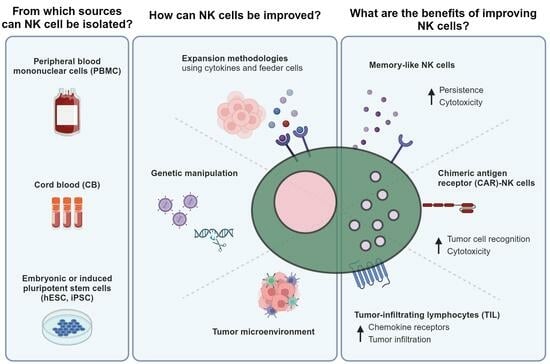
Building a Better Defense: Expanding and Improving Natural Killer Cells for Adoptive Cell Therapy
Abstract
:1. What Are Natural Killer Cells?
2. How Do NK Cells Modulate Their Immune Response?
3. What Are the Major Advantages of NK Cells and Their Respective Sources for Adoptive Cell Therapy?
3.1. Peripheral Blood (PB)
3.2. Cord Blood (CB)
3.3. Embryonic and Induced Pluripotent Stem Cells

4. What Are the Major NK Cell Expansion Approaches?
4.1. Cytokine-Based Expansion
4.2. Feeder-Cell-Based Expansion
| Ref. | Cell Source | Culture Factors | Feeders | Time (Days) | Culture Material | Results |
|---|---|---|---|---|---|---|
| [76] | PBMC-NK cells | TexMACS containing 5% HS type AB, 500 U/mL of IL-2, 100 ng/mL of IL-21, and feeder cells. | EBV-LCL cells (ratio 1:20). | 7 | N/A | NK cells reached 22-fold expansion in 1 week, which increased to 53-fold with IL-21. |
| [77] | CB-NK cells | X-VIVO 20 media with 10% heat-inactivated human AB serum, 500 IU/mL rhIL-2 and 2 mM GlutaMAX-1. | EBV-LCL cells. | Up to 40 | T75 flasks. | CD3− CD56+ cells reached a median of 6092-fold expansion. |
| [87] | Isolated NK cells | AIM-V medium supplemented with 5% HS, 1000 U/mL of IL-2 and OKT3. | NK cell—negative fraction of PBMC. | 14 | 24-well plates. | The patient’s NK cells co-cultured with healthy donor feeder cells reached 300-fold expansion. |
| [79] | PBMC or purified NK cells | Media with 10 ng/mL IL-2. | Genetically modified K562 cells. | 24 | 24-well plates. | K562-MICA-41BBL-IL-15 cells induced 550-fold NK cell expansion in 24 days. |
| [80] | PBMC or purified NK cells | RPMI-1640 media with 10 IU/mL human IL-2 and 10% FBS. | K562-mb15-41BBL cells. | 21 | 24-well plates. | PBMC showed a 21.6-fold expansion of NK cells, while a 277-fold was reached in purified NK cells after 21 days. |
| [81] | CB-NK cells | RPMI-1640 media with 10% FBS and 10 IU/mL of recombinant IL-2. | K562-mbIL15-41BBL cells. | 14 | 24-well plates. | K562-mbIL15-41BBL cells induced a 35-fold NK cell expansion. |
| [82] | PBMC | RPMI-1640 media with 50 IU/mL IL-2, 10% FBS, L-glutamine, and P/S. | K562-mbIL21 cells. | 42 | T75 flasks. | K562-mbIL21 cells induced a 47,967-fold expansion of NK cells by day 21. |
| [57] | iNK cells | B0 media supplemented with cytokines. | K562-IL21-4-1BBL cells. | 42 | At day 42, 98.5% of cells had a CD3− CD56+ phenotype and reached 105 to 106-fold expansion. | |
| [83] | PBMC | RPMI-1640 media with 10% FBS, 2mM L-glutamine, 100 U/mL P/S, 100 U/mL IL-2 and 5 ng/mL of IL-15. | 221-mbIL21 cells. | 20 | G-Rex 6 Multiwell cell culture plates. | NK cells showed a 39,663-fold increase with 221-mbIL21 cells compared to a 3588-fold expansion with K562-mbIL21 cells. |
| [84] | PBMC | RPMI 1640 media with 10% FBS, P/S, 4 mmol/L of L-glutamine, and 10 U/mL of IL-2. After one week, IL-2 was increased to 100 U/mL, and 5 ng/mL of IL-15 was added. IL-21 was added at different concentrations. | K562-OX40L cells. | 38 | 24-well plate | After four weeks, K562-OX40L cells and short exposure to IL-21 induced a 2000-fold expansion of NK cells. |
| [85] | Purified NK cells | AIM-V media with cytokines or feeder cells and 100 ng/mL OKT3 in the first culture cycle. | NK92-Neo2/15-OX40L cells. | 21 | N/A | NK92-Neo2/15-OX40L cells induced a 2180-fold increase of NK cells after 21 days without additional cytokines. |
| [86] | Isolated NK cells | CellGro SCGM with 5% HS, P/S and 10 ng/mL of OKT3. 200 U/mL of IL-2 was added alone or with 10 ng/mL of IL-15. | Autologous feeder cells. | 19 | Baxter LifeCell culture bags. | NK cells reached 62.7-fold expansion with IL-2 and 117-fold when IL-15 was added. |
| [99] | CB-NK cells | RPMI-1640 media with IL-2, 10% human AB, 1 mM L-glutamina, 10 U/mL Pen, and 0.01 mg/mL of streptomycin. | Autologous PBMC. | 26 | 24-well plate. | CB-CD56+ cytotoxic NK cells reached 156.3-fold increased on day 26. |
| [88] | Isolated NK cells | TexMACS media with 5% human AB serum, 1000 U/mL of IL-2, and 10 ng/mL of OKT3. | Autologous CD56-depleted PBMC. | 12 | 24-well plate, T25 and T75 flasks. | NK cells reached a 212-fold expansion with feeder cells, while only IL-2 showed a 22.5-fold expansion. |
| [78] | PBMC | GT-T507 with 1% plasma, IL-2, and OK-432. | FN-CH296 cells. | 21–22 | Flasks and culture bags. | A median of 4720-fold expansion was reached after 22 days with 90.96% purity. |
4.3. Culture Materials Used for NK Cell Expansion
5. How Do Cell Culture Strategies Improve NK Cell Activity?
5.1. Phenotype
5.2. Telomere Length
5.3. Cytotoxicity
6. Which Other Strategies Improve NK Cell Antitumor Response?
6.1. Memory-like NK Cells
6.2. Chimeric Antigen Receptor (CAR) Technology
6.3. Tumor-Infiltrating Lymphocytes (TILs), including TIL-NK Cells
7. Which Are Currently the Major NK-Cell-Based Clinical Trials?
7.1. Autologous NK Cells
7.2. Allogeneic NK Cells
7.3. Allogeneic CB-NK Cells
8. What Is the Future of NK-Cell-Based Therapy?
Author Contributions
Funding
Conflicts of Interest
References
- Del Zotto, G.; Antonini, F.; Pesce, S.; Moretta, F.; Moretta, L.; Marcenaro, E. Comprehensive Phenotyping of Human PB NK Cells by Flow Cytometry. J. Quant. Cell Sci. 2020, 97, 891–899. [Google Scholar] [CrossRef] [PubMed]
- Lam, R.A.; Chwee, J.Y.; Le Bert, N.; Sauer, M.; Pogge Von Strandmann, E.; Gasser, S. Regulation of Self-Ligands for Activating Natural Killer Cell Receptors. Ann. Med. 2013, 45, 384–394. [Google Scholar] [CrossRef] [PubMed]
- Cui, F.; Qu, D.; Sun, R.; Nan, K. Circulating CD16+CD56+ Nature Killer Cells Indicate the Prognosis of Colorectal Cancer after Initial Chemotherapy. Med. Oncol. 2018, 36, 84. [Google Scholar] [CrossRef] [PubMed]
- Ferlazzo, G.; Münz, C. NK Cell Compartments and Their Activation by Dendritic Cells. J. Immunol. 2004, 172, 1333–1339. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Lau, J.K.C.; Yu, J. The Role of Natural Killer Cell in Gastrointestinal Cancer: Killer or Helper. Oncogene 2021, 40, 717–730. [Google Scholar] [CrossRef]
- Barnes, S.A.; Trew, I.; De Jong, E.; Foley, B. Making a Killer: Selecting the Optimal Natural Killer Cells for Improved Immunotherapies. Front. Immunol. 2021, 12, 765705. [Google Scholar] [CrossRef] [PubMed]
- Wagner, J.A.; Rosario, M.; Romee, R.; Berrien-Elliott, M.M.; Schneider, S.E.; Leong, J.W.; Sullivan, R.P.; Jewell, B.A.; Becker-Hapak, M.; Schappe, T.; et al. CD56bright NK Cells Exhibit Potent Antitumor Responses Following IL-15 Priming. J. Clin. Investig. 2017, 127, 4042–4058. [Google Scholar] [CrossRef] [PubMed]
- Bald, T.; Krummel, M.F.; Smyth, M.J.; Barry, K.C. The NK Cell–Cancer Cycle: Advances and New Challenges in NK Cell–Based Immunotherapies. Nat. Immunol. 2020, 21, 835–847. [Google Scholar] [CrossRef]
- Valipour, B.; Velaei, K.; Abedelahi, A.; Karimipour, M.; Darabi, M.; Charoudeh, H.N. NK Cells: An Attractive Candidate for Cancer Therapy. J. Cell. Physiol. 2019, 234, 19352–19365. [Google Scholar] [CrossRef]
- Lo Nigro, C.; Macagno, M.; Sangiolo, D.; Bertolaccini, L.; Aglietta, M.; Carlo Merlano, M. NK-Mediated Antibody-Dependent Cell-Mediated Cytotoxicity in Solid Tumors: Biological Evidence and Clinical Perspectives. Ann. Transl. Med. 2019, 7, 105. [Google Scholar] [CrossRef]
- Judge, S.J.; Murphy, W.J.; Canter, R.J. Characterizing the Dysfunctional NK Cell: Assessing the Clinical Relevance of Exhaustion, Anergy, and Senescence. Front. Cell. Infect. Microbiol. 2020, 10, 49. [Google Scholar] [CrossRef]
- Fang, F.; Xiao, W.; Tian, Z. NK Cell-Based Immunotherapy for Cancer. Semin. Immunol. 2017, 31, 37–54. [Google Scholar] [CrossRef] [PubMed]
- Meckawy, G.R.; Mohamed, A.M.; Zaki, W.K.; Khattab, M.A.; Amin, M.M.; ElDeeb, M.A.; El-Najjar, M.R.; Safwat, N.A. Natural Killer NKG2A and NKG2D in Patients with Colorectal Cancer. J. Gastrointest. Oncol. 2019, 10, 218–225. [Google Scholar] [CrossRef] [PubMed]
- Chester, C.; Fritsch, K.; Kohrt, H.E. Natural Killer Cell Immunomodulation: Targeting Activating, Inhibitory, and Co-Stimulatory Receptor Signaling for Cancer Immunotherapy. Front. Immunol. 2015, 6, 601. [Google Scholar] [CrossRef] [PubMed]
- Béziat, V.; Hilton, H.G.; Norman, P.J.; Traherne, J.A. Deciphering the Killer-Cell Immunoglobulin-like Receptor System at Super-Resolution for Natural Killer and T-Cell Biology. Immunology 2017, 150, 248–264. [Google Scholar] [CrossRef] [PubMed]
- Zingoni, A.; Ardolino, M.; Santoni, A.; Cerboni, C. NKG2D and DNAM-1 Activating Receptors and Their Ligands in NK-T Cell Interactions: Role in the NK Cell-Mediated Negative Regulation of T Cell Responses. Front. Immunol. 2012, 3, 408. [Google Scholar] [CrossRef] [PubMed]
- Dhar, P.; Wu, J.D. NKG2D and Its Ligands in Cancer. Curr. Opin. Immunol. 2018, 51, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Gumá, M.; Busch, L.K.; Salazar-Fontana, L.I.; Bellosillo, B.; Morte, C.; García, P.; López-Botet, M. The CD94/NKG2C Killer Lectin-like Receptor Constitutes an Alternative Activation Pathway for a Subset of CD8+ T Cells. Eur. J. Immunol. 2005, 35, 2071–2080. [Google Scholar] [CrossRef] [PubMed]
- Barrow, A.D.; Martin, C.J.; Colonna, M. The Natural Cytotoxicity Receptors in Health and Disease. Front. Immunol. 2019, 10, 909. [Google Scholar] [CrossRef]
- Littera, R.; Piredda, G.; Argiolas, D.; Lai, S.; Congeddu, E.; Ragatzu, P.; Melis, M.; Carta, E.; Michittu, M.B.; Valentini, D.; et al. KIR and Their HLA Class I Ligands: Two More Pieces towards Completing the Puzzle of Chronic Rejection and Graft Loss in Kidney Transplantation. PLoS ONE 2017, 12, e0180831. [Google Scholar] [CrossRef]
- Sen Santara, S.; Lee, D.-J.; Crespo, Â.; Hu, J.J.; Walker, C.; Ma, X.; Zhang, Y.; Chowdhury, S.; Meza-Sosa, K.F.; Lewandrowski, M.; et al. The NK Cell Receptor NKp46 Recognizes Ecto-Calreticulin on ER-Stressed Cells. Nature 2023, 616, 348–356. [Google Scholar] [CrossRef] [PubMed]
- Ljunggren, H.; Kirre, K. The Search of the ‘Missing Self’: MHC Molecules NK Cell Recognition. Immunol. Today 1990, 11, 237–244. [Google Scholar] [CrossRef]
- Long, E.O.; Rajagopalan, S. Stress Signals Activate Natural Killer Cells. J. Exp. Med. 2002, 196, 1399–1402. [Google Scholar] [CrossRef]
- Khan, M.; Arooj, S.; Wang, H.; Wang, H. NK Cell-Based Immune Checkpoint Inhibition. Front. Genet. 2020, 11, 167. [Google Scholar] [CrossRef] [PubMed]
- Hsu, J.; Hodgins, J.J.; Marathe, M.; Nicolai, C.J.; Bourgeois-Daigneault, M.C.; Trevino, T.N.; Azimi, C.S.; Scheer, A.K.; Randolph, H.E.; Thompson, T.W.; et al. Contribution of NK Cells to Immunotherapy Mediated by PD-1/PD-L1 Blockade. J. Clin. Investig. 2018, 128, 4654–4668. [Google Scholar] [CrossRef]
- Concha-Benavente, F.; Kansy, B.; Moskovitz, J.; Moy, J.; Chandran, U.; Ferris, R.L. PD-L1 Mediates Dysfunction in Activated PD-1 þ NK Cells in Head and Neck Cancer Patients. Cancer Immunol. Res. 2018, 6, 1548–1560. [Google Scholar] [CrossRef]
- Neelapu, S.S.; Locke, F.L.; Bartlett, N.L.; Lekakis, L.J.; Miklos, D.B.; Jacobson, C.A.; Braunschweig, I.; Oluwole, O.O.; Siddiqi, T.; Lin, Y.; et al. Axicabtagene Ciloleucel CAR T-Cell Therapy in Refractory Large B-Cell Lymphoma. N. Engl. J. Med. 2017, 377, 2531–2544. [Google Scholar] [CrossRef]
- Schuster, S.J.; Svoboda, J.; Chong, E.A.; Nasta, S.D.; Mato, A.R.; Anak, Ö.; Brogdon, J.L.; Pruteanu-Malinici, I.; Bhoj, V.; Landsburg, D.; et al. Chimeric Antigen Receptor T Cells in Refractory B-Cell Lymphomas. N. Engl. J. Med. 2017, 377, 2545–2554. [Google Scholar] [CrossRef] [PubMed]
- Sterner, R.C.; Sterner, R.M. CAR-T Cell Therapy: Current Limitations and Potential Strategies. Blood Cancer J. 2021, 11, 69. [Google Scholar] [CrossRef]
- Maude, S.L.; Laetsch, T.W.; Buechner, J.; Rives, S.; Boyer, M.; Bittencourt, H.; Bader, P.; Verneris, M.R.; Stefanski, H.E.; Myers, G.D.; et al. Tisagenlecleucel in Children and Young Adults with B-Cell Lymphoblastic Leukemia. N. Engl. J. Med. 2018, 378, 439–448. [Google Scholar] [CrossRef]
- Locke, F.L.; Ghobadi, A.; Jacobson, C.A.; Miklos, D.B.; Lekakis, L.J.; Oluwole, O.O.; Lin, Y.; Braunschweig, I.; Hill, B.T.; Timmerman, J.M.; et al. Long-Term Safety and Activity of Axicabtagene Ciloleucel in Refractory Large B-Cell Lymphoma (ZUMA-1): A Single-Arm, Multicentre, Phase 1–2 Trial. Lancet Oncol. 2019, 20, 31–42. [Google Scholar] [CrossRef]
- Abramson, J.S.; Palomba, M.L.; Gordon, L.I.; Lunning, M.A.; Wang, M.; Arnason, J.; Mehta, A.; Purev, E.; Maloney, D.G.; Andreadis, C.; et al. Lisocabtagene Maraleucel for Patients with Relapsed or Refractory Large B-Cell Lymphomas (TRANSCEND NHL 001): A Multicentre Seamless Design Study. Lancet 2020, 396, 839–852. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Munoz, J.; Goy, A.; Locke, F.L.; Jacobson, C.A.; Hill, B.T.; Timmerman, J.M.; Holmes, H.; Jaglowski, S.; Flinn, I.W.; et al. KTE-X19 CAR T-Cell Therapy in Relapsed or Refractory Mantle-Cell Lymphoma. N. Engl. J. Med. 2020, 382, 1331–1342. [Google Scholar] [CrossRef] [PubMed]
- Munshi, N.C.; Anderson, L.D.; Shah, N.; Madduri, D.; Berdeja, J.; Lonial, S.; Raje, N.; Lin, Y.; Siegel, D.; Oriol, A.; et al. Idecabtagene Vicleucel in Relapsed and Refractory Multiple Myeloma. N. Engl. J. Med. 2021, 384, 705–716. [Google Scholar] [CrossRef] [PubMed]
- Martin, T.; Usmani, S.Z.; Berdeja, J.G.; Agha, M.; Cohen, A.D.; Hari, P.; Avigan, D.; Deol, A.; Htut, M.; Lesokhin, A.; et al. Ciltacabtagene Autoleucel, an Anti-B-Cell Maturation Antigen Chimeric Antigen Receptor T-Cell Therapy, for Relapsed/Refractory Multiple Myeloma: CARTITUDE-1 2-Year Follow-Up. J. Clin. Oncol. 2022, 41, 1265–1274. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, N.; Mo, F.; McKenna, M.K. Impact of Manufacturing Procedures on CAR T Cell Functionality. Front. Immunol. 2022, 13, 876339. [Google Scholar] [CrossRef] [PubMed]
- Brudno, J.N.; Kochenderfer, J.N. Toxicities of Chimeric Antigen Receptor T Cells: Recognition and Management. Blood 2016, 127, 3321–3330. [Google Scholar] [CrossRef] [PubMed]
- Hirayama, A.V.; Turtle, C.J. Toxicities of CD19 CAR-T Cell Immunotherapy. Am. J. Hematol. 2019, 94, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Allen, E.S.; Stroncek, D.F.; Ren, J.; Eder, A.F.; West, K.A.; Fry, T.J.; Lee, D.W.; Mackall, C.L.; Conry-Cantilena, C. Autologous Lymphapheresis for the Production of Chimeric Antigen Receptor T Cells. Transfusion 2017, 57, 1133–1141. [Google Scholar] [CrossRef]
- Marin, D.; Li, Y.; Basar, R.; Rafei, H.; Daher, M.; Dou, J.; Mohanty, V.; Dede, M.; Nieto, Y.; Uprety, N.; et al. Safety, Efficacy and Determinants of Response of Allogeneic CD19-Specific CAR-NK Cells in CD19+ B Cell Tumors: A Phase 1/2 Trial. Nat. Med. 2024. [Google Scholar] [CrossRef]
- Aptsiauri, N.; Ruiz-Cabello, F.; Garrido, F. The Transition from HLA-I Positive to HLA-I Negative Primary Tumors: The Road to Escape from T-Cell Responses. Curr. Opin. Immunol. 2018, 51, 123–132. [Google Scholar] [CrossRef]
- Garrido, F.; Aptsiauri, N.; Doorduijn, E.M.; Garcia Lora, A.M.; van Hall, T. The Urgent Need to Recover MHC Class I in Cancers for Effective Immunotherapy. Curr. Opin. Immunol. 2016, 39, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Angelo, L.S.; Banerjee, P.P.; Monaco-Shawver, L.; Rosen, J.B.; Makedonas, G.; Forbes, L.R.; Mace, E.M.; Orange, J.S. Practical NK Cell Phenotyping and Variability in Healthy Adults. Immunol. Res. 2015, 62, 341–356. [Google Scholar] [CrossRef]
- Laskowski, T.J.; Biederstädt, A.; Rezvani, K. Natural Killer Cells in Antitumour Adoptive Cell Immunotherapy. Nat. Rev. Cancer 2022, 22, 557–575. [Google Scholar] [CrossRef]
- Sarvaria, A.; Jawdat, D.; Madrigal, J.A.; Saudemont, A. Umbilical Cord Blood Natural Killer Cells, Their Characteristics, and Potential Clinical Applications. Front. Immunol. 2017, 8, 329. [Google Scholar] [CrossRef]
- Dalle, J.; Menezes, J.; Wagner, É.; Blagdon, M.; Champagne, J.; Champagne, M.A.; Duval, M.; Hematology-oncology, D.P. Characterization of Cord Blood Natural Killer Cells: Implications for Transplantation and Neonatal Infections. Pediatr. Res. 2005, 57, 649–655. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, H.; Kai, S.; Yamaguchi, M.; Misawa, M.; Fujimori, Y.; Yamamoto, M.; Hara, H. Analysis of Natural Killer ( NK ) Cell Activity and Adhesion Molecules on NK Cells from Umbilical Cord Blood. Eur. J. Haematol. 2003, 71, 29–38. [Google Scholar] [CrossRef]
- Fan, Y.; Yang, B.; Wu, C. Phenotypic and Functional Heterogeneity of Natural Killer Cells from Umbilical Cord Blood Mononuclear Cells. Immunol. Investig. 2008, 37, 79–96. [Google Scholar] [CrossRef] [PubMed]
- Horwitz, M.E.; Stiff, P.J.; Cutler, C.; Brunstein, C.; Hanna, R.; Mariarz, R.T.; Rezvani, A.R.; Karris, N.A.; McGuirk, J.; Valcarcel, D.; et al. Omidubicel vs Standard Myeloablative Umbilical Cord Blood Transplantation: Results of a Phase 3 Randomized Study. Blood 2021, 138, 1429–1440. [Google Scholar] [CrossRef] [PubMed]
- Shpall, E.J.; Rezvani, K. Cord Blood Expansion Has Arrived. Blood 2021, 138, 1381–1382. [Google Scholar] [CrossRef]
- Liu, E.; Marin, D.; Banerjee, P.; MacApinlac, H.A.; Thompson, P.; Basar, R.; Kerbauy, L.N.; Overman, B.; Thall, P.; Kaplan, M.; et al. Use of CAR-Transduced Natural Killer Cells in CD19-Positive Lymphoid Tumors. N. Engl. J. Med. 2020, 382, 545–553. [Google Scholar] [CrossRef] [PubMed]
- Spanholtz, J.; Preijers, F.; Tordoir, M.; Trilsbeek, C.; Paardekooper, J.; de Witte, T.; Schaap, N.; Dolstra, H. Clinical-Grade Generation of Active NK Cells from Cord Blood Hematopoietic Progenitor Cells for Immunotherapy Using a Closed-System Culture Process. PLoS ONE 2011, 6, e20740. [Google Scholar] [CrossRef] [PubMed]
- Cany, J.; van der Waart, A.B.; Spanholtz, J.; Tordoir, M.; Jansen, J.H.; van der Voort, R.; Schaap, N.M.; Dolstra, H. Combined IL-15 and IL-12 Drives the Generation of CD34+-Derived Natural Killer Cells with Superior Maturation and Alloreactivity Potential Following Adoptive Transfer. Oncoimmunology 2015, 4, e1017701. [Google Scholar] [CrossRef] [PubMed]
- Herrera, L.; Salcedo, J.M.; Santos, S.; Vesga, M.Á.; Borrego, F.; Eguizabal, C. OP9 Feeder Cells Are Superior to M2-10B4 Cells for the Generation of Mature and Functional Natural Killer Cells from Umbilical Cord Hematopoietic Progenitors. Front. Immunol. 2017, 8, 755. [Google Scholar] [CrossRef] [PubMed]
- Luevano, M.; Madrigal, A.; Saudemont, A. Generation of Natural Killer Cells from Hematopoietic Stem Cells in Vitro for Immunotherapy. Cell. Mol. Immunol. 2012, 9, 310–320. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Hermanson, D.L.; Moriarity, B.S.; Kaufman, D.S.; Li, Y.; Hermanson, D.L.; Moriarity, B.S.; Kaufman, D.S. Human IPSC-Derived Natural Killer Cells Engineered with Chimeric Antigen Receptors Enhance Anti-Tumor Activity. Cell Stem Cell 2018, 23, 181–192.e5. [Google Scholar] [CrossRef] [PubMed]
- Cichocki, F.; Bjordahl, R.; Gaidarova, S.; Mahmood, S.; Abujarour, R.; Wang, H.; Tuininga, K.; Felices, M.; Davis, Z.B.; Bendzick, L.; et al. IPSC-Derived NK Cells Maintain High Cytotoxicity and Enhance in Vivo Tumor Control in Concert with T Cells and Anti–PD-1 Therapy. Sci. Transl. Med. 2020, 12, eaaz5618. [Google Scholar] [CrossRef]
- Knorr, D.A.; Bock, A.; Brentjens, R.J.; Kaufman, D.S. Engineered Human Embryonic Stem Cell-Derived Lymphocytes to Study in Vivo Trafficking and Immunotherapy. Stem Cells Dev. 2013, 22, 1861–1869. [Google Scholar] [CrossRef]
- Knorr, D.A.; Ni, Z.; Hermanson, D.L.; Hexum, M.K.; Bendzick, L.; Cooper, L.J.; Lee, D.A.; Kaufman, D.S. Clinical-Scale Derivation of Natural Killer Cells From Human Pluripotent Stem Cells for Cancer Therapy. Stem Cells Transl. Med. 2013, 2, 274–283. [Google Scholar] [CrossRef]
- Goldenson, B.H.; Hor, P.; Kaufman, D.S. IPSC-Derived Natural Killer Cell Therapies—Expansion and Targeting. Front. Immunol. 2022, 13, 841107. [Google Scholar] [CrossRef]
- Euchner, J.; Sprissler, J.; Cathomen, T.; Fürst, D.; Schrezenmeier, H.; Debatin, K.M.; Schwarz, K.; Felgentreff, K. Natural Killer Cells Generated From Human Induced Pluripotent Stem Cells Mature to CD56brightCD16+NKp80+/− In-Vitro and Express KIR2DL2/DL3 and KIR3DL1. Front. Immunol. 2021, 12, 640672. [Google Scholar] [CrossRef] [PubMed]
- Woll, P.S.; Martin, C.H.; Miller, J.S.; Kaufman, D.S. Human Embryonic Stem Cell-Derived NK Cells Acquire Functional Receptors and Cytolytic Activity. J. Immunol. 2005, 175, 5095–5103. [Google Scholar] [CrossRef] [PubMed]
- Goldenson, B.H.; Zhu, H.; Wang, Y.Z.M.; Heragu, N.; Bernareggi, D.; Ruiz-Cisneros, A.; Bahena, A.; Ask, E.H.; Hoel, H.J.; Malmberg, K.J.; et al. Umbilical Cord Blood and IPSC-Derived Natural Killer Cells Demonstrate Key Differences in Cytotoxic Activity and KIR Profiles. Front. Immunol. 2020, 11, 561553. [Google Scholar] [CrossRef]
- Woll, P.S.; Grzywacz, B.; Tian, X.; Marcus, R.K.; Knorr, D.A.; Verneris, M.R.; Kaufman, D.S. Human Embryonic Stem Cells Differentiate into a Homogeneous Population of Natural Killer Cells with Potent In Vivo Antitumor Activity. Blood 2009, 113, 6094–6101. [Google Scholar] [CrossRef]
- Eguizabal, C.; Zenarruzabeitia, O.; Monge, J.; Santos, S.; Vesga, M.A.; Maruri, N.; Arrieta, A.; Riñón, M.; Tamayo-orbegozo, E.; Amo, L.; et al. Natural Killer Cells for Cancer Immunotherapy: Pluripotent Stem Cells-Derived NK Cells as an Immunotherapeutic Perspective. Front. Immunol. 2014, 5, 439. [Google Scholar] [CrossRef]
- Maddineni, S.; Silberstein, J.L.; Sunwoo, J.B. Emerging NK Cell Therapies for Cancer and the Promise of next Generation Engineering of IPSC-Derived NK Cells. J. Immunother. Cancer 2022, 10, e004693. [Google Scholar] [CrossRef] [PubMed]
- Nayar, S.; Dasgupta, P.; Galustian, C. Extending the Lifespan and Efficacies of Immune Cells Used in Adoptive Transfer for Cancer Immunotherapies—A Review. Oncoimmunology 2015, 4, e1002720. [Google Scholar] [CrossRef]
- Zhang, Y.; Wallace, D.L.; De Lara, C.M.; Ghattas, H.; Asquith, B.; Worth, A.; Griffin, G.E.; Taylor, G.P.; Tough, D.F.; Beverley, P.C.L.; et al. In Vivo Kinetics of Human Natural Killer Cells: The Effects of Ageing and Acute and Chronic Viral Infection. Immunology 2007, 121, 258–265. [Google Scholar] [CrossRef]
- Leong, J.W.; Chase, J.M.; Romee, R.; Schneider, S.E.; Sullivan, R.P.; Cooper, M.A.; Fehniger, T.A. Preactivation with IL-12, IL-15, and IL-18 Induces Cd25 and a Functional High-Affinity Il-2 Receptor on Human Cytokine-Induced Memory-like Natural Killer Cells. Biol. Blood Marrow Transplant. 2014, 20, 463–473. [Google Scholar] [CrossRef]
- Heinze, A.; Grebe, B.; Bremm, M.; Huenecke, S.; Munir, T.A.; Graafen, L.; Frueh, J.T.; Merker, M.; Rettinger, E.; Soerensen, J.; et al. The Synergistic Use of IL-15 and IL-21 for the Generation of NK Cells From CD3/CD19-Depleted Grafts Improves Their Ex Vivo Expansion and Cytotoxic Potential Against Neuroblastoma: Perspective for Optimized Immunotherapy Post Haploidentical Stem Cell Transplantation. Front. Immunol. 2019, 10, 2816. [Google Scholar] [CrossRef]
- Carlens, S.; Gilljam, M.; Chambers, B.J.; Aschan, J.; Guven, H.; Ljunggren, H.; Christensson, B.; Dilber, M.S. A New Method for In Vitro Expansion of Cytotoxic Human CD3−CD56+ Natural Killer Cells. Hum. Immunol. 2001, 62, 1092–1098. [Google Scholar] [CrossRef]
- Deng, X.; Terunuma, H.; Nieda, M.; Xiao, W.; Nicol, A. Synergistic Cytotoxicity of Ex Vivo Expanded Natural Killer Cells in Combination with Monoclonal Antibody Drugs against Cancer Cells. Int. Immunopharmacol. 2012, 14, 593–605. [Google Scholar] [CrossRef]
- Koehl, U.; Brehm, C.; Huenecke, S.; Zimmermann, S.Y.; Kloess, S.; Bremm, M.; Ullrich, E.; Soerensen, J.; Quaiser, A.; Erben, S.; et al. Clinical Grade Purification and Expansion of NK Cell Products for an Optimized Manufacturing Protocol. Front. Oncol. 2013, 3, 118. [Google Scholar] [CrossRef]
- Wagner, J.; Pfannenstiel, V.; Waldmann, A.; Bergs, J.W.J.; Brill, B.; Huenecke, S.; Klingebiel, T.; Rödel, F.; Buchholz, C.J.; Wels, W.S.; et al. A Two-Phase Expansion Protocol Combining Interleukin (IL)-15 and IL-21 Improves Natural Killer Cell Proliferation and Cytotoxicity against Rhabdomyosarcoma. Front. Immunol. 2017, 8, 676. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, J.; Sugita, J.; Shiratori, S.; Shigematu, A.; Asanuma, S.; Fujimoto, K.; Nishio, M.; Kondo, T.; Imamura, M. Expansion of NK Cells from Cord Blood with Antileukemic Activity Using GMP-Compliant Substances without Feeder Cells. Leukemia 2012, 26, 1149–1152. [Google Scholar] [CrossRef]
- Granzin, M.; Stojanovic, A.; Miller, M.; Childs, R.; Huppert, V.; Cerwenka, A. Highly Efficient IL-21 and Feeder Cell-Driven Ex Vivo Expansion of Human NK Cells with Therapeutic Activity in a Xenograft Mouse Model of Melanoma. Oncoimmunology 2016, 5, e1219007. [Google Scholar] [CrossRef]
- Vasu, S.; Berg, M.; Davidson-Moncada, J.; Tian, X.; Cullis, H.; Childs, R.W. A Novel Method to Expand Large Numbers of CD56+ Natural Killer Cells from a Minute Fraction of Selectively Accessed Cryopreserved Cord Blood for Immunotherapy Post-Transplantation. Cytotherapy 2015, 17, 1582–1593. [Google Scholar] [CrossRef]
- Sakamoto, N.; Ishikawa, T.; Kokura, S.; Okayama, T.; Oka, K.; Ideno, M.; Sakai, F.; Kato, A.; Tanabe, M.; Enoki, T.; et al. Phase I Clinical Trial of Autologous NK Cell Therapy Using Novel Expansion Method in Patients with Advanced Digestive Cancer. J. Transl. Med. 2015, 13, 277. [Google Scholar] [CrossRef] [PubMed]
- Gong, W.; Xiao, W.; Hu, M.; Weng, X.; Qian, L.; Pan, X.; Ji, M. Ex Vivo Expansion of Natural Killer Cells with High Cytotoxicity by K562 Cells Modified to Co-Express Major Histocompatibility Complex Class I Chain-Related Protein A, 4-1BB Ligand, and Interleukin-15. Tissue Antigens 2010, 76, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Fujisaki, H.; Kakuda, H.; Shimasaki, N.; Imai, C.; Ma, J.; Lockey, T.; Eldridge, P.; Leung, W.H.; Campana, D. Expansion of Highly Cytotoxic Human Natural Killer Cells for Cancer Cell Therapy. Cancer Res. 2009, 69, 4010–4017. [Google Scholar] [CrossRef]
- Ayello, J.; Hochberg, J.; Flower, A.; Chu, Y.; Baxi, L.V.; Quish, W.; van de Ven, C.; Cairo, M.S. Genetically Re-Engineered K562 Cells Significantly Expand and Functionally Activate Cord Blood Natural Killer Cells: Potential for Adoptive Cellular Immunotherapy. Exp. Hematol. 2017, 46, 38–47. [Google Scholar] [CrossRef]
- Denman, C.J.; Senyukov, V.V.; Somanchi, S.S.; Phatarpekar, P.V.; Kopp, L.M.; Johnson, J.L.; Singh, H.; Hurton, L.; Maiti, S.N.; Huls, M.H.; et al. Membrane-Bound IL-21 Promotes Sustained Ex Vivo Proliferation of Human Natural Killer Cells. PLoS ONE 2012, 7, e30264. [Google Scholar] [CrossRef]
- Yang, Y.; Badeti, S.; Tseng, H.C.; Ma, M.T.; Liu, T.; Jiang, J.G.; Liu, C.; Liu, D. Superior Expansion and Cytotoxicity of Human Primary NK and CAR-NK Cells from Various Sources via Enriched Metabolic Pathways. Mol. Ther. Methods Clin. Dev. 2020, 18, 428–445. [Google Scholar] [CrossRef]
- Kweon, S.; Phan, M.T.T.; Chun, S.; Yu, H.B.; Kim, J.; Kim, S.; Lee, J.; Ali, A.K.; Lee, S.H.; Kim, S.K.; et al. Expansion of Human NK Cells Using K562 Cells Expressing OX40 Ligand and Short Exposure to IL-21. Front. Immunol. 2019, 10, 879. [Google Scholar] [CrossRef]
- Guo, M.; Sun, C.; Qian, Y.; Zhu, L.; Ta, N.; Wang, G.; Zheng, J.; Guo, F.; Liu, Y. Proliferation of Highly Cytotoxic Human Natural Killer Cells by OX40L Armed NK-92 With Secretory Neoleukin-2/15 for Cancer Immunotherapy. Front. Oncol. 2021, 11, 632540. [Google Scholar] [CrossRef]
- Siegler, U.; Meyer-Monard, S.; Jrger, S.; Stern, M.; Tichelli, A.; Gratwohl, A.; Wodnar-Filipowicz, A.; Kalberer, C.P. Good Manufacturing Practice-Compliant Cell Sorting and Large-Scale Expansion of Single KIR-Positive Alloreactive Human Natural Killer Cells for Multiple Infusions to Leukemia Patients. Cytotherapy 2010, 12, 750–763. [Google Scholar] [CrossRef]
- Kim, E.; Ahn, Y.; Kim, S.; Kim, T.A.E.M.I.N.; Keam, B.; Heo, D.A.E.S. Ex Vivo Activation and Expansion of Natural Killer Cells from Patients with Advanced Cancer with Feeder Cells from Healthy Volunteers. J. Cytotherapy 2013, 15, 231–241.e1. [Google Scholar] [CrossRef]
- Delso-vallejo, M.; Kollet, J.; Koehl, U.; Huppert, V. Influence of Irradiated Peripheral Blood Mononuclear Cells on Both Ex Vivo Proliferation of Human Natural Killer Cells and Change in Cellular Property. Front. Immunol. 2017, 8, 854. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Lundqvist, A. Immunomodulatory Effects of IL-2 and IL-15; Implications for Cancer Immunotherapy. Cancers 2020, 12, 3586. [Google Scholar] [CrossRef] [PubMed]
- Isvoranu, G.; Surcel, M.; Munteanu, A.; Bratu, O.; Ionita-Radu, F.; Neagu, M.; Chiritoiu-Butnaru, M. Therapeutic Potential of Interleukin-15 in Cancer. Exp. Ther. Med. 2021, 22, 675. [Google Scholar] [CrossRef] [PubMed]
- Liao, W.; Lin, J.X.; Leonard, W.J. Interleukin-2 at the Crossroads of Effector Responses, Tolerance, and Immunotherapy. Immunity 2013, 38, 13–25. [Google Scholar] [CrossRef]
- Fehniger, T.A.; Cooper, M.A.; Caligiuri, M.A. Interleukin-2 and Interleukin-15 Immunotherapy for Cancer. Cytokine Growth Factor Rev. 2002, 13, 169–183. [Google Scholar] [CrossRef]
- Lehmann, D.; Spanholtz, J.; Sturtzel, C.; Tordoir, M.; Schlechta, B.; Groenewegen, D.; Hofer, E. IL-12 Directs Further Maturation of Ex Vivo Differentiated NK Cells with Improved Therapeutic Potential. PLoS ONE 2014, 9, e87131. [Google Scholar] [CrossRef]
- Hodge, D.L.; Subleski, J.J.; Reynolds, D.A.; Buschman, M.D.; Schill, W.B.; Burkett, M.W.; Malyguine, A.M.; Young, H.A. The Proinflammatory Cytokine Interleukin-18 Alters Multiple Signaling Pathways to Inhibit Natural Killer Cell Death. J. Interferon Cytokine Res. 2006, 26, 706–718. [Google Scholar] [CrossRef] [PubMed]
- Skak, K.; Frederiksen, K.S.; Lundsgaard, D. Interleukin-21 Activates Human Natural Killer Cells and Modulates Their Surface Receptor Expression. Immunology 2007, 123, 575–583. [Google Scholar] [CrossRef] [PubMed]
- Peighambarzadeh, F.; Najafalizadeh, A.; Esmaeil, N.; Rezaei, A.; Ashrafi, F.; Hakemi, M.G. Optimization of in Vitro Expansion and Activation of Human Natural Killer Cells against Breast Cancer Cell Line. Avicenna J. Med. Biotechnol. 2020, 12, 17–23. [Google Scholar] [PubMed]
- Haroun-Izquierdo, A.; Vincenti, M.; Netskar, H.; Van Ooijen, H.; Zhang, B.; Bendzick, L.; Kanaya, M.; Momayyezi, P.; Li, S.; Wiiger, M.T.; et al. Adaptive Single-KIR + NKG2C + NK Cells Expanded from Select Superdonors Show Potent Missing-Self Reactivity and Efficiently Control HLA-Mismatched Acute Myeloid Leukemia. J. Immunother. Cancer 2022, 10, e005577. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.L.; Béziat, V.; Oei, V.Y.S.; Pfefferle, A.; Schaffer, M.; Lehmann, S.; Hellström-Lindberg, E.; Söderhäll, S.; Heyman, M.; Grandér, D.; et al. Ex Vivo Expanded Adaptive NK Cells Effectively Kill Primary Acute Lymphoblastic Leukemia Cells. Cancer Immunol. Res. 2017, 5, 654–665. [Google Scholar] [CrossRef]
- Condiotti, R.; Zakai, Y.B.; Barak, V.; Nagler, A. Ex Vivo Expansion of CD56 Cytotoxic Cells from Human Umbilical Cord Blood. Exp. Hematol. 2001, 29, 104–113. [Google Scholar] [CrossRef]
- Meehan, K.R.; Wu, J.; Webber, S.M.; Barber, A.; Szczepiorkowski, Z.M.; Sentman, C. Development of a Clinical Model for Ex Vivo Expansion of Multiple Populations of Effector Cells for Adoptive Cellular Therapy. Cytotherapy 2008, 10, 30–37. [Google Scholar] [CrossRef]
- Sutlu, T.; Stellan, B.; Gilljam, M.; Quezada, H.C.; Nahi, H.; Gahrton, G.Ö.S.T.A.; Alici, E. Clinical-Grade, Large-Scale, Feeder-Free Expansion of Highly Active Human Natural Killer Cells for Adoptive Immunotherapy Using an Automated Bioreactor. Cytotherapy 2010, 12, 1044–1055. [Google Scholar] [CrossRef] [PubMed]
- Lapteva, N.; Durett, A.G.; Sun, J.; Rollins, L.A.; Huye, L.L.; Fang, J.; Dandekar, V.; Mei, Z.; Jackson, K.; Vera, J.; et al. Large-Scale Ex Vivo Expansion and Characterization of Natural Killer Cells for Clinical Applications. Cytotherapy 2012, 14, 1131–1143. [Google Scholar] [CrossRef] [PubMed]
- Zwirner, N.W.; Domaica, C.I.; Fuertes, M.B. Regulatory Functions of NK Cells during Infections and Cancer. J. Leukoc. Biol. 2020, 109, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Somanchi, S.S.; Senyukov, V.V.; Denman, C.J.; Lee, D.A. Expansion, Purification, and Functional Assessment of Human Peripheral Blood NK Cells. J. Vis. Exp. 2010, e2540. [Google Scholar] [CrossRef]
- Streltsova, M.A.; Ustiuzhanina, M.O.; Barsov, E.V.; Kust, S.A.; Velichinskii, R.A.; Kovalenko, E.I. Telomerase Reverse Transcriptase Increases Proliferation and Lifespan of Human NK Cells without Immortalization. Biomedicines 2021, 9, 662. [Google Scholar] [CrossRef] [PubMed]
- Fujisaki, H.; Kakuda, H.; Imai, C.; Mullighan, C.G.; Campana, D. Replicative Potential of Human Natural Killer Cells. Br. J. Haematol. 2009, 145, 606–613. [Google Scholar] [CrossRef]
- Konnikova, L.; Simeone, M.C.; Kruger, M.M.; Kotecki, M.; Cochran, B.H. Signal Transducer and Activator of Transcription 3 (STAT3) Regulates Human Telomerase Reverse Transcriptase (HTERT) Expression in Human Cancer and Primary Cells. Cancer Res. 2005, 65, 6516–6520. [Google Scholar] [CrossRef] [PubMed]
- Gurney, M.; Kundu, S.; Pandey, S.; O’Dwyer, M. Feeder Cells at the Interface of Natural Killer Cell Activation, Expansion and Gene Editing. Front. Immunol. 2022, 13, 802906. [Google Scholar] [CrossRef]
- Wang, K.S.; Frank, D.A.; Ritz, J. Interleukin-2 Enhances the Response of Natural Killer Cells to Interleukin-12 through up-Regulation of the Interleukin-12 Receptor and STAT4. Blood 2000, 95, 3183–3190. [Google Scholar] [CrossRef]
- Heipertz, E.L.; Zynda, E.R.; Stav-Noraas, T.E.; Hungler, A.D.; Boucher, S.E.; Kaur, N.; Vemuri, M.C. Current Perspectives on “Off-The-Shelf” Allogeneic NK and CAR-NK Cell Therapies. Front. Immunol. 2021, 12, 732135. [Google Scholar] [CrossRef]
- Maia, A.; Tarannum, M.; Romee, R. Genetic Manipulation Approaches to Enhance the Clinical Application of NK Cell-Based Immunotherapy. Stem Cells Transl. Med. 2023, szad087. [Google Scholar] [CrossRef]
- Maia, A.; Tarannum, M.; Romee, R. Cytokine-Induced Memory-Like NK Cells for Improved Cancer Immunotherapy. ASHI Q. Second Quart. 2023, 47, 30–34. [Google Scholar]
- Wang, S.; Sun, J.; Chen, K.; Ma, P.; Lei, Q.; Xing, S.; Cao, Z.; Sun, S.; Yu, Z.; Liu, Y.; et al. Perspectives of Tumor-Infiltrating Lymphocyte Treatment in Solid Tumors. BMC Med. 2021, 19, 140. [Google Scholar] [CrossRef]
- Tarannum, M.; Romee, R. Cytokine-Induced Memory-like Natural Killer Cells for Cancer Immunotherapy. Stem Cell Res. Ther. 2021, 12, 592. [Google Scholar] [CrossRef] [PubMed]
- Malone, D.F.G.; Lunemann, S.; Hengst, J.; Ljunggren, H.G.; Manns, M.P.; Sandberg, J.K.; Cornberg, M.; Wedemeyer, H.; Björkström, N.K. Cytomegalovirus-Driven Adaptive-like Natural Killer Cell Expansions Are Unaffected by Concurrent Chronic Hepatitis Virus Infections. Front. Immunol. 2017, 8, 525. [Google Scholar] [CrossRef] [PubMed]
- Stary, V.; Stary, G. NK Cell-Mediated Recall Responses: Memory-Like, Adaptive, or Antigen-Specific? Front. Cell. Infect. Microbiol. 2020, 10, 208. [Google Scholar] [CrossRef] [PubMed]
- Terrén, I.; Orrantia, A.; Mosteiro, A.; Vitallé, J.; Zenarruzabeitia, O.; Borrego, F. Metabolic Changes of Interleukin-12/15/18-Stimulated Human NK Cells. Sci. Rep. 2021, 11, 6472. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-H.; Fragoso, M.F.; Biron, C.A. Cutting Edge: A Novel Mechanism Bridging Innate and Adaptive Immunity: IL-12 Induction of CD25 To Form High-Affinity IL-2 Receptors on NK Cells. J. Immunol. 2012, 189, 2712–2716. [Google Scholar] [CrossRef] [PubMed]
- Romee, R.; Schneider, S.E.; Leong, J.W.; Chase, J.M.; Keppel, C.R.; Sullivan, R.P.; Cooper, M.A.; Fehniger, T.A. Cytokine Activation Induces Human Memory-like NK Cells. Blood 2012, 120, 4751–4760. [Google Scholar] [CrossRef] [PubMed]
- Cooper, M.A.; Elliott, J.M.; Keyel, P.A.; Yang, L.; Carrero, J.A.; Yokoyama, W.M. Cytokine-Induced Memory-like Natural Killer Cells. Proc. Natl. Acad. Sci. USA 2009, 106, 1915–1919. [Google Scholar] [CrossRef]
- Uppendahl, L.D.; Felices, M.; Bendzick, L.; Ryan, C.; Kodal, B.; Hinderlie, P.; Boylan, K.L.M.; Skubitz, A.P.N.; Miller, J.S.; Geller, M.A. Cytokine-Induced Memory-like Natural Killer Cells Have Enhanced Function, Proliferation, and In Vivo Expansion against Ovarian Cancer Cells. Gynecol. Oncol. 2019, 153, 149–157. [Google Scholar] [CrossRef]
- Romee, R.; Rosario, M.; Berrien-Elliott, M.M.; Wagner, J.A.; Jewell, B.A.; Schappe, T.; Leong, J.W.; Abdel-Latif, S.; Schneider, S.E.; Willey, S.; et al. Cytokine-Induced Memory-like Natural Killer Cells Exhibit Enhanced Responses against Myeloid Leukemia. Sci. Transl. Med. 2016, 8, 357ra123. [Google Scholar] [CrossRef] [PubMed]
- Barnes, S.; Schilizzi, O.; Audsley, K.M.; Newnes, H.V.; Foley, B. Deciphering the Immunological Phenomenon of Adaptive Natural Killer Cells and Cytomegalovirus. Int. J. Mol. Sci. 2020, 21, 8864. [Google Scholar] [CrossRef]
- Gumá, M.; Angulo, A.; Vilches, C.; Gómez-Lozano, N.; Malats, N.; López-Botet, M. Imprint of Human Cytomegalovirus Infection on the NK Cell Receptor Repertoire. Blood 2004, 104, 3664–3671. [Google Scholar] [CrossRef]
- Fesnak, A.D.; June, C.H.; Levine, B.L. Engineered T Cells: The Promise and Challenges of Cancer Immunotherapy. Nat. Rev. Cancer 2016, 16, 566–581. [Google Scholar] [CrossRef] [PubMed]
- Porter, D.L.; Levine, B.L.; Kalos, M.; Bagg, A.; June, C.H. Chimeric Antigen Receptor–Modified T Cells in Chronic Lymphoid Leukemia. N. Engl. J. Med. 2011, 365, 725–733. [Google Scholar] [CrossRef] [PubMed]
- Mikkilineni, L.; Kochenderfer, J.N. CAR T Cell Therapies for Patients with Multiple Myeloma. Nat. Rev. Clin. Oncol. 2021, 18, 71–84. [Google Scholar] [CrossRef]
- Pan, K.; Farrukh, H.; Chittepu, V.C.S.R.; Xu, H.; Pan, C.-X.; Zhu, Z. CAR Race to Cancer Immunotherapy: From CAR T, CAR NK to CAR Macrophage Therapy. J. Exp. Clin. Cancer Res. 2022, 41, 119. [Google Scholar] [CrossRef]
- Gang, M.; Marin, N.D.; Wong, P.; Neal, C.C.; Marsala, L.; Foster, M.; Schappe, T.; Meng, W.; Tran, J.; Schaettler, M.; et al. CAR-Modified Memory-like NK Cells Exhibit Potent Responses to NK-Resistant Lymphomas. Blood 2020, 136, 2308–2318. [Google Scholar] [CrossRef]
- Dong, H.; Ham, J.D.; Hu, G.; Xie, G.; Vergara, J.; Liang, Y.; Ali, A.; Tarannum, M.; Donner, H.; Baginska, J.; et al. Memory-like NK Cells Armed with a Neoepitope-Specific CAR Exhibit Potent Activity against NPM1 Mutated Acute Myeloid Leukemia. Proc. Natl. Acad. Sci. USA 2022, 119, e2122379119. [Google Scholar] [CrossRef]
- Daher, M.; Basar, R.; Gokdemir, E.; Baran, N.; Uprety, N.; Nunez Cortes, A.K.; Mendt, M.; Kerbauy, L.N.; Banerjee, P.P.; Shanley, M.; et al. Targeting a Cytokine Checkpoint Enhances the Fitness of Armored Cord Blood CAR-NK Cells. Blood 2021, 137, 624–636. [Google Scholar] [CrossRef]
- Zhu, H.; Blum, R.H.; Bernareggi, D.; Ask, E.H.; Wu, Z.; Hoel, H.J.; Meng, Z.; Wu, C.; Guan, K.L.; Malmberg, K.J.; et al. Metabolic Reprograming via Deletion of CISH in Human IPSC-Derived NK Cells Promotes In Vivo Persistence and Enhances Anti-Tumor Activity. Cell Stem Cell 2020, 27, 224–237.e6. [Google Scholar] [CrossRef]
- Bernard, P.L.; Delconte, R.; Pastor, S.; Laletin, V.; Costa Da Silva, C.; Goubard, A.; Josselin, E.; Castellano, R.; Krug, A.; Vernerey, J.; et al. Targeting CISH Enhances Natural Cytotoxicity Receptor Signaling and Reduces NK Cell Exhaustion to Improve Solid Tumor Immunity. J. Immunother. Cancer 2022, 10, e004244. [Google Scholar] [CrossRef]
- Shaim, H.; Shanley, M.; Basar, R.; Daher, M.; Gumin, J.; Zamler, D.B.; Uprety, N.; Wang, F.; Huang, Y.; Gabrusiewicz, K.; et al. Targeting the Av Integrin/TGF-β Axis Improves Natural Killer Cell Function against Glioblastoma Stem Cells. J. Clin. Investig. 2021, 131, e142116. [Google Scholar] [CrossRef]
- Kararoudi, M.; Nagai, Y.; Elmas, E.; Pereira, M.; Ali, S.; Imus, P.; Wethington, D.; Borrello, I.; Lee, D.; Ghiaur, G. CD38 Deletion of Human Primary NK Cells Eliminates Daratumumab-Induced Fratricide and Boosts Their Effector Activity. Blood 2020, 136, 2416–2427. [Google Scholar] [CrossRef]
- Ng, Y.Y.; Tay, J.C.K.; Wang, S. CXCR1 Expression to Improve Anti-Cancer Efficacy of Intravenously Injected CAR-NK Cells in Mice with Peritoneal Xenografts. Mol. Ther. Oncolytics 2020, 16, 75–85. [Google Scholar] [CrossRef]
- Ng, Y.Y.; Du, Z.; Zhang, X.; Chng, W.J.; Wang, S. CXCR4 and Anti-BCMA CAR Co-Modified Natural Killer Cells Suppress Multiple Myeloma Progression in a Xenograft Mouse Model. Cancer Gene Ther. 2022, 29, 475–483. [Google Scholar] [CrossRef] [PubMed]
- Schomer, N.T.; Jiang, Z.K.; Lloyd, M.I.; Klingemann, H.; Boissel, L. CCR7 Expression in CD19 Chimeric Antigen Receptor-Engineered Natural Killer Cells Improves Migration toward CCL19-Expressing Lymphoma Cells and Increases Tumor Control in Mice with Human Lymphoma. Cytotherapy 2022, 24, 827–834. [Google Scholar] [CrossRef] [PubMed]
- Brummel, K.; Eerkens, A.L.; de Bruyn, M.; Nijman, H.W. Tumour-Infiltrating Lymphocytes: From Prognosis to Treatment Selection. Br. J. Cancer 2023, 128, 451–458. [Google Scholar] [CrossRef] [PubMed]
- Dafni, U.; Michielin, O.; Lluesma, S.M.; Tsourti, Z.; Polydoropoulou, V.; Karlis, D.; Besser, M.J.; Haanen, J.; Svane, I.M.; Ohashi, P.S.; et al. Efficacy of Adoptive Therapy with Tumor-Infiltrating Lymphocytes and Recombinant Interleukin-2 in Advanced Cutaneous Melanoma: A Systematic Review and Meta-Analysis. Ann. Oncol. 2019, 30, 1902–1913. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, S.A.; Yannelli, J.R.; Yang, J.C.; Topalian, S.L.; Schwartzentruber, D.J.; Weber, J.S.; Parkinson, D.R.; Seipp, C.A.; Einhorn, J.H.; White, D.E. Treatment of Patients with Metastatic Melanoma with Autologous Tumor-Infiltrating Lymphocytes and Interleukin 2. J. Natl. Cancer Cent. 1994, 86, 1159–1166. [Google Scholar] [CrossRef]
- Chandran, S.S.; Somerville, R.P.T.; Yang, J.C.; Sherry, R.M.; Klebanoff, C.A.; Goff, S.L.; Wunderlich, J.R.; Danforth, D.N.; Zlott, D.; Paria, B.C.; et al. Treatment of Metastatic Uveal Melanoma with Adoptive Transfer of Tumour-Infiltrating Lymphocytes: A Single-Centre, Two-Stage, Single-Arm, Phase 2 Study. Lancet Oncol. 2017, 18, 792–802. [Google Scholar] [CrossRef]
- Zacharakis, N.; Chinnasamy, H.; Black, M.; Xu, H.; Lu, Y.C.; Zheng, Z.; Pasetto, A.; Langhan, M.; Shelton, T.; Prickett, T.; et al. Immune Recognition of Somatic Mutations Leading to Complete Durable Regression in Metastatic Breast Cancer. Nat. Med. 2018, 24, 724–730. [Google Scholar] [CrossRef]
- Zhao, Y.; Deng, J.; Rao, S.; Guo, S.; Shen, J.; Du, F.; Wu, X.; Chen, Y.; Li, M.; Chen, M.; et al. Tumor Infiltrating Lymphocyte (TIL) Therapy for Solid Tumor Treatment: Progressions and Challenges. Cancers 2022, 14, 4160. [Google Scholar] [CrossRef] [PubMed]
- Coca, S.; Perez-Piqueras, J.; Martinez, D.; Colmenarejo, A.; Saez, M.A.; Vallejo, C.; Martos, J.A.; Moreno, M. The Prognostic Significance of Intratumoral Natural Killer Cells in Patients with Colerectal Carcinoma. Cancer 1997, 79, 2320–2328. [Google Scholar] [CrossRef]
- Donadon, M.; Hudspeth, K.; Cimino, M.; Di Tommaso, L.; Preti, M.; Tentorio, P.; Roncalli, M.; Mavilio, D.; Torzilli, G. Increased Infiltration of Natural Killer and T Cells in Colorectal Liver Metastases Improves Patient Overall Survival. J. Gastrointest. Surg. 2017, 21, 1226–1236. [Google Scholar] [CrossRef]
- Ishigami, S.; Natsugoe, S.; Tokuda, K.; Nakajo, A.; Che, X.; Iwashige, H.; Aridome, K.; Hokita, S.; Aikou, T. Prognostic Value of Intratumoral Natural Killer Cells in Gastric Carcinoma. Cancer 2000, 88, 577–583. [Google Scholar] [CrossRef]
- Hoshikawa, M.; Aoki, T.; Matsushita, H.; Karasaki, T.; Hosoi, A.; Odaira, K.; Fujieda, N.; Kobayashi, Y.; Kambara, K.; Ohara, O.; et al. NK Cell and IFN Signatures Are Positive Prognostic Biomarkers for Resectable Pancreatic Cancer. Biochem. Biophys. Res. Commun. 2018, 495, 2058–2065. [Google Scholar] [CrossRef] [PubMed]
- Nersesian, S.; Schwartz, S.L.; Grantham, S.R.; MacLean, L.K.; Lee, S.N.; Pugh-Toole, M.; Boudreau, J.E. NK Cell Infiltration Is Associated with Improved Overall Survival in Solid Cancers: A Systematic Review and Meta-Analysis. Transl. Oncol. 2021, 14, 100930. [Google Scholar] [CrossRef] [PubMed]
- Cózar, B.; Greppi, M.; Carpentier, S.; Narni-Mancinelli, E.; Chiossone, L.; Vivier, E. Tumor-Infiltrating Natural Killer Cells. Cancer Discov. 2021, 11, 34–44. [Google Scholar] [CrossRef] [PubMed]
- Carrega, P.; Morandi, B.; Costa, R.; Frumento, G.; Forte, G.; Altavilla, G.; Ratto, G.B.; Mingari, M.C.; Moretta, L.; Ferlazzo, G. Natural Killer Cells Infiltrating Human Nonsmall-Cell Lung Cancer Are Enriched in CD56brightCD16− Cells and Display an Impaired Capability to Kill Tumor Cells. Cancer 2008, 112, 863–875. [Google Scholar] [CrossRef] [PubMed]
- Judge, S.J.; Darrow, M.A.; Thorpe, S.W.; Gingrich, A.A.; O’Donnell, E.F.; Bellini, A.R.; Sturgill, I.R.; Vick, L.V.; Dunai, C.; Stoffel, K.M.; et al. Analysis of Tumor-Infiltrating NK and T Cells Highlights IL-15 Stimulation and TIGIT Blockade as a Combination Immunotherapy Strategy for Soft Tissue Sarcomas. J. Immunother. Cancer 2020, 8, e001355. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.A.; Kim, J.; Jeon, S.; Shin, M.H.; Kwon, J.; Kim, T.J.; Im, K.; Han, Y.; Kwon, W.; Kim, S.W.; et al. Defective Localization with Impaired Tumor Cytotoxicity Contributes to the Immune Escape of NK Cells in Pancreatic Cancer Patients. Front. Immunol. 2019, 10, 496. [Google Scholar] [CrossRef] [PubMed]
- Kremer, V.; Ligtenberg, M.; Zendehdel, R.; Seitz, C.; Duivenvoorden, A.; Wennerberg, E.; Colón, E.; Scherman-Plogell, A.H.; Lundqvist, A. Genetic Engineering of Human NK Cells to Express CXCR2 Improves Migration to Renal Cell Carcinoma. J. Immunother. Cancer 2017, 5, 73. [Google Scholar] [CrossRef]
- Giraldo, N.A.; Sanchez-Salas, R.; Peske, J.D.; Vano, Y.; Becht, E.; Petitprez, F.; Validire, P.; Ingels, A.; Cathelineau, X.; Fridman, W.H.; et al. The Clinical Role of the TME in Solid Cancer. Br. J. Cancer 2019, 120, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Melaiu, O.; Lucarini, V.; Cifaldi, L.; Fruci, D. Influence of the Tumor Microenvironment on NK Cell Function in Solid Tumors. Front. Immunol. 2020, 10, 3038. [Google Scholar] [CrossRef] [PubMed]
- FDA. FDA Approves First Cellular Therapy to Treat Patients with Unresectable or Metastatic Melanoma; FDA: Silver Spring, MD, USA; Rockville, MD, USA, 2024.
- Mullard, A. FDA Approves First Tumour-Infiltrating Lymphocyte (TIL) Therapy, Bolstering Hopes for Cell Therapies in Solid Cancers. Nat. Rev. Drug Discov. 2024. [Google Scholar] [CrossRef]
- Liang, S.; Xu, K.; Niu, L.; Wang, X.; Liang, Y.; Zhang, M.; Chen, J.; Lin, M. Comparison of Autogeneic and Allogeneic Natural Killer Cells Immunotherapy on the Clinical Outcome of Recurrent Breast Cancer. OncoTargets Ther. 2017, 10, 4273–4281. [Google Scholar] [CrossRef]
- Yang, Y.; Lim, O.; Kim, T.M.; Ahn, Y.; Choi, H.; Chung, H.; Min, B.; Her, J.H.; Cho, S.Y.; Keam, B.; et al. Phase I Study of Random Healthy Donor—Derived Allogeneic Natural Killer Cell Therapy in Patients with Malignant Lymphoma or Advanced Solid Tumors. Cancer Immunol. Res. 2016, 4, 215–224. [Google Scholar] [CrossRef]
- Liu, S.; Galat, V.; Galat4, Y.; Lee, Y.K.A.; Wainwright, D.; Wu, J. NK Cell-Based Cancer Immunotherapy: From Basic Biology to Clinical Development. J. Hematol. Oncol. 2021, 14, 7. [Google Scholar] [CrossRef] [PubMed]
- Hermanson, D.L.; Bendzick, L.; Pribyl, L.; McCullar, V.; Vogel, R.I.; Miller, J.S.; Geller, M.A.; Kaufman, D.S. Induced Pluripotent Stem Cell-Derived Natural Killer Cells for Treatment of Ovarian Cancer. Stem Cells 2016, 34, 93–101. [Google Scholar] [CrossRef]
- Lee, S.C.; Shimasaki, N.; Lim, J.S.J.; Wong, A.; Yadav, K.; Yong, W.P.; Tan, L.K.; Koh, L.P.; Poon, M.L.M.; Tan, S.H.; et al. Phase I Trial of Expanded, Activated Autologous NK-Cell Infusions with Trastuzumab in Patients with HER2-Positive Cancers. Clin. Cancer Res. 2020, 26, 4494–4502. [Google Scholar] [CrossRef]
- Lim, C.M.; Liou, A.; Poon, M.; Koh, L.P.; Tan, L.K.; Loh, K.S.; Petersson, B.F.; Ting, E.; Campana, D.; Goh, B.C.; et al. Phase I Study of Expanded Natural Killer Cells in Combination with Cetuximab for Recurrent/Metastatic Nasopharyngeal Carcinoma. Cancer Immunol. Immunother. 2022, 71, 2277–2286. [Google Scholar] [CrossRef]
- Nahi, H.; Chrobok, M.; Meinke, S.; Gran, C.; Marquardt, N.; Afram, G.; Sutlu, T.; Gilljam, M.; Stellan, B.; Wagner, A.K.; et al. Autologous NK Cells as Consolidation Therapy Following Stem Cell Transplantation in Multiple Myeloma. Cell Rep. Med. 2022, 3, 100508. [Google Scholar] [CrossRef]
- Parkhurst, M.R.; Riley, J.P.; Dudley, M.E.; Rosenberg, S.A. Adoptive Transfer of Autologous Natural Killer Cells Leads to High Levels of Circulating Natural Killer Cells but Does Not Mediate Tumor Regression. Clin. Cancer Res. 2011, 17, 6287–6297. [Google Scholar] [CrossRef]
- Chu, J.; Gao, F.; Yan, M.; Zhao, S.; Yan, Z.; Shi, B.; Liu, Y. Natural Killer Cells: A Promising Immunotherapy for Cancer. J. Transl. Med. 2022, 20, 240. [Google Scholar] [CrossRef]
- Ruggeri, L.; Capanni, M.; Urbani, E.; Perruccio, K.; Shlomchik, W.D.; Tosti, A.; Posati, S.; Rogaia, D.; Frassoni, F.; Aversa, F.; et al. Effectiveness of Donor Natural Killer Cell Alloreactivity in Mismatched Hematopoietic Transplants. Science 2002, 295, 2097–2100. [Google Scholar] [CrossRef]
- Miller, J.S.; Cooley, S.; Parham, P.; Farag, S.S.; Verneris, M.R.; Mcqueen, K.L.; Guethlein, L.A.; Trachtenberg, E.A.; Haagenson, M.; Horowitz, M.M.; et al. Missing KIR Ligands Are Associated with Less Relapse and Increased Graft-versus-Host Disease (GVHD) Following Unrelated Donor Allogeneic HCT. Blood 2007, 109, 5058–5061. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.S.; Soignier, Y.; Panoskaltsis-Mortari, A.; McNearney, S.A.; Yun, G.H.; Fautsch, S.K.; McKenna, D.; Le, C.; Defor, T.E.; Burns, L.J.; et al. Successful Adoptive Transfer and in Vivo Expansion of Human Haploidentical NK Cells in Patients with Cancer. Blood 2005, 105, 3051–3057. [Google Scholar] [CrossRef] [PubMed]
- Bachanova, V.; Sarhan, D.; Defor, T.; Cooley, S.; Panoskaltsis-, A.; Blazar, B.R.; Curtsinger, J.; Burns, L.; Weisdorf, D.J.; Miller, S.; et al. Haploidentical Natural Killer Cells Induce Remissions in Non-Hodgkin Lymphoma Patients With Low Levels of Immune-Suppressor Cells. Cancer Immunol. Immunother. 2018, 67, 483–494. [Google Scholar] [CrossRef] [PubMed]
- Bachanova, V.; Cooley, S.; Defor, T.E.; Verneris, M.R.; Zhang, B.; Mckenna, D.H.; Curtsinger, J.; Panoskaltsis-Mortari, A.; Lewis, D.; Hippen, K.; et al. Clearance of Acute Myeloid Leukemia by Haploidentical Natural Killer Cells Is Improved Using IL-2 Diphtheria Toxin Fusion Protein. Blood 2014, 123, 3855–3863. [Google Scholar] [CrossRef]
- Cooley, S.; He, F.; Bachanova, V.; Vercellotti, G.M.; DeFor, T.E.; Curtsinger, J.M.; Robertson, P.; Grzywacz, B.; Conlon, K.C.; Waldmann, T.A.; et al. First-in-Human Trial of RhIL-15 and Haploidentical Natural Killer Cell Therapy for Advanced Acute Myeloid Leukemia. Blood Adv. 2019, 3, 1970–1980. [Google Scholar] [CrossRef] [PubMed]
- Romee, R.; Cooley, S.; Berrien-Elliott, M.M.; Westervelt, P.; Verneris, M.R.; Wagner, J.E.; Weisdorf, D.J.; Blazar, B.R.; Ustun, C.; Defor, T.E.; et al. First-in-Human Phase 1 Clinical Study of the IL-15 Superagonist Complex ALT-803 to Treat Relapse after Transplantation. Blood 2018, 131, 2515–2527. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, R.M.; Birch, G.C.; Hu, G.; Vergara Cadavid, J.; Nikiforow, S.; Baginska, J.; Ali, A.K.; Tarannum, M.; Sheffer, M.; Abdulhamid, Y.Z.; et al. Expansion, Persistence, and Efficacy of Donor Memory-like NK Cells Infused for Posttransplant Relapse. J. Clin. Investig. 2022, 132, e154334. [Google Scholar] [CrossRef] [PubMed]
- Shah, N.; Li, L.; McCarty, J.; Kaur, I.; Yvon, E.; Shaim, H.; Muftuoglu, M.; Liu, E.; Orlowski, R.Z.; Cooper, L.; et al. Phase I Study of Cord Blood-Derived Natural Killer Cells Combined with Autologous Stem Cell Transplantation in Multiple Myeloma. Br. J. Haematol. 2017, 177, 457–466. [Google Scholar] [CrossRef]
- Hoogstad-van Evert, J.; Bekkers, R.; Ottevanger, N.; Schaap, N.; Hobo, W.; Jansen, J.H.; Massuger, L.; Dolstra, H. Intraperitoneal Infusion of Ex Vivo-Cultured Allogeneic NK Cells in Recurrent Ovarian Carcinoma Patients (a Phase I Study). Medicine 2019, 98, e14290. [Google Scholar] [CrossRef]
- Dolstra, H.; Roeven, M.W.H.; Spanholtz, J.; Hangalapura, B.N.; Tordoir, M.; Maas, F.; Leenders, M.; Bohme, F.; Kok, N.; Trilsbeek, C.; et al. Successful Transfer of Umbilical Cord Blood CD34+ Hematopoietic Stem and Progenitor-Derived NK Cells in Older Acute Myeloid Leukemia Patients. Clin. Cancer Res. 2017, 23, 4107–4118. [Google Scholar] [CrossRef]
- Burga, R.A.; Nguyen, T.; Zulovich, J.; Madonna, S.; Ylisastigui, L.; Fernandes, R.; Yvon, E. Improving Efficacy of Cancer Immunotherapy by Genetic Modification of Natural Killer Cells. Cytotherapy 2016, 18, 1410–1421. [Google Scholar] [CrossRef]
- Cichocki, F.; Miller, J.S. In Vitro Development of Human Killer-Immunoglobulin Receptor-Positive NK Cells. Methods Mol. Biol. 2010, 612, 15–26. [Google Scholar] [CrossRef]
- Rafei, H.; Daher, M.; Katayoun, R. Chimeric Antigen Receptor (CAR) Natural Killer (NK)-Cell Therapy: Leveraging the Power of Innate Immunity. Br. J. Haematol. 2020, 193, 216–230. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, T.; Wang, X.; Zhang, Y.; Song, S.; Ma, C. Improving the Ability of CAR-T Cells to Hit Solid Tumors: Challenges and Strategies. Pharmacol. Res. 2021, 175, 106036. [Google Scholar] [CrossRef] [PubMed]
- Yeku, O.; Li, X.; Brentjens, R.J. Adoptive T-Cell Therapy for Solid Tumors. Am. Soc. Clin. Oncol. Educ. Book 2021, 37, 193–204. [Google Scholar] [CrossRef]
- Tong, L.; Jiménez-Cortegana, C.; Tay, A.H.M.; Wickström, S.; Galluzzi, L.; Lundqvist, A. NK Cells and Solid Tumors: Therapeutic Potential and Persisting Obstacles. Mol. Cancer 2022, 21, 206. [Google Scholar] [CrossRef] [PubMed]
- Rafiq, S.; Hackett, C.S.; Brentjens, R.J. Engineering Strategies to Overcome the Current Roadblocks in CAR T Cell Therapy. Nat. Rev. Clin. Oncol. 2020, 17, 147–167. [Google Scholar] [CrossRef] [PubMed]
- Xin, T.; Cheng, L.; Zhou, C.; Zhao, Y.; Hu, Z.; Wu, X. In-Vivo Induced CAR-T Cell for the Potential Breakthrough to Overcome the Barriers of Current CAR-T Cell Therapy. Front. Oncol. 2022, 12, 809754. [Google Scholar] [CrossRef]
- Hamilton, J.R.; Chen, E.; Perez, B.S.; Sandoval Espinoza, C.R.; Kang, M.H.; Trinidad, M.; Ngo, W.; Doudna, J.A. In Vivo Human T Cell Engineering with Enveloped Delivery Vehicles. Nat. Biotechnol. 2024. [Google Scholar] [CrossRef]



| Ref. | Cell Source | Culture Factors | Time (Days) | Culture Material | Results |
|---|---|---|---|---|---|
| [71] | PBMC | CellGro SCGM with 5% HS, anti-CD3 Ab for the first 5 days with 500 U/mL of IL-2. | 21 | 6-well plates and T25 flasks. | CD3− CD56+ cells reached 193-fold expansion. |
| [72] | PBMC | Serum-free medium with 700 IU/mL IL-2, 0.01 KE/mL OK432, 10% human plasma, and an anti-CD16 Ab. | 21 | Flask and culture bag. | Fold expansion ranges from 637 to 5712, with a purity of 76.9%. |
| [73] | Isolated NK cells | X-VIVO 10 media with 5% heat-inactivated human FFP and 1000 U/mL of rhIL-2. | 12 | GMP-grade VueLife culture bags. | The mean expansion rate of NK cells was 4-fold, while two donors reached 30-fold. |
| [74] | Isolated NK cells | X-VIVO 10 media with 5% heat-inactivated human FFP, P/S, 100 or 1000 U/mL of IL-2, 10 ng/mL of IL-15, 25 ng/mL of IL-21 or combinations of those. | 42 | T25 flasks. | IL-15 induced NK cell expansion, while IL-21 triggered NK cell maturation and functionality. |
| [75] | CB-NK cells | SCGM with 5% human AB serum, IL-15, IL-2, anti-CD3 ab, tacrolimus, and dalteparin sodium. | 20 | 24-well plates and T25 flasks. | 1700-fold expansion with 72.8% purity. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maia, A.; Tarannum, M.; Lérias, J.R.; Piccinelli, S.; Borrego, L.M.; Maeurer, M.; Romee, R.; Castillo-Martin, M. Building a Better Defense: Expanding and Improving Natural Killer Cells for Adoptive Cell Therapy. Cells 2024, 13, 451. https://doi.org/10.3390/cells13050451
Maia A, Tarannum M, Lérias JR, Piccinelli S, Borrego LM, Maeurer M, Romee R, Castillo-Martin M. Building a Better Defense: Expanding and Improving Natural Killer Cells for Adoptive Cell Therapy. Cells. 2024; 13(5):451. https://doi.org/10.3390/cells13050451
Chicago/Turabian StyleMaia, Andreia, Mubin Tarannum, Joana R. Lérias, Sara Piccinelli, Luis Miguel Borrego, Markus Maeurer, Rizwan Romee, and Mireia Castillo-Martin. 2024. "Building a Better Defense: Expanding and Improving Natural Killer Cells for Adoptive Cell Therapy" Cells 13, no. 5: 451. https://doi.org/10.3390/cells13050451