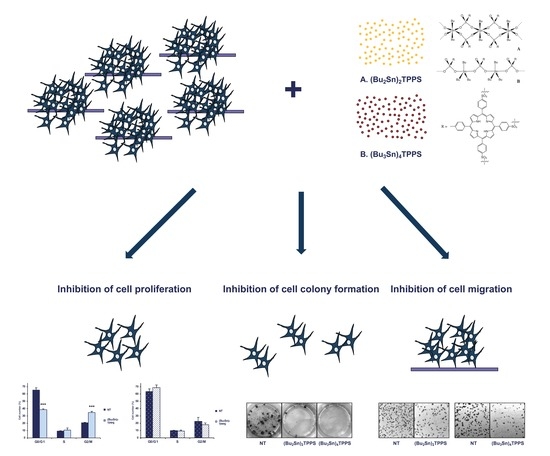
Dibutyltin(IV) and Tributyltin(IV) Derivatives of meso-Tetra(4-sulfonatophenyl)porphine Inhibit the Growth and the Migration of Human Melanoma Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Lines, Antibodies, Chemicals and Reagents
2.2. Cell Viability Assay (MTS Assay)
2.3. Total Cell Extracts and Western Blot Analysis
2.4. Cell Cycle Analysis
2.5. Cell Colony Assay
2.6. Cell Migration Assay
3. Results
3.1. Inhibition of Melanoma Cell Proliferation
3.2. Cell Cycle Arrest of Treated Melanoma Cells
3.3. Cell Colony Formation
3.4. Expression of Adhesion Receptors and FAK Signalling Protein
3.5. Analysis of Melanoma Cell Migration
3.6. Expression of STAT3 Signalling Protein
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2019. Ca A Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef] [PubMed]
- Schadendorf, D.; van Akkooi, A.C.J.; Berking, C.; Griewank, K.G.; Gutzmer, R.; Hauschild, A.; Stang, A.; Roesch, A.; Ugurel, S. Melanoma. Lancet 2018, 392, 971–984. [Google Scholar] [CrossRef]
- Shain, A.H.; Bastian, B.C. From melanocytes to melanomas. Nat. Rev. Cancer 2016, 16, 345–358. [Google Scholar] [CrossRef] [PubMed]
- Mattia, G.; Puglisi, R.; Ascione, B.; Malorni, W.; Care, A.; Matarrese, P. Cell death-based treatments of melanoma: Conventional treatments and new therapeutic strategies. Cell Death Dis. 2018, 9, 112. [Google Scholar] [CrossRef]
- Gershenwald, J.E.; Scolyer, R.A.; Hess, K.R.; Sondak, V.K.; Long, G.V.; Ross, M.I.; Lazar, A.J.; Faries, M.B.; Kirkwood, J.M.; McArthur, G.A.; et al. Melanoma staging: Evidence-based changes in the American Joint Committee on Cancer eighth edition cancer staging manual. Ca A Cancer J. Clin. 2017, 67, 472–492. [Google Scholar] [CrossRef]
- Devi, J.; Yadav, J. Recent Advancements in Organotin(IV) Complexes as Potential Anticancer Agents. Anti-Cancer Agents Med. Chem. 2018, 18, 335–353. [Google Scholar] [CrossRef]
- Arjmand, F.P.S.; Tabassum, S.; Pettinari, C. Organo-tin antitumor compounds: Their present status in drug development and future perspectives. Inorg. Chim. Acta 2014, 423, 26–37. [Google Scholar] [CrossRef]
- Pellerito, A.; Fiore, T.; Giuliani, A.M.; Maggio, F.; Pellerito, L.; Vitturi, R.; Colomba, M.S.; Barbieri, R. Organometallic complexes with biological molecules. 8. Synthesis, solid state and in vivo investigation of triorganotin(lV) derivatives of L-homocysteic acid. Appl. Organomet. Chem. 1997, 11, 601–616. [Google Scholar] [CrossRef]
- Ruisi, G.; Silvestri, A.; Lo Guidice, M.T.; Barbieri, R.; Atassi, G.; Huber, F.; Gratz, K.; Lamartina, L. The antitumor activity of di-n-butyltin(IV) glycylglycinate, and the correlation with the structure of dialkyltin(IV) glycylglycinates in solution studied by conductivity measurements and by infrared, nuclear magnetic resonance, and Mossbauer spectroscopic methods. J. Inorg. Biochem. 1985, 25, 229–245. [Google Scholar]
- Huber, F.; Roge, G.; Carl, L.; Atassi, G.; Spreafico, F.; Filippeschi, S.; Barbieri, R.; Silvestri, A.; Rivarola, E.; Ruisi, G.; et al. Studies on the Anti-Tumor Activity of Di-Organotin(Iv) and Tri-Organotin(Iv) Complexes of Amino-Acids and Related-Compounds, of 2-Mercaptoethanesulphonate, and of Purine-6-Thiol. J. Chem. Soc. Dalton 1985. [Google Scholar] [CrossRef]
- Casini, A.; Messori, L.; Orioli, P.; Gielen, M.; Kemmer, M.; Willem, R. Interactions of two cytotoxic organotin(IV) compounds with calf thymus DNA. J. Inorg. Biochem. 2001, 85, 297–300. [Google Scholar] [CrossRef]
- Devi, J.; Pachwania, S. Recent advancements in DNA interaction studies of organotin (IV) complexes. Inorg. Chem. Commun. 2018, 91, 44–62. [Google Scholar] [CrossRef]
- Barbieri, R.; Silvestri, A. The interaction of native DNA with dimethyltin(IV) species. J. Inorg. Biochem. 1991, 41, 31–35. [Google Scholar] [CrossRef]
- Costa, M.A.; Pellerito, L.; Izzo, V.; Fiore, T.; Pellerito, C.; Melis, M.; Musmeci, M.T.; Barbieri, G. Diorganotin(IV) and triorganotin(IV) complexes of meso-tetra(4-sulfonatophenyl)porphine induce apoptosis in A375 human melanoma cells. Cancer Lett. 2006, 238, 284–294. [Google Scholar] [CrossRef] [PubMed]
- Aydinoglu, S.; Biver, T.; Figuccia, S.; Fiore, T.; Montanaro, S.; Pellerito, C. Studies on DNA interaction of organotin(IV) complexes of meso-tetra(4-sulfonatophenyl)porphine that show cellular activity. J. Inorg. Biochem. 2016, 163, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Pellerito, C.; Morana, O.; Ferrante, F.; Calvaruso, G.; Notaro, A.; Sabella, S.; Fiore, T. Synthesis, chemical characterization, computational studies and biological activity of new DNA methyltransferases (DNMTs) specific inhibitor. Epigenetic regulation as a new and potential approach to cancer therapy. J. Inorg. Biochem. 2015, 150, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Barbieri, R. QSAR approach to understand the antitumour activity of organotins. Inorg. Chim. Acta 1992, 191, 253–259. [Google Scholar] [CrossRef]
- Chwilkowska, A.; Saczko, J.; Modrzycka, T.; Marcinkowska, A.; Malarska, A.; Bielewicz, J.; Patalas, D.; Banas, T. Uptake of photofrin II, a photosensitizer used in photodynamic therapy, by tumour cells in vitro. Acta Biochim. Pol. 2003, 50, 509–513. [Google Scholar]
- Dong, X.; Chen, H.; Qin, J.; Wei, C.; Liang, J.; Liu, T.; Kong, D.; Lv, F. Thermosensitive porphyrin-incorporated hydrogel with four-arm PEG-PCL copolymer (II): Doxorubicin loaded hydrogel as a dual fluorescent drug delivery system for simultaneous imaging tracking in vivo. Drug Deliv. 2017, 24, 641–650. [Google Scholar] [CrossRef]
- Huang, H.; Song, W.; Rieffel, J.; Lovell, J.F. Emerging applications of porphyrins in photomedicine. Front. Phys. 2015, 3. [Google Scholar] [CrossRef]
- Xiao, X.; Liang, J.W.; Xie, J.Y.; Liu, X.; Zhu, D.S.; Dong, Y. Organotin(IV) carboxylates based on 2-(1,3-dioxo-1H-benzo[de] isoquinolin-2(3H)-yl)acetic acid: Syntheses, crystal structures, luminescent properties and antitumor activities. J. Mol. Struct. 2017, 1146, 233–241. [Google Scholar] [CrossRef]
- Barbieri, F.; Viale, M.; Sparatore, F.; Favre, A.; Cagnoli, M.; Bruzzo, C.; Novelli, F.; Alama, A. Cytotoxicity in vitro and preliminary antitumor activity in vivo of a novel organotin compound. Anticancer Res. 2000, 20, 977–980. [Google Scholar] [PubMed]
- Costa, M.A.; Zito, F.; Emma, M.R.; Pellerito, L.; Fiore, T.; Pellerito, C.; Barbieri, G. Apoptosis and cell growth arrest in A375 human melanoma cells by diorganotin(IV) and triorganotin(IV) complexes of [meso-Tetra(4-sulfonatophenyl)porphine] manganese(III)chloride. Int. J. Oncol. 2011, 38, 693–700. [Google Scholar] [CrossRef] [PubMed]
- Mazzaglia, A.; Bondi, M.L.; Scala, A.; Zito, F.; Barbieri, G.; Crea, F.; Vianelli, G.; Mineo, P.; Fiore, T.; Pellerito, C.; et al. Supramolecular assemblies based on complexes of nonionic amphiphilic cyclodextrins and a meso-tetra(4-sulfonatophenyl)porphine tributyltin(IV) derivative: Potential nanotherapeutics against melanoma. Biomacromolecules 2013, 14, 3820–3829. [Google Scholar] [CrossRef]
- Costa, M.A.; Gulino, L.; Pellerito, L.; Fiore, T.; Pellerito, C.; Barbieri, G. Effects of two organotin(IV)(sulfonatophenyl)porphinates on MAPKs and on the growth of A375 human melanoma cells. Oncol. Rep. 2009, 21, 593–599. [Google Scholar] [CrossRef]
- Giard, D.J.; Aaronson, S.A.; Todaro, G.J.; Arnstein, P.; Kersey, J.H.; Dosik, H.; Parks, W.P. In vitro cultivation of human tumors: Establishment of cell lines derived from a series of solid tumors. J. Natl. Cancer Inst. 1973, 51, 1417–1423. [Google Scholar] [CrossRef]
- Tsuruo, T.; Fidler, I.J. Differences in drug sensitivity among tumor cells from parental tumors, selected variants, and spontaneous metastases. Cancer Res. 1981, 41, 3058–3064. [Google Scholar]
- Fogh, J.; Fogh, J.M.; Orfeo, T. One hundred and twenty-seven cultured human tumor cell lines producing tumors in nude mice. J. Natl. Cancer Inst. 1977, 59, 221–226. [Google Scholar] [CrossRef]
- Gervois, N.; Heuze, F.; Diez, E.; Jotereau, F. Selective expansion of a specific anti-tumor CD8+ cytotoxic T lymphocyte clone in the bulk culture of tumor-infiltrating lymphocytes from a melanoma patient: Cytotoxic activity and T cell receptor gene rearrangements. Eur. J. Immunol. 1990, 20, 825–831. [Google Scholar] [CrossRef]
- Costantini, F.; Barbieri, G. The HLA-DR mediated signalling increases the migration and invasion of melanoma cells, the expression and lipid raft recruitment of adhesion receptors, PD-L1 and signal transduction proteins. Cell Signal 2017, 36, 189–203. [Google Scholar] [CrossRef]
- Valster, A.; Tran, N.L.; Nakada, M.; Berens, M.E.; Chan, A.Y.; Symons, M. Cell migration and invasion assays. Methods 2005, 37, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Vallarelli, A.F.; Rachakonda, P.S.; Andre, J.; Heidenreich, B.; Riffaud, L.; Bensussan, A.; Kumar, R.; Dumaz, N. TERT promoter mutations in melanoma render TERT expression dependent on MAPK pathway activation. Oncotarget 2016, 7, 53127–53136. [Google Scholar] [CrossRef] [PubMed]
- Ramsay, J.; Birrell, G.; Baumann, K.; Bodero, A.; Parsons, P.; Lavin, M. Radiosensitive melanoma cell line with mutation of the gene for ataxia telangiectasia. Br. J. Cancer 1998, 77, 11–14. [Google Scholar] [CrossRef] [PubMed]
- Gnjatic, S.; Cai, Z.; Viguier, M.; Chouaib, S.; Guillet, J.G.; Choppin, J. Accumulation of the p53 protein allows recognition by human CTL of a wild-type p53 epitope presented by breast carcinomas and melanomas. J. Immunol. 1998, 160, 328–333. [Google Scholar] [PubMed]
- Shiloh, Y.; Ziv, Y. The ATM protein kinase: Regulating the cellular response to genotoxic stress, and more. Nat. Rev. Mol. Cell Biol. 2013, 14, 197–210. [Google Scholar] [CrossRef]
- Jackson, S.P.; Bartek, J. The DNA-damage response in human biology and disease. Nature 2009, 461, 1071–1078. [Google Scholar] [CrossRef] [Green Version]
- Khanna, K.K.; Jackson, S.P. DNA double-strand breaks: Signaling, repair and the cancer connection. Nat. Genet. 2001, 27, 247–254. [Google Scholar] [CrossRef]
- Lazebnik, Y.A.; Kaufmann, S.H.; Desnoyers, S.; Poirier, G.G.; Earnshaw, W.C. Cleavage of poly(ADP-ribose) polymerase by a proteinase with properties like ICE. Nature 1994, 371, 346–347. [Google Scholar] [CrossRef]
- Satyamoorthy, K.; Li, G.; Gerrero, M.R.; Brose, M.S.; Volpe, P.; Weber, B.L.; Van Belle, P.; Elder, D.E.; Herlyn, M. Constitutive mitogen-activated protein kinase activation in melanoma is mediated by both BRAF mutations and autocrine growth factor stimulation. Cancer Res. 2003, 63, 756–759. [Google Scholar]
- Hustedt, N.; Durocher, D. The control of DNA repair by the cell cycle. Nat. Cell Biol. 2016, 19, 1–9. [Google Scholar] [CrossRef]
- Bai, J.; Li, Y.; Zhang, G. Cell cycle regulation and anticancer drug discovery. Cancer Biol. Med. 2017, 14, 348–362. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Qin, Y.; Yang, C.; Zhang, H.; Li, Y.; Wu, B.; Huang, J.; Zhou, X.; Huang, B.; Yang, K.; et al. Cardamonin induces ROS-mediated G2/M phase arrest and apoptosis through inhibition of NF-kappaB pathway in nasopharyngeal carcinoma. Cell Death Dis. 2017, 8, e3024. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh, A.M.; Shiri, S.; Farsinejad, S. Metastasis review: From bench to bedside. Tumour Biol. J. Int. Soc. Oncodev. Biol. Med. 2014, 35, 8483–8523. [Google Scholar] [CrossRef] [PubMed]
- Bellis, S.L.; Miller, J.T.; Turner, C.E. Characterization of tyrosine phosphorylation of paxillin in vitro by focal adhesion kinase. J. Biol. Chem. 1995, 270, 17437–17441. [Google Scholar] [CrossRef] [Green Version]
- Xie, T.X.; Wei, D.; Liu, M.; Gao, A.C.; Ali-Osman, F.; Sawaya, R.; Huang, S. Stat3 activation regulates the expression of matrix metalloproteinase-2 and tumor invasion and metastasis. Oncogene 2004, 23, 3550–3560. [Google Scholar] [CrossRef] [Green Version]
- Niu, G.; Heller, R.; Catlett-Falcone, R.; Coppola, D.; Jaroszeski, M.; Dalton, W.; Jove, R.; Yu, H. Gene therapy with dominant-negative Stat3 suppresses growth of the murine melanoma B16 tumor in vivo. Cancer Res. 1999, 59, 5059–5063. [Google Scholar]
- De Vos, M.; Schreiber, V.; Dantzer, F. The diverse roles and clinical relevance of PARPs in DNA damage repair: Current state of the art. Biochem. Pharmacol. 2012, 84, 137–146. [Google Scholar] [CrossRef]
- Montero, J.; Dutta, C.; van Bodegom, D.; Weinstock, D.; Letai, A. p53 regulates a non-apoptotic death induced by ROS. Cell Death Differ. 2013, 20, 1465–1474. [Google Scholar] [CrossRef] [Green Version]
- Hydbring, P.; Malumbres, M.; Sicinski, P. Non-canonical functions of cell cycle cyclins and cyclin-dependent kinases. Nat. Rev. Mol. Cell Biol. 2016, 17, 280–292. [Google Scholar] [CrossRef]
- Jirawatnotai, S.; Sittithumcharee, G. Paradoxical roles of cyclin D1 in DNA stability. Dna Repair 2016, 42, 56–62. [Google Scholar] [CrossRef]
- Pathria, G.; Garg, B.; Garg, K.; Wagner, C.; Wagner, S.N. Dual c-Jun N-terminal kinase-cyclin D1 and extracellular signal-related kinase-c-Jun disjunction in human melanoma. Br. J. Dermatol. 2016, 175, 1221–1231. [Google Scholar] [CrossRef] [PubMed]
- Gennari, A.; Viviani, B.; Galli, C.L.; Marinovich, M.; Pieters, R.; Corsini, E. Organotins induce apoptosis by disturbance of [Ca(2+)](i) and mitochondrial activity, causing oxidative stress and activation of caspases in rat thymocytes. Toxicol. Appl. Pharmacol. 2000, 169, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Lutticken, C.; Wegenka, U.M.; Yuan, J.P.; Buschmann, J.; Schindler, C.; Ziemiecki, A.; Harpur, A.G.; Wilks, A.F.; Yasukawa, K.; Taga, T.; et al. Association of Transcription Factor Aprf and Protein-Kinase Jak1 with the Interleukin-6 Signal Transducer Gp130. Science 1994, 263, 89–92. [Google Scholar] [CrossRef] [PubMed]












© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Costantini, F.; Di Leo, F.; Di Sano, C.; Fiore, T.; Pellerito, C.; Barbieri, G. Dibutyltin(IV) and Tributyltin(IV) Derivatives of meso-Tetra(4-sulfonatophenyl)porphine Inhibit the Growth and the Migration of Human Melanoma Cells. Cells 2019, 8, 1547. https://doi.org/10.3390/cells8121547
Costantini F, Di Leo F, Di Sano C, Fiore T, Pellerito C, Barbieri G. Dibutyltin(IV) and Tributyltin(IV) Derivatives of meso-Tetra(4-sulfonatophenyl)porphine Inhibit the Growth and the Migration of Human Melanoma Cells. Cells. 2019; 8(12):1547. https://doi.org/10.3390/cells8121547
Chicago/Turabian StyleCostantini, Francesca, Fabiana Di Leo, Caterina Di Sano, Tiziana Fiore, Claudia Pellerito, and Giovanna Barbieri. 2019. "Dibutyltin(IV) and Tributyltin(IV) Derivatives of meso-Tetra(4-sulfonatophenyl)porphine Inhibit the Growth and the Migration of Human Melanoma Cells" Cells 8, no. 12: 1547. https://doi.org/10.3390/cells8121547