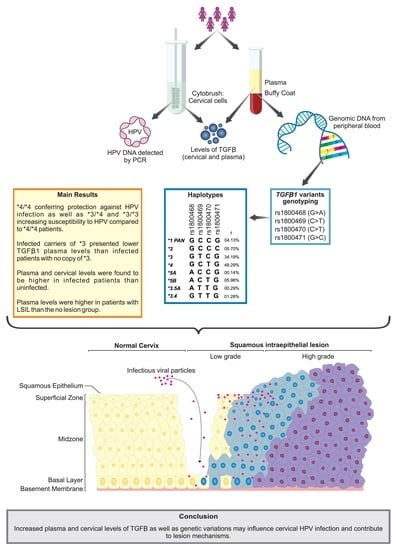
Haplotype Structures and Protein Levels of TGFB1 in HPV Infection and Cervical Lesion: A Case-Control Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients and Samples
2.2. DNA Extraction
2.3. HPV Detection by PCR
2.4. Cervical Cytology
2.5. TGFB1 Genetic Variants Genotyping
2.6. Haplotype Analysis
2.7. TGFB1 Levels
2.8. Statistical Analysis
3. Results
3.1. Participant Characterization According to HPV Infection, Sociodemographic, and Clinical Data
3.2. Distribution of Alleles, Genotypes, and Haplotypes of TGFB1 Genetic Variations and Susceptibility to HPV Infection and Cervical Lesions
3.3. Impact of the TGFB1 Haplotypes on Plasma and Cervical Levels of Protein
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tommasino, M. The Human Papillomavirus Family and Its Role in Carcinogenesis. Semin. Cancer Biol. 2014, 26, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, Q.; Allouch, S.; Gupta, I.; Elmakaty, I.; Elzawawi, K.E.; Amarah, A.; Al-Thawadi, H.; Al-Farsi, H.; Vranic, S.; al Moustafa, A.-E.; et al. Human Papillomaviruses-Related Cancers: An Update on the Presence and Prevention Strategies in the Middle East and North African Regions. Pathogens 2022, 11, 1380. [Google Scholar] [CrossRef] [PubMed]
- Bernard, H.U.; Burk, R.D.; Chen, Z.; van Doorslaer, K.; zur Hausen, H.; de Villiers, E.M. Classification of Papillomaviruses (PVs) Based on 189 PV Types and Proposal of Taxonomic Amendments. Virology 2010, 401, 70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- HPV and Cancer—NCI. Available online: https://www.cancer.gov/about-cancer/causes-prevention/risk/infectious-agents/hpv-and-cancer (accessed on 15 December 2022).
- Chan, C.K.; Aimagambetova, G.; Ukybassova, T.; Kongrtay, K.; Azizan, A. Human Papillomavirus Infection and Cervical Cancer: Epidemiology, Screening, and Vaccination-Review of Current Perspectives. J. Oncol. 2019, 2019, 3257939. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Hu, Z.; Ma, D. The Precision Prevention and Therapy of HPV-Related Cervical Cancer: New Concepts and Clinical Implications. Cancer Med. 2018, 7, 5217–5236. [Google Scholar] [CrossRef]
- Bosch, F.X.; De Sanjosé, S. The epidemiology of human papillomavirus infection and cervical cancer. Dis. Markers 2007, 23, 213–227. [Google Scholar] [CrossRef] [Green Version]
- Hardikar, S.; Johnson, L.G.; Malkki, M.; Petersdorf, E.W.; Galloway, D.A.; Schwartz, S.M.; Madeleine, M.M. A population-based case-control study of genetic variation in cytokine genes associated with risk of cervical and vulvar cancers. Gynecol. Oncol. 2015, 139, 90–96. [Google Scholar] [CrossRef] [Green Version]
- Chin, D.; Boyle, G.M.; Parsons, P.G.; Coman, W.B. What is transforming growth factor-beta (TGF-b)? Br. J Plast. Surg. 2004, 57, 215–221. [Google Scholar] [CrossRef]
- Kubiczkova, L.; Sedlarikova, L.; Hajek, R.; Sevcikova, S. TGF-β—An excellent servant but a bad master. J. Transl. Med. 2012, 10, 183. [Google Scholar] [CrossRef]
- Pickup, M.; Novitskiy, S.; Moses, H.L. The roles of TGFβ in the tumour microenvironment. Nat. Rev. Cancer 2013, 13, 788–799. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Derynck, R.; Rhee, L.; Chen, E.Y.; Tilburg, A.V. Intron-exon structure of the human transforming growth factor-beta precursor gene. Nucleic Acids Res. 1987, 15, 3188. [Google Scholar] [CrossRef] [PubMed]
- Cebinelli, G.C.M.; Trugilo, K.P.; Garcia, S.B.; Oliveira, K.B. TGF-β1 functional polymorphisms: A review. Eur. Cytokine Netw. 2016, 27, 81–89. [Google Scholar] [CrossRef] [Green Version]
- Shastry, B.S. SNPs: Impact on gene function and phenotype. Methods Mol. Biol. 2009, 578, 3–22. [Google Scholar] [CrossRef]
- Bauer, H.M.; Ting, Y.; Greer, C.E.; Chambers, J.C.; Tashiro, C.J.; Chimera, J.; Reingold, A.; Manos, M.M. Genital Human Papillomavirus Infection in Female University Students as Determined by a PCR-Based Method. JAMA 1991, 265, 472–477. [Google Scholar] [CrossRef]
- Da Silva, M.C.; Martins, H.P.R.; Souza, J.L.; Tognim, M.C.B.; Svidzinski, T.I.E.; Teixeira, J.J.V.; Consolaro, M.E.L. Prevalence of HPV infection and genotypes in women with normal cervical cytology in the state of Paraná, Brazil. Arch. Gynecol. Obstet. 2012, 286, 1015–1022. [Google Scholar] [CrossRef]
- Jin, Q.; Hemminki, K.; Grzybowska, E.; Klaes, R.; Söderberg, M.; Zientek, H.; Rogozinska-Szczepka, J.; Utracka-Hutka, B.; Pamuła-Piłat, J.; Pekala, W.; et al. Polymorphisms and haplotype structures in genes for transforming growth factorβ1 and its receptors in familial and unselected breast cancers. Int. J. Cancer 2004, 112, 94–99. [Google Scholar] [CrossRef]
- Stephens, M.; Scheet, P. Accounting for Decay of Linkage Disequilibrium in Haplotype Inference and Missing-Data Imputation. Am. J. Hum. Genet. 2005, 76, 449–462. [Google Scholar] [CrossRef] [Green Version]
- Stephens, M.; Smith, N.J.; Donnelly, P. A New Statistical Method for Haplotype Reconstruction from Population Data. Am. J. Hum. Genet. 2001, 68, 978–989. [Google Scholar] [CrossRef] [Green Version]
- Barrett, J.C.; Fry, B.; Maller, J.; Daly, M.J. Haploview: Analysis and visualization of LD and haplotype maps. Bioinformatics 2005, 21, 263–265. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coser, J.; Boeira, T.D.R.; Wolf, J.M.; Cerbaro, K.; Simon, D.; Lunge, V.R. Cervical human papillomavirus infection and persistence: A clinic-based study in the countryside from South Brazil. Braz. J. Infect. Dis. 2016, 20, 61–68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Sanjosé, S.; Diaz, M.; Castellsagué, X.; Clifford, G.; Bruni, L.; Muñoz, N.; Bosch, F.X. Worldwide prevalence and genotype distribution of cervical human papillomavirus DNA in women with normal cytology: A meta-analysis. Lancet Infect. Dis. 2007, 7, 453–459. [Google Scholar] [CrossRef] [PubMed]
- Cezar-Dos-Santos, F.; Ferreira, R.S.; Okuyama, N.C.M.; Trugilo, K.P.; Sena, M.M.; Pereira, R.; Pereira, A.P.L.; Watanabe, M.A.E.; de Oliveira, K.B. FOXP3 immunoregulatory gene variants are independent predictors of human papillomavirus infection and cervical cancer precursor lesions. J. Cancer Res. Clin. Oncol. 2019, 145, 2013–2025. [Google Scholar] [CrossRef] [PubMed]
- Okuyama, N.C.M.; Cezar-Dos-Santos, F.; Pereira, R.; Trugilo, K.P.; Cebinelli, G.C.M.; Sena, M.M.; Pereira, A.P.L.; Aranome, A.M.F.; Mangieri, L.F.L.; Ferreira, R.S.; et al. Genetic variant in CXCL12 gene raises susceptibility to HPV infection and squamous intraepithelial lesions development: A case-control study. J. Biomed. Sci. 2018, 25, 69. [Google Scholar] [CrossRef] [Green Version]
- Berti, F.C.B.; Pereira, A.P.L.; Trugilo, K.P.; Cebinelli, G.C.M.; Silva, L.F.D.R.S.; Lozovoy, M.A.B.; Simão, A.N.C.; Watanabe, M.A.E.; de Oliveira, K.B. IL-10 gene polymorphism c.-592C > A increases HPV infection susceptibility and influences IL-10 levels in HPV infected women. Infect. Genet. Evol. 2017, 53, 128–134. [Google Scholar] [CrossRef]
- Trugilo, K.P.; Cebinelli, G.C.M.; Berti, F.C.B.; Okuyama, N.C.M.; Cezar-Dos-Santos, F.; Sena, M.M.; Mangieri, L.F.L.; Watanabe, M.A.E.; de Oliveira, K.B. Polymorphisms in the TGFB1 signal peptide influence human papillomavirus infection and development of cervical lesions. Med. Microbiol. Immunol. 2019, 208, 49–58. [Google Scholar] [CrossRef]
- Shah, R.; Hurley, C.K.; Posch, P.E. A molecular mechanism for the differential regulation of TGF-β1 expression due to the common SNP-509C-T (c.-1347C > T). Hum. Genet. 2006, 120, 461–469. [Google Scholar] [CrossRef]
- Grainger, D.J.; Heathcote, K.; Chiano, M.; Snieder, H.; Kemp, P.R.; Metcalfe, J.C.; Carter, N.D.; Spector, T.D. Genetic control of the circulating concentration of transforming growth factor type beta1. Hum. Mol. Genet. 1999, 8, 93–97. [Google Scholar] [CrossRef] [Green Version]
- Wood, N.A.; Thomson, S.C.; Smith, R.M.; Bidwell, J.L. Identification of human TGF-β1 signal (leader) sequence polymorphisms by PCR-RFLP. J. Immunol. Methods 2000, 234, 117–122. [Google Scholar] [CrossRef]
- Awad, M.R.; El-Gamel, A.; Hasleton, P.; Turner, D.M.; Sinnott, P.J.; Hutchinson, I.V. Genotypic variation in the transforming growth factor-beta1 gene: Association with transforming growth factor-beta1 production, fibrotic lung disease, and graft fibrosis after lung transplantation. Transplantation 1998, 66, 1014–1020. [Google Scholar] [CrossRef] [PubMed]
- Taubenschuss, E.; Marton, E.; Mogg, M.; Frech, B.; Ehart, L.; Muin, D.; Schreiber, M. The L10P polymorphism and serum levels of transforming growth factor beta1 in human breast cancer. Int. J. Mol. Sci. 2013, 14, 15376–15385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yokota, M.; Ichihara, S.; Lin, T.L.; Nakashima, N.; Yamada, Y. Association of a T29 → C Polymorphism of the Transforming Growth Factor-β1 Gene With Genetic Susceptibility to Myocardial Infarction in Japanese. Circulation 2000, 101, 2783–2787. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Genome Aggregation Database (gnomAD). Available online: https://gnomad.broadinstitute.org/ (accessed on 18 December 2021).
- Karczewski, K.J.; Francioli, L.C.; Tiao, G.; Cummings, B.B.; Alfoldi, J.; Wang, Q.; Collins, R.L.; Laricchia, K.M.; Ganna, A.; Birnbaum, D.P.; et al. The mutational constraint spectrum quantified from variation in 141,456 humans. Nature 2020, 581, 434–443. [Google Scholar] [CrossRef] [PubMed]
- Lins, T.C.; Vieira, R.G.; Abreu, B.S.; Grattapaglia, D.; Pereira, R.W. Genetic composition of Brazilian population samples based on a set of twenty-eight ancestry informative SNPs. Am. J. Hum. Biol. 2010, 22, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Parra, F.C.; Amado, R.C.; Lambertucci, J.R.; Rocha, J.; Antunes, C.M.; Pena, S.D.J. Color and genomic ancestry in Brazilians. Proc. Natl. Acad. Sci. USA 2003, 100, 177–182. [Google Scholar] [CrossRef] [Green Version]
- Guan, X.; Sturgis, E.M.; Lei, D.; Liu, Z.; Dahlstrom, K.R.; Wei, Q.; Li, G. Association of TGF-beta1 genetic variants with HPV16-positive oropharyngeal cancer. Clin. Cancer Res. 2010, 16, 1416–1422. [Google Scholar] [CrossRef] [Green Version]
- Fernandes, A.P.M.; Gonçalves, M.A.G.; Simões, R.T.; Mendes-Junior, C.T.; Duarte, G.; Donadi, E.A. A pilot case-control association study of cytokine polymorphisms in Brazilian women presenting with HPV-related cervical lesions. Eur. J. Obstet. Gynecol. Reprod. Biol. 2008, 140, 241–244. [Google Scholar] [CrossRef]
- Júnior, S.F.D.L.; Tavares, M.M.F.; de Macedo, J.L.; de Oliveira, R.S.; Heráclio, S.D.A.; Maia, M.D.M.D.; de Souza, P.R.E.; Moura, R.; Crovella, S. Influence of IL-6, IL-8, and TGF-β1 gene polymorphisms on the risk of human papillomavirus-infection in women from Pernambuco, Brazil. Mem. Inst. Oswaldo Cruz. 2016, 111, 663–669. [Google Scholar] [CrossRef] [Green Version]
- Marangon, A.V.; Guelsin, G.A.S.; Visentainer, J.E.L.; Borelli, S.D.; Watanabe, M.A.E.; Consolaro, M.E.L.; Caleffi-Ferracioli, K.R.; Rudnick, C.C.C.; Sell, A.M. The Association of the Immune Response Genes to Human Papillomavirus-Related Cervical Disease in a Brazilian Population. Biomed. Res. Int. 2013, 2013, 146079. [Google Scholar] [CrossRef]
- Singh, H.; Jain, M.; Mittal, B. Role of TGF-β1 (−509C > T) Promoter Polymorphism in Susceptibility to Cervical Cancer. Oncol. Res. 2009, 18, 41–45. [Google Scholar] [CrossRef] [PubMed]
- Vitiello, G.A.F.; Guembarovski, R.L.; Hirata, B.K.B.; Amarante, M.K.; de Oliveira, C.E.C.; de Oliveira, K.B.; Cebinelli, G.C.M.; Guembarovski, A.L.; Campos, C.Z.; Watanabe, M.A.E. Transforming growth factor-beta 1 (TGFβ1) polymorphisms and haplotype structures have dual roles in breast cancer pathogenesis. J. Cancer Res. Clin. Oncol. 2018, 144, 645–655. [Google Scholar] [CrossRef] [PubMed]
- Peralta-Zaragoza, O.; Bermúdez-Morales, V.; Gutiérrez-Xicotencatl Alcocer-González, J.; Recillas-Targa, F.; Madrid-Marina, V. E6 and E7 Oncoproteins from Human Papillomavirus Type 16 Induce Activation of Human Transforming Growth Factor β 1 Promoter throughout Sp1 Recognition Sequence. Viral. Immunol. Summer 2006, 19, 468–480. [Google Scholar] [CrossRef]
- Torres-Poveda, K.; Bahena-Román, M.; Madrid-González, C.; Burguete-García, A.I.; Bermúdez-Morales, V.H.; Peralta-Zaragoza, O.; Madrid-Marina, V. Role of IL-10 and TGF-β1 in local immunosuppression in HPV-associated cervical neoplasia. World J. Clin. Oncol. 2014, 5, 753–763. [Google Scholar] [CrossRef] [PubMed]
- Kowli, S.; Velidandla, R.; Creek, K.E.; Pirisi, L. TGF-β regulation of gene expression at early and late stages of HPV16-mediated transformation of human keratinocytes. Virology 2013, 447, 63–73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woodworth, C.D.; Notario, V.; DiPaolo, J.A. Transforming growth factors beta 1 and 2 transcriptionally regulate human papillomavirus (HPV) type 16 early gene expression in HPV-immortalized human genital epithelial cells. J. Virol. 1990, 64, 4767–4775. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonin, C.M.; Padovani, C.T.J.; Da Costa, I.P.; Ávila, L.S.; Ferreira, A.M.T.; Fernandes, C.E.S.; Dos Santos, A.R.; Tozetti, I.A. Detection of regulatory T cell phenotypic markers and cytokines in patients with human papillomavirus infection. J. Med. Virol. 2019, 91, 317–325. [Google Scholar] [CrossRef]
- Ansa-Addo, E.A.; Zhang, Y.; Yang, Y.; Hussey, G.S.; Howley, B.V.; Salem, M.; Riesenberg, B.; Sun, S.; Rockey, N.C.; Karvar, S.; et al. Membrane-organizing protein moesin controls Treg differentiation and antitumor immunity via TGF-β signaling. J. Clin. Investig. 2017, 127, 1321–1337. [Google Scholar] [CrossRef] [Green Version]
- Papageorgis, P. TGFβ Signaling in Tumor Initiation, Epithelial-to-Mesenchymal Transition, and Metastasis. J. Oncol. 2015, 2015, 587193. [Google Scholar] [CrossRef]



| Characteristics | HPV | Lesion Grade (Infected Patients) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Uninfected (n = 190) | Infected (n = 161) | OR (CI95%) | NL (n = 80) | LSIL (n = 23) | HSIL (n = 53) | ORLSIL (CI95%) | ORHSIL (CI95%) | ||
| Age range (years) | <35 | 57 (30.0) | 80 (49.7) | 2.29 (1.35–3.90) | 40 (50.0) | 12 (52.2) | 26 (49.1) | 1.79 (0.51–6.35) | 1.16 (0.45–2.95) |
| ≥35 | 133 (70.0) | 81 (50.3) | Reference | 40 (50.0) | 11 47.8) | 27 (50.9) | Reference | Reference | |
| Age at menarche (years) | <13 | 86 (45.5) | 84 (52.2) | 1.16 (0.74–1.83) | 42 (52.5) | 11 (47.8) | 29 (54.7) | Reference | Reference |
| ≥13 | 103 (54.5) | 77 (47.8) | Reference | 38 (47.5) | 12 (52.2) | 24 (45.3) | 1.26 (0.45–3.54) | 0.79 (0.37–1.71) | |
| Age at first sexual intercourse (years) | <18 | 92 (48.4) | 99 (61.5) | Reference | 47 (58.8) | 16 (69.6) | 32 (60.4) | Reference | Reference |
| ≥18 | 98 (51.6) | 62 (38.5) | 0.89 (0.54–1.46) | 33 (41.3) | 7 (30.4) | 21 (39.6) | 0.87 (0.27–2.74) | 1.37 (0.60–3.16) | |
| Pregnancies | <3 | 108 (56.8) | 95 (59.0) | Reference | 56 (70.0) | 10 (43.5) | 28 (52.8) | Reference | Reference |
| ≥3 | 82 (43.2) | 66 (41.0) | 1.27 (0.76–2.12) | 24 (30.0) | 13 (56.5) | 25 (47.2) | 5.64 (1.56–20.39) | 3.30 (1.31–8.28) | |
| Oral contraceptive usage | No | 132 (69.5) | 105 (65.2) | Reference | 56 (70.0) | 13 (56.5) | 32 (60.4) | Reference | Reference |
| Yes | 58 (30.5) | 56 (34.8) | 0.96 (0.57–1.62) | 24 (30.0) | 10 (43.5) | 21 (39.6) | 3.11 (0.97–9.90) | 1.83 (0.78–4.29) | |
| Marital status | Married a | 139 (73.2) | 97 (60.2) | Reference | 48 (60.0) | 10 (43.5) | 36 (67.9) | Reference | Reference |
| Single b | 51 (26.8) | 64 (39.8) | 1.58 (0.98–2.56) | 32 (40.0) | 13 (56.5) | 17 (32.1) | 2.47 (0.85–7.15) | 0.59 (0.26–1.37) | |
| Sexual partners during the lifetime | <3 | 111 (58.4) | 65 (40.4) | 0.63 (0.39–1.00) | 37 (46.3) | 10 (43.5) | 17 (32.1) | 1.29 (0.41–4.05) | 0.41 (0.17–0.99) |
| ≥3 | 79 (41.6) | 96 (59.6) | Reference | 43 (53.8) | 13 (56.5) | 36 (67.9) | Reference | Reference | |
| Smoking status | No | 142 (74.7) | 114 (70.8) | Reference | 62 (77.5) | 13 (56.5) | 37 (69.8) | Reference | Reference |
| Yes | 48 (25.3) | 47 (29.2) | 1.06 (0.64–1.76) | 18 (22.5) | 10 (43.5) | 16 (30.2) | 2.33 (0.74–7.31) | 1.12 (0.46–2.76) | |
| TGFB1 SNVs | HPV | Lesion Grade (Infected Patients) | ||||||
|---|---|---|---|---|---|---|---|---|
| Uninfected (n = 190) | Infected (n = 161) | p | NL (n = 80) | LSIL (n = 23) | HSIL (n = 53) | p | ||
| c.–1638G>A | ||||||||
| GG | 169 (88.9) | 140 (87.0) | 0.708 | 68 (85.0) | 23 (100.0) | 45 (84.9) | 0.213 | |
| GA | 19 (10.0) | 20 (12.4) | 12 (15.0) | 0 | 7 (13.2) | |||
| AA | 2 (1.1) | 1 (0.6) | 0 | 0 | 1 (1.9) | |||
| Allele G | 357 (94.0) | 300 (93.2) | 0.674 | 148 (92.5) | 46 (100.0) | 97 (91.5) | 0.136 | |
| Allele A | 23 (6.0) | 22 (6.8) | 12 (7.5) | 0 | 9 (8.5) | |||
| c.–1347C>T | ||||||||
| TT | 22 (11.6) | 28 (17.4) | 0.104 | 10 (12.7) | 6 (26.1) | 11 (20.8) | 0.398 | |
| TC | 78 (41.1) | 73 (45.3) | 38 (48.1) | 7 (30.4) | 24 (45.3) | |||
| CC | 90 (47.4) | 60 (37.3) | 31 (39.2) | 10 (43.5) | 18 (34.0) | |||
| Allele T | 122 (32.1) | 129 (40.1) | 0.028 | 58 (36.7) | 19 (41.3) | 46 (43.4) | 0.537 | |
| Allele C | 258 (67.9) | 193 (59.9) | 100 (63.3) | 27 (58.7) | 60 (56.6) | |||
| c.29C>T | ||||||||
| CC | 33 (17.4) | 40 (24.8) | 0.023 | 17 (21.3) | 5 (21.7) | 16 (30.2) | 0.768 | |
| CT | 85 (44.7) | 81 (50.4) | 41 (51.2) | 13 (56.5) | 25 (47.2) | |||
| TT | 72 (37.9) | 40 (24.8) | 22 (27.5) | 5 (21.7) | 12 (22.6) | |||
| Allele C | 151 (39.7) | 161 (50.0) | 0.006 | 75 (46.9) | 23 (50.0) | 57 (53.8) | 0.544 | |
| Allele T | 229 (60.3) | 161 (50.0) | 85 (53.1) | 23 (50.0) | 49 (46.2) | |||
| c.74G>C | ||||||||
| GG | 173 (91.1) | 138 (85.7) | 0.117 | 71 (88.8) | 19 (82.6) | 45 (84.9) | 0.683 | |
| GC | 17 (8.9) | 23 (14.3) | 9 (11.3) | 4 (17.4) | 8 (15.1) | |||
| Allele G | 363 (95.5) | 299 (92.9) | 0.128 | 151 (94.4) | 42 (91.3) | 98 (92.4) | 0.702 | |
| Allele C | 17 (4.5) | 23 (7.1) | 9 (5.6) | 4 (8.7) | 8 (7.6) | |||
| TGFB1 Haplotypes | All | HPV | Cervical Lesion Grade (Infected Patients) | |||||
|---|---|---|---|---|---|---|---|---|
| (n = 702) | Uninfected (n = 380) | Infected (n = 322) | p | NL (n = 160) | LSIL (n = 46) | HSIL (n = 106) | p | |
| *1 PAN | 0.0413 | 0.0395 | 0.0435 | 0.790 | 0.0500 | 0.0435 | 0.0283 | 0.685 |
| *2 | 0.0570 | 0.0448 | 0.0714 | 0.128 | 0.0688 | 0.0870 | 0.0755 | 0.914 |
| *3 | 0.3419 | 0.3105 | 0.3789 | 0.057 | 0.3562 | 0.3695 | 0.4151 | 0.620 |
| *4 | 0.4829 | 0.5342 | 0.4224 | 0.003 | 0.4375 | 0.4565 | 0.3868 | 0.634 |
| *5A | 0.0014 | 0.0026 | 0 | 1.000 | 0 | 0 | 0 | - |
| *5B | 0.0598 | 0.0579 | 0.0621 | 0.814 | 0.0750 | 0 | 0.0660 | 0.166 |
| *3.4 | 0.0128 | 0.0105 | 0.0155 | 0.739 | 0.0125 | 0.0435 | 0.0094 | 0.270 |
| *3.5A | 0.0029 | 0 | 0.0062 | 0.210 | 0 | 0 | 0.0189 | 0.141 |
| TGFB1 SNVs | Adjusted Odds Ratio (OR (CI95%)) | |||
|---|---|---|---|---|
| HPV Infected | Lesion Grade (Infected Patients) | |||
| LSIL | HSIL | |||
| c.–1638G>A | ||||
| GA vs. GG | 1.13 (0.56–2.26) | - | - | |
| AA vs. GG | 1.03 (0.09–11.91) | - | - | |
| GA + AA vs. GG | 1.12 (0.57–2.19) | - | - | |
| c.–1347C>T | ||||
| TC vs. CC | 1.47 (0.91–2.37) | 0.44 (0.14–1.45) | 0.95 (0.42–2.17) | |
| TT vs. CC | 2.16 (1.10–4.25) * | 1.50 (0.39–5.78) | 1.76 (0.57–5.38) | |
| TC + TT vs. CC | 1.62 (1.03–2.54) * | 0.66 (0.23–1.88) | 1.11 (0.51–2.43) | |
| c.29C>T | ||||
| CT vs. TT | 1.77 (1.06–2.97) * | 1.52 (0.42–5.44) | 1.01 (0.41–2.52) | |
| CC vs. TT | 2.31 (1.23–4.34) ** | 1.33 (0.29–6.07) | 1.48 (0.52–4.19) | |
| CT + CC vs. TT | 1.92 (1.18–3.12) ** | 1.46 (0.43–4.96) | 1.15 (0.49–2.71) | |
| c.74G>C | ||||
| GC vs. GG | 1.60 (0.80–3.20) | 1.67 (0.44–6.38) | 1.02 (0.37–2.85) | |
| *4 (GCTG) | ||||
| Ht vs. no copy | 0.93 (0.55–1.56) | 0.95 (0.30–3.04) | 0.84 (0.37–1.90) | |
| Hm vs. no copy | 0.39 (0.21–0.72) ** | 1.15 (0.27–4.87) | 0.66 (0.21–2.06) | |
| Ht + Hm vs. no copy | 0.69 (0.42–1.11) | 1.00 (0.33–3.00) | 0.79 (0.36–1.74) | |
| *3 (GTCG) | ||||
| Ht vs. no copy | 1.48 (0.92–2.38) | 0.73 (0.24–2.21) | 1.02 (0.46–2.28) | |
| Hm vs. no copy | 1.81 (0.91–3.58) § | 1.18 (0.28–5.09) | 1.62 (0.53–4.95) | |
| Ht + Hm vs. no copy | 1.56 (1.00–2.43) § | 0.83 (0.30–2.30) | 1.14 (0.53–2.43) | |
| *5B (ACTG) | ||||
| Ht + Hm vs. no copy | 1.12 (0.56–2.23) | - | 1.16 (0.40–3.37) | |
| *2 (GCCC) | ||||
| Ht + Hm vs. no copy | 1.60 (0.80–3.20) | 1.67 (0.44–6.38) | 1.02 (0.37–2.85) | |
| *3/*4 | ||||
| Ht vs. *4Hm | 2.13 (1.13–4.00) * | 0.83 (0.16–4.19) | 1.77 (0.53–5.88) | |
| *3Hm vs. *4Hm | 2.81 (1.29–6.10) ** | 1.34 (0.20–8.66) | 2.39 (0.61–9.45) | |
| TGFB1 Haplotypes | TGFB1 Plasma Level (pg/mL) | ||||||
|---|---|---|---|---|---|---|---|
| n | HPV-Uninfected | p | n | HPV-Infected | p | ||
| *4 (GCTG) | |||||||
| Hm | 60 | 3369.30 (3167.17) | 28 | 4762.51 (4959.49) | |||
| Ht | 77 | 2888.33 (2858.02) | 0.55 | 73 | 4565.70 (4093.63) | 0.72 | |
| No copy | 48 | 2891.69 (3338.91) | 46 | 4352.38 (4697.96) | |||
| Ht + Hm | 137 | 3004.68 (3076.96) | 0.75 | 101 | 4576.46 (4289.30) | 0.73 | |
| No copy | 48 | 2891.69 (3338.91) | 46 | 4352.38 (4697.96) | |||
| *3 (GTCG) | |||||||
| Hm | 22 | 2344.52 (3189.62) | 22 | 4766.67 (4046.06) | |||
| Ht | 72 | 3084.49 (3667.38) | 0.27 | 63 | 3067.13 (4200.20) A | 0.03 | |
| No copy | 91 | 3114.03 (3151.74) | 62 | 4836.23 (4313.38) A | |||
| Hm + Ht | 94 | 2891.69 (3124.40) | 0.45 | 85 | 3993.99 (4173.05) | 0.04 | |
| No copy | 91 | 3114.03 (3151.74) | 62 | 4836.23 (4313.38) | |||
| *5B (ACTG) | |||||||
| Hm + Ht | 20 | 3528.98 (3796.48) | 0.17 | 18 | 2474.66 (4087.90) | 0.27 | |
| No copy | 165 | 2908.18 (2983.04) | 129 | 4706.97 (4414.91) | |||
| *2 (GCCC) | |||||||
| Hm + Ht | 16 | 2298.79 (2713.65) | 0.45 | 21 | 4831.49 (4719.33) | 0.45 | |
| No copy | 169 | 2974.81 (3205.66) | 126 | 4548.31 (4189.74) | |||
| *3 vs. *4 | |||||||
| *3 Hm | 22 | 2344.52 (3189.62) | 22 | 4766.67 (4046.06) | |||
| Ht | 54 | 2984.74 (3091.14) | 0.22 | 44 | 3403.58 (3793.59) | 0.23 | |
| *4 Hm | 60 | 3369.30 (3167.17) | 28 | 4762.51 (4959.49) | |||
| *5B vs. *4 | |||||||
| *5B Hm | 2 | 4838.50 (---) | - | - | |||
| Ht | 7 | 3023.64 (3337.03) | 9 | 3452.63 (3521.60) | 0.39 | ||
| *4 Hm | 60 | 3369.30 (3167.17) | 28 | 4762.51 (4959.49) | |||
| TGFB1 Haplotypes | TGFB1 Cervical Level (pg/mL) | ||||||
|---|---|---|---|---|---|---|---|
| n | HPV-Uninfected | p | n | HPV-Infected | p | ||
| *4 (GCTG) | |||||||
| Hm | 38 | 35.28 (50.71) | 11 | 66.67 (112.24) | |||
| Ht | 46 | 29.02 (75.07) | 0.87 | 20 | 53.17 (49.93) | 0.28 | |
| No copy | 27 | 36.37 (38.99) | 17 | 47.45 (64.76) | |||
| Ht + Hm | 84 | 31.29 (68.09) | 0.88 | 31 | 60.33 (49.99) | 0.24 | |
| No copy | 27 | 36.37 (38.99) | 17 | 47.45 (64.76) | |||
| *3 (GTCG) | |||||||
| Hm | 13 | 29.42 (31.88) | 7 | 47.45 (64.83) | |||
| Ht | 39 | 29.06 (77.90) | 0.68 | 23 | 52.85 (42.88) | 0.68 | |
| No copy | 59 | 35.34 (51.42) | 18 | 63.50 (69.55) | |||
| Hm + Ht | 52 | 29.24 (53.16) | 0.43 | 30 | 52.82 (55.31) | 0.38 | |
| No copy | 59 | 35.34 (51.42) | 18 | 63.50 (69.55) | |||
| *5B (ACTG) | |||||||
| Hm + Ht | 9 | 30.00 (56.74) | 0.83 | 5 | 68.05 (111.38) | 0.87 | |
| No copy | 102 | 33.90 (55.08) | 43 | 53.15 (51.66) | |||
| *2 (GCCC) | |||||||
| Hm + Ht | 12 | 65.05 (86.80) | 0.10 | 5 | 52.84 (67.79) | 0.95 | |
| No copy | 99 | 29.42 (53.92) | 43 | 53.19 (58.06) | |||
| *3 vs. *4 | |||||||
| *3 Hm | 13 | 29.42 (31.88) | 7 | 47.45 (64.83) | |||
| Ht | 30 | 25.03 (98.54) | 0.69 | 14 | 53.17 (55.35) | 0.45 | |
| *4 Hm | 38 | 35.28 (50.71) | 11 | 66.67 (112.24) | |||
| *5B vs. *4 | |||||||
| *5B Hm | 1 | - | |||||
| Ht | 3 | 30.00 (---) | 3 | 68.05 (---) | --- | ||
| *4 Hm | 38 | 35.28 (50.71) | 11 | 66.67 (112.24) | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trugilo, K.P.; Cebinelli, G.C.M.; Pereira, É.R.; Okuyama, N.C.M.; Cezar-dos-Santos, F.; Castilha, E.P.; Flauzino, T.; Hoch, V.B.-B.; Watanabe, M.A.E.; Guembarovski, R.L.; et al. Haplotype Structures and Protein Levels of TGFB1 in HPV Infection and Cervical Lesion: A Case-Control Study. Cells 2023, 12, 84. https://doi.org/10.3390/cells12010084
Trugilo KP, Cebinelli GCM, Pereira ÉR, Okuyama NCM, Cezar-dos-Santos F, Castilha EP, Flauzino T, Hoch VB-B, Watanabe MAE, Guembarovski RL, et al. Haplotype Structures and Protein Levels of TGFB1 in HPV Infection and Cervical Lesion: A Case-Control Study. Cells. 2023; 12(1):84. https://doi.org/10.3390/cells12010084
Chicago/Turabian StyleTrugilo, Kleber Paiva, Guilherme Cesar Martelossi Cebinelli, Érica Romão Pereira, Nádia Calvo Martins Okuyama, Fernando Cezar-dos-Santos, Eliza Pizarro Castilha, Tamires Flauzino, Valéria Bumiller-Bini Hoch, Maria Angelica Ehara Watanabe, Roberta Losi Guembarovski, and et al. 2023. "Haplotype Structures and Protein Levels of TGFB1 in HPV Infection and Cervical Lesion: A Case-Control Study" Cells 12, no. 1: 84. https://doi.org/10.3390/cells12010084