, V.; Wagh, S.Y.; Vellotare, P.K.; Damodaran, D.; Viswanathan, P.; et al. Efficacy and safety of adult human bone marrow-derived, cultured, pooled, allogeneic mesenchymal stromal cells (Stempeucel®): Preclinical and clinical trial in osteoarthritis of the knee joint. Arthritis Res. Ther. 2016, 18, 301. [Google Scholar] [CrossRef] [Green Version]








© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tschon, M.; Salamanna, F.; Martini, L.; Giavaresi, G.; Lorenzini, L.; Calzà, L.; Fini, M. Boosting the Intra-Articular Efficacy of Low Dose Corticosteroid through a Biopolymeric Matrix: An In Vivo Model of Osteoarthritis. Cells 2020, 9, 1571. https://doi.org/10.3390/cells9071571
Tschon M, Salamanna F, Martini L, Giavaresi G, Lorenzini L, Calzà L, Fini M. Boosting the Intra-Articular Efficacy of Low Dose Corticosteroid through a Biopolymeric Matrix: An In Vivo Model of Osteoarthritis. Cells. 2020; 9(7):1571. https://doi.org/10.3390/cells9071571
Chicago/Turabian StyleTschon, Matilde, Francesca Salamanna, Lucia Martini, Gianluca Giavaresi, Luca Lorenzini, Laura Calzà, and Milena Fini. 2020. "Boosting the Intra-Articular Efficacy of Low Dose Corticosteroid through a Biopolymeric Matrix: An In Vivo Model of Osteoarthritis" Cells 9, no. 7: 1571. https://doi.org/10.3390/cells9071571