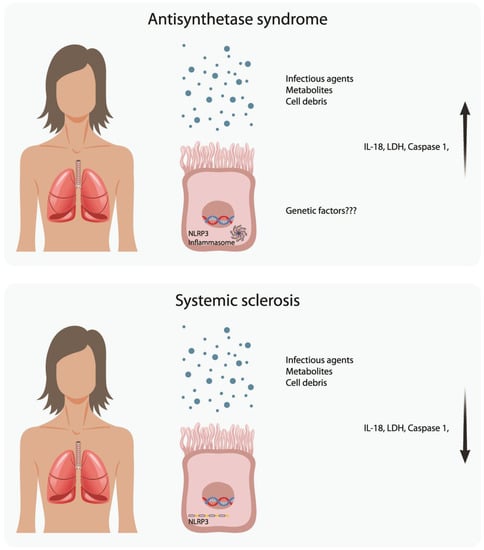
Enhanced Activity of NLRP3 Inflammasome in the Lung of Patients with Anti-Synthetase Syndrome
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients, Period, and Treatment
2.2. Bronchoalveolar Lavage (BAL) Collection
2.3. High-Resolution Computed Tomography (HRCT) Evaluation
2.4. Autoantibodies
2.5. Cytokines’ Immunoassays
2.6. Caspase-1 Activity
2.7. Enzyme-Linked Immunosorbent Assay (ELISA)
2.8. Statistical Analysis
3. Results
3.1. Patients
3.2. IL-18 and IFN-γ Levels Were Higher in the BAL of ASSD Patients
3.3. LDH Concentration and Caspase-1 Activity Were Higher in the BAL of ASSD Patients
3.4. LDH Was Significantly Correlated with Caspase-1 and IL-18 in ASSD
3.5. The Extent of Fibrosis Was Higher in SSc Patients
3.6. NLRP3 Was Significantly Correlated with the Extent of Lung Disease in ASSD Patients
4. Discussion
4.1. Autoantibodies
4.2. Scanning for Inflammasome Activation
4.3. Comparison with SSc
4.4. Other Autoimmune Entities and the Possible Role of the Inflammasome in the Progression of ILD
4.5. HRCT Findings
4.6. Therapeutic Perspective
4.7. Strengths and Limitations of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Patel, H.C.; Lauder, N.N. The antisynthetase syndrome. Am. J. Med. 2011, 124, e3–e4. [Google Scholar] [CrossRef] [PubMed]
- Witt, L.J.; Curran, J.J.; Strek, M.E. The Diagnosis and Treatment of Antisynthetase Syndrome. Clin. Pulm. Med. 2016, 23, 218–226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Debray, M.P.; Borie, R.; Revel, M.P.; Naccache, J.M.; Khalil, A.; Toper, C.; Israel-Biet, D.; Estellat, C.; Brillet, P.Y. Interstitial lung disease in anti-synthetase syndrome: Initial and follow-up CT findings. Eur. J. Radiol. 2015, 84, 516–523. [Google Scholar] [CrossRef] [PubMed]
- Kalluri, M.; Sahn, S.A.; Oddis, C.V.; Gharib, S.L.; Christopher-Stine, L.; Danoff, S.K.; Casciola-Rosen, L.; Hong, G.; Dellaripa, P.F.; Highland, K.B. Clinical profile of anti-PL-12 autoantibody. Cohort study and review of the literature. Chest 2009, 135, 1550–1556. [Google Scholar] [CrossRef]
- Richards, T.J.; Eggebeen, A.; Gibson, K.; Yousem, S.; Fuhrman, C.; Gochuico, B.R.; Fertig, N.; Oddis, C.V.; Kaminski, N.; Rosas, I.O.; et al. Characterization and peripheral blood biomarker assessment of anti-Jo-1 antibody-positive interstitial lung disease. Arthritis Rheum. 2009, 60, 2183–2192. [Google Scholar] [CrossRef] [Green Version]
- Danoff, S.K.; Casciola-Rosen, L. The lung as a possible target for the immune reaction in myositis. Arthritis Res. Ther. 2011, 13, 230. [Google Scholar] [CrossRef] [Green Version]
- Ponce-Gallegos, M.A.; Ramos-Martinez, E.; Garcia-Carmona, A.; Mejia, M.; Nava-Quiroz, K.J.; Perez-Rubio, G.; Ambrocio-Ortiz, E.; Gonzalez-Perez, M.I.; Buendia-Roldan, I.; Rojas-Serrano, J.; et al. Genetic Susceptibility to Antisynthetase Syndrome Associated With Single-Nucleotide Variants in the IL1B Gene That Lead Variation in IL-1beta Serum Levels. Front. Med. (Lausanne) 2020, 7, 547186. [Google Scholar] [CrossRef]
- Levine, S.M.; Raben, N.; Xie, D.; Askin, F.B.; Tuder, R.; Mullins, M.; Rosen, A.; Casciola-Rosen, L.A. Novel conformation of histidyl-transfer RNA synthetase in the lung: The target tissue in Jo-1 autoantibody-associated myositis. Arthritis Rheum. 2007, 56, 2729–2739. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, Z.; Ruan, J.; Pan, Y.; Magupalli, V.G.; Wu, H.; Lieberman, J. Inflammasome-activated gasdermin D causes pyroptosis by forming membrane pores. Nature 2016, 535, 153–158. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Huang, H.; Liu, B.; Zhang, Y.; Pan, X.; Yu, X.Y.; Shen, Z.; Song, Y.H. Inflammasomes as therapeutic targets in human diseases. Signal Transduct. Target. Ther. 2021, 6, 247. [Google Scholar] [CrossRef]
- Ramos-Martinez, E.; Falfan-Valencia, R.; Perez-Rubio, G.; Mejia, M.; Buendia-Roldan, I.; Gonzalez-Perez, M.I.; Mateos-Toledo, H.N.; Rojas Serrano, J. Anti-Aminoacyl Transfer-RNA-Synthetases (Anti-tRNA) Autoantibodies Associated with Interstitial Lung Disease: Pulmonary Disease Progression has a Persistent Elevation of the Th17 Cytokine Profile. J. Clin. Med. 2020, 9, 1356. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, H.M.; Wanderer, A.A. Inflammasome and IL-1beta-mediated disorders. Curr. Allergy Asthma Rep. 2010, 10, 229–235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guey, B.; Bodnar, M.; Manie, S.N.; Tardivel, A.; Petrilli, V. Caspase-1 autoproteolysis is differentially required for NLRP1b and NLRP3 inflammasome function. Proc. Natl. Acad. Sci. USA 2014, 111, 17254–17259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lundberg, I.E.; Tjarnlund, A.; Bottai, M.; Werth, V.P.; Pilkington, C.; Visser, M.; Alfredsson, L.; Amato, A.A.; Barohn, R.J.; Liang, M.H.; et al. 2017 European League Against Rheumatism/American College of Rheumatology classification criteria for adult and juvenile idiopathic inflammatory myopathies and their major subgroups. Ann. Rheum. Dis. 2017, 76, 1955–1964. [Google Scholar] [CrossRef] [PubMed]
- Bohan, A.; Peter, J.B. Polymyositis and dermatomyositis (first of two parts). N. Engl. J. Med. 1975, 292, 344–347. [Google Scholar] [CrossRef]
- Bohan, A.; Peter, J.B. Polymyositis and dermatomyositis (second of two parts). N. Engl. J. Med. 1975, 292, 403–407. [Google Scholar] [CrossRef] [Green Version]
- Gallay, L.; Gayed, C.; Hervier, B. Antisynthetase syndrome pathogenesis: Knowledge and uncertainties. Curr. Opin. Rheumatol. 2018, 30, 664–673. [Google Scholar] [CrossRef]
- Van Den Hoogen, F.; Khanna, D.; Fransen, J.; Johnson, S.R.; Baron, M.; Tyndall, A.; Matucci-Cerinic, M.; Naden, R.P.; Medsger, T.A., Jr.; Carreira, P.E. 2013 classification criteria for systemic sclerosis: An American College of Rheumatology/European League against Rheumatism collaborative initiative. Arthritis Rheum. 2013, 65, 2737–2747. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez-Perez, M.I.; Mejia-Hurtado, J.G.; Perez-Roman, D.I.; Buendia-Roldan, I.; Mejia, M.; Falfan-Valencia, R.; Mateos-Toledo, H.N.; Rojas-Serrano, J. Evolution of Pulmonary Function in a Cohort of Patients with Interstitial Lung Disease and Positive for Antisynthetase Antibodies. J. Rheumatol. 2020, 47, 415–423. [Google Scholar] [CrossRef]
- Liaskos, C.; Marou, E.; Simopoulou, T.; Gkoutzourelas, A.; Barmakoudi, M.; Efthymiou, G.; Scheper, T.; Meyer, W.; Katsiari, C.G.; Bogdanos, D.P.; et al. Multiparametric autoantibody profiling of patients with systemic sclerosis in Greece. Mediterr. J. Rheumatol. 2018, 29, 120–126. [Google Scholar] [CrossRef]
- Marie, I.; Josse, S.; Decaux, O.; Diot, E.; Landron, C.; Roblot, P.; Jouneau, S.; Hatron, P.Y.; Hachulla, E.; Vittecoq, O.; et al. Clinical manifestations and outcome of anti-PL7 positive patients with antisynthetase syndrome. Eur. J. Intern. Med. 2013, 24, 474–479. [Google Scholar] [CrossRef] [PubMed]
- Marie, I.; Josse, S.; Decaux, O.; Dominique, S.; Diot, E.; Landron, C.; Roblot, P.; Jouneau, S.; Hatron, P.Y.; Tiev, K.P.; et al. Comparison of long-term outcome between anti-Jo1- and anti-PL7/PL12 positive patients with antisynthetase syndrome. Autoimmun. Rev. 2012, 11, 739–745. [Google Scholar] [CrossRef] [PubMed]
- Yi, Y.S. Role of inflammasomes in inflammatory autoimmune rheumatic diseases. Korean J. Physiol. Pharmacol. Off. J. Korean Physiol. Soc. Korean Soc. Pharmacol. 2018, 22, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Evavold, C.L.; Kagan, J.C. How Inflammasomes Inform Adaptive Immunity. J. Mol. Biol. 2018, 430, 217–237. [Google Scholar] [CrossRef] [PubMed]
- Alehashemi, S.; Goldbach-Mansky, R. Human Autoinflammatory Diseases Mediated by NLRP3-, Pyrin-, NLRP1-, and NLRC4-Inflammasome Dysregulation Updates on Diagnosis, Treatment, and the Respective Roles of IL-1 and IL-18. Front. Immunol. 2020, 11, 1840. [Google Scholar] [CrossRef]
- Hayward, J.A.; Mathur, A.; Ngo, C.; Man, S.M. Cytosolic Recognition of Microbes and Pathogens: Inflammasomes in Action. Microbiol. Mol. Biol. Rev. MMBR 2018, 82, e00015-18. [Google Scholar] [CrossRef] [Green Version]
- Perelas, A.; Silver, R.M.; Arrossi, A.V.; Highland, K.B. Systemic sclerosis-associated interstitial lung disease. Lancet. Respir. Med. 2020, 8, 304–320. [Google Scholar] [CrossRef]
- Denton, C.P.; Khanna, D. Systemic sclerosis. Lancet 2017, 390, 1685–1699. [Google Scholar] [CrossRef]
- Lin, C.; Jiang, Z.; Cao, L.; Zou, H.; Zhu, X. Role of NLRP3 inflammasome in systemic sclerosis. Arthritis Res. Ther. 2022, 24, 196. [Google Scholar] [CrossRef]
- Dieude, P.; Guedj, M.; Wipff, J.; Ruiz, B.; Riemekasten, G.; Airo, P.; Melchers, I.; Hachulla, E.; Cerinic, M.M.; Diot, E.; et al. NLRP1 influences the systemic sclerosis phenotype: A new clue for the contribution of innate immunity in systemic sclerosis-related fibrosing alveolitis pathogenesis. Ann. Rheum. Dis. 2011, 70, 668–674. [Google Scholar] [CrossRef]
- Bhattacharyya, S.; Kelley, K.; Melichian, D.S.; Tamaki, Z.; Fang, F.; Su, Y.; Feng, G.; Pope, R.M.; Budinger, G.R.; Mutlu, G.M.; et al. Toll-like receptor 4 signaling augments transforming growth factor-beta responses: A novel mechanism for maintaining and amplifying fibrosis in scleroderma. Am. J. Pathol. 2013, 182, 192–205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pattanaik, D.; Brown, M.; Postlethwaite, B.C.; Postlethwaite, A.E. Pathogenesis of Systemic Sclerosis. Front. Immunol. 2015, 6, 272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Reilly, S. Innate immunity in systemic sclerosis pathogenesis. Clin. Sci. 2014, 126, 329–337. [Google Scholar] [CrossRef] [PubMed]
- Okamura, Y.; Watari, M.; Jerud, E.S.; Young, D.W.; Ishizaka, S.T.; Rose, J.; Chow, J.C.; Strauss, J.F., 3rd. The extra domain A of fibronectin activates Toll-like receptor 4. J. Biol. Chem. 2001, 276, 10229–10233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gonzalo-Gil, E.; Galindo-Izquierdo, M. Role of transforming growth factor-beta (TGF) beta in the physiopathology of rheumatoid arthritis. Reumatol. Clin. 2014, 10, 174–179. [Google Scholar] [CrossRef]
- Wong, C.K.; Ho, C.Y.; Li, E.K.; Tam, L.S.; Lam, C.W. Elevated production of interleukin-18 is associated with renal disease in patients with systemic lupus erythematosus. Clin. Exp. Immunol. 2002, 130, 345–351. [Google Scholar] [CrossRef]
- Kiripolsky, J.; McCabe, L.G.; Kramer, J.M. Innate immunity in Sjogren’s syndrome. Clin. Immunol. 2017, 182, 4–13. [Google Scholar] [CrossRef]
- Harden, L.M.; du Plessis, I.; Poole, S.; Laburn, H.P. Interleukin (IL)-6 and IL-1 beta act synergistically within the brain to induce sickness behavior and fever in rats. Brain Behav. Immun. 2008, 22, 838–849. [Google Scholar] [CrossRef]
- Mansoori, M.N.; Shukla, P.; Kakaji, M.; Tyagi, A.M.; Srivastava, K.; Shukla, M.; Dixit, M.; Kureel, J.; Gupta, S.; Singh, D. IL-18BP is decreased in osteoporotic women: Prevents Inflammasome mediated IL-18 activation and reduces Th17 differentiation. Sci. Rep. 2016, 6, 33680. [Google Scholar] [CrossRef] [Green Version]
- Alyaseer, A.A.A.; de Lima, M.H.S.; Braga, T.T. The Role of NLRP3 Inflammasome Activation in the Epithelial to Mesenchymal Transition Process During the Fibrosis. Front. Immunol. 2020, 11, 883. [Google Scholar] [CrossRef]
- Liu, Y.; Gao, X.; Miao, Y.; Wang, Y.; Wang, H.; Cheng, Z.; Wang, X.; Jing, X.; Jia, L.; Dai, L.; et al. NLRP3 regulates macrophage M2 polarization through up-regulation of IL-4 in asthma. Biochem. J. 2018, 475, 1995–2008. [Google Scholar] [CrossRef] [PubMed]
- De Carvalho, R.V.H.; Silva, A.L.N.; Santos, L.L.; Andrade, W.A.; de Sa, K.S.G.; Zamboni, D.S. Macrophage priming is dispensable for NLRP3 inflammasome activation and restriction of Leishmania amazonensis replication. J. Leukoc. Biol. 2019, 106, 631–640. [Google Scholar] [CrossRef] [PubMed]
- Abbate, A.; Trankle, C.R.; Buckley, L.F.; Lipinski, M.J.; Appleton, D.; Kadariya, D.; Canada, J.M.; Carbone, S.; Roberts, C.S.; Abouzaki, N.; et al. Interleukin-1 Blockade Inhibits the Acute Inflammatory Response in Patients With ST-Segment-Elevation Myocardial Infarction. J. Am. Heart Assoc. 2020, 9, e014941. [Google Scholar] [CrossRef]
- Abbate, A.; Toldo, S.; Marchetti, C.; Kron, J.; Van Tassell, B.W.; Dinarello, C.A. Interleukin-1 and the Inflammasome as Therapeutic Targets in Cardiovascular Disease. Circ. Res. 2020, 126, 1260–1280. [Google Scholar] [CrossRef] [PubMed]
- Vidal-Vanaclocha, F.; Amezaga, C.; Asumendi, A.; Kaplanski, G.; Dinarello, C.A. Interleukin-1 receptor blockade reduces the number and size of murine B16 melanoma hepatic metastases. Cancer Res. 1994, 54, 2667–2672. [Google Scholar] [PubMed]
- Ruscitti, P.; Masedu, F.; Alvaro, S.; Airo, P.; Battafarano, N.; Cantarini, L.; Cantatore, F.P.; Carlino, G.; D’Abrosca, V.; Frassi, M.; et al. Anti-interleukin-1 treatment in patients with rheumatoid arthritis and type 2 diabetes (TRACK): A multicentre, open-label, randomised controlled trial. PLoS Med. 2019, 16, e1002901. [Google Scholar] [CrossRef]
- Quartier, P.; Alexeeva, E.; Constantin, T.; Chasnyk, V.; Wulffraat, N.; Palmblad, K.; Wouters, C.; H, I.B.; Marzan, K.; Schneider, R.; et al. Tapering Canakinumab Monotherapy in Patients With Systemic Juvenile Idiopathic Arthritis in Clinical Remission: Results From a Phase IIIb/IV Open-Label, Randomized Study. Arthritis Rheumatol. 2021, 73, 336–346. [Google Scholar] [CrossRef]


| Variable | ASSD n = 19 | SSc n = 17 | p-Value |
|---|---|---|---|
| Women * | 12 (63%) | 12 (70%) | 0.7 |
| Age ** | 63 (52–69) | 65 (63–70) | 0.15 |
| Arterial * hypertension | 6(31%) | 5(26%) | 0.61 |
| Type 2 diabetes * | 4 (22%) | 6 (33%) | 0.35 |
| Obesity * | 5 (26%) | 2 (10%) | 0.26 |
| PH * | 3 (15%) | 3 (15%) | 0.67 |
| Clinical characteristics | |||
| Fever * | 5 (27%) | 0 | 0.047 |
| Cough * | 15 (83%) | 15 (83%) | 0.73 |
| Dyspnoea * | 16 (88%) | 15(83%) | 0.54 |
| Mechanic’s Hands * | 6 (33%) | 0 | 0.02 |
| Arthralgias * | 6 (33%) | 4 (22%) | 0.71 |
| Muscular Weakness * | 5 (26%) | 1(6%) | 0.18 |
| Xerostomy * | 7 (38%) | 7 (38%) | 0.92 |
| Dysphagia * | 0 | 4 (23%) | 0.02 |
| Cutaneous sclerosis * | 0 | 5 (29%) | 0.01 |
| Clubbing * | 3 (16%) | 4 (24%) | 0.56 |
| Bronchoalveolar lavage characteristics | |||
| Macrophages (%) ** | 76 (45–90) | 90 (81–94) | 0.02 |
| Lymphocytes (%) ** | 22 (9.5–43) | 9.75 (5–18) | 0.03 |
| Neutrophils (%) ** | 0 (0–0.5) | 0 (0–1) | 0.1 |
| Eosinophils (%) ** | 0.25 (0–1.5) | 0.25 (0–0.75) | 0.7 |
| Autoantibody | ASSDn = 19 | SScn = 17 | p-Value |
|---|---|---|---|
| Anti-Jo1 | 4 (22%) | 0 | 0.06 |
| Anti-OJ | 6 (33%) | 0 (0%) | 0.014 |
| Anti-PL7 | 8 (42%) | 0 | 0.002 |
| Anti-PL12 | 4 (22%) | 0 | 0.06 |
| Anti-Ej | 3 (11%) | 0 | 0.13 |
| Anti-PM SCL-75 | 3 (16%) | 1 (5.5%) | 0.34 |
| Anti-Mi-2 | 3 (16%) | 3 (17%) | 0.61 |
| Anti-PM SCL-100 | 2 (11%) | 2 (12%) | 0.67 |
| Anti-TH/To | 1 (5.5%) | 12 (70%) | 0.001 |
| Anti-Fibrillarin | 1 (5.5%) | 5 (29%) | 0.067 |
| Anti-Ro52 | 3 (16%) | 2 (11%) | 0.55 |
| Anti-Nor90 | 1 (5.5%) | 4 (23%) | 0.13 |
| Anti-SCL-70 | 0 | 2 (11%) | 0.21 |
| Variable | ASSD n = 19 | SSc n = 17 | p-Value |
|---|---|---|---|
| LDH OD | 0.15 (0.14–0.19) | 0.09 (0.09–0.10) | 0.001 |
| Caspase-1 RFU | 1.25 (1.15–1.35) | 0.75 (0.62–1.14) | 0.003 |
| NLRP3 | 10.34 (9.76–10.93) | 10.69 (10.34–13.02) | 0.15 |
| IL-1β pg/mL | 1.44 (0.5–4.1) | 0.58 (0.45–1.11) | 0.07 |
| IL-2 pg/mL | 0.75 (0.75–0.79) | 0.75 (0.71–0.79) | 0.87 |
| IL-4 pg/mL | 0.31 (0.26–0.46) | 0.29 (0.22–0.36) | 0.25 |
| IL-5 pg/mL (n = 13/5) | 0.33 (0.13–0.64) | 0.34 (0.33–0.44) | 0.72 |
| IL-6pg/mL (n =18/15) | 7.9 (4.4–21.24) | 8.18 (5.77–28.84) | 0.61 |
| IL-12 pg/mL | 0.41 (0.36–0.45) | 0.38 (0.34–0.39) | 0.11 |
| IL-13 pg/mL (n = 2/0) | 0.14 (0.14–0.31) | ------------------------- | ------- |
| IL-18 pg/mL | 1.42 (0.98–2.65) | 0.87 (0.62–1.14) | 0.02 |
| IFN-γ | 0.9 (0.9–1.09) | 0.86 (0.81–0.95) | 0.01 |
| TNF-α pg/mL | 0.47 (0.38–0.69) | 0.45 (0.38–0.52) | 0.50 |
| Variable | Both Groups n = 38 | ASSD n = 19 | SSc n = 17 |
|---|---|---|---|
| Caspase-1/LDH | Rho = 0.59 p = 0.0001 | Rho = 0.58 p = 0.008 | Rho = 0.15 p = 0.56 |
| Caspase-1/IL-1β | Rho = 0.43 p = 0.007 | Rho = 0.34 p = 0.14 | Rho = 0.28 p = 0.25 |
| Caspase-1/IL-18 | Rho = 0.69 p = 0.001 | Rho = 0.19 p = 0.42 | Rho = 0.40 p = 0.10 |
| NLRP3/Caspase-1 | Rho = -0.15 p = 0.38 | Rho = -0.27 p = 0.27 | Rho = 0.11 p = 0.65 |
| NLRP3/LDH | Rho = −0.32 p = 0.05 | Rho = −0.42 p = 0.07 | Rho = −0.06 p = 0.81 |
| NLRP3/IL-1β | Rho = −0.10 p = 0.55 | Rho = 0.06 p = 0.80 | Rho = −0.12 p = 0.63 |
| NLRP3/IL-18 | Rho = −0.17 p = 0.30 | Rho = −0.29 p = 0.23 | Rho = 0.15 p = 0.56 |
| IL-1β/IL-18 | Rho = 0.69 p = >0.001 | Rho = 0.60 p = 0.006 | Rho = 0.72 p = 0.001 |
| IL-1β/LHD | Rho = 0.36 p = 0.02 | Rho = 0.26 p = 0.26 | Rho = 0.15 p = 0.55 |
| IL-18/LHD | Rho = 0.55 p = 0.0004 | Rho = 0.55 p = 0.01 | Rho = 0.39 p = 0.11 |
| IFN-γ/IL-18 | Rho = 0.57 p = >0.001 | Rho = 0.67 p = 0.001 | Rho = 0.24 p = 0.33 |
| Variable | Both Groups n = 38 | ASSD n = 19 | SSc n = 17 |
|---|---|---|---|
| Caspase-1/macrophages | Rho = 0.36 p = 0.83 | Rho = 0.10 p = 0.66 | Rho = 0.39 p = 0.12 |
| Caspase-1/neutrophils | Rho = 0.12 p = 0.48 | Rho = −0.3 p = 0.87 | Rho = 0.85 p = 0.00 |
| Caspase-1/lymphocytes | Rho = −0.05 p = 0.75 | Rho = −0.08 p = 0.72 | Rho = −0.37 p = 0.13 |
| Caspase-1/eosinophils | Rho = −0.01 p = 0.91 | Rho = −0.11 p = 0.63 | Rho = −0.26 p = 0.30 |
| NLRP3/macrophages | Rho = 0.04 p = 0.80 | Rho = −0.09 p = 0.70 | Rho = −0.02 p = 0.91 |
| NLRP3/neutrophils | Rho = 0.28 p = 0.09 | Rho = 0.49 p = 0.03 | Rho = 0.53 p = 0.02 |
| NLRP3/lymphocytes | Rho = −0.14 p = 0.40 | Rho = −0.17 p = 0.49 | Rho = 0.03 p = 0.89 |
| NLRP3/eosinophils | Rho = −0.28 p = 0.18 | Rho = −0.21 p = 0.39 | Rho = −0.23 p = 0−35 |
| LDH/macrophages | Rho = −0.35 p = 0.03 | Rho = −0.11 p = 0.64 | Rho = 0.17 p = 0.50 |
| LDH/neutrophils | Rho = 0.05 p = 0.75 | Rho = −0.06 p = 0.77 | Rho = −0.18 p = 0.48 |
| LDH/lymphocytes | Rho = 0.35 p = 0.03 | Rho = 0.16 p = 0.51 | Rho = −0.14 p = 0.58 |
| LDH/eosinophils | Rho = 0.20 p = 0.22 | Rho = 0.10 p = 0.66 | Rho = −0.52 p = 0.02 |
| IL-1β/macrophages | Rho = −0.17 p = 0.32 | Rho = −0.08 p = 0.72 | Rho = 0.24 p = 0.33 |
| IL-1β/neutrophils | Rho = 0.42 p = 0.01 | Rho = 0.41 p = 0.08 | Rho = 0.11 p = 0.65 |
| IL-1β/lymphocytes | Rho = −0.01 p = 0.92 | Rho = −0.13 p = 0.58 | Rho = −0.24 p = 0.33 |
| IL-1β/eosinophils | Rho = −0.02 p = 0.88 | Rho = −0.08 p = 0.72 | Rho = 0.03 p = 0.89 |
| IL-18/macrophages | Rho = 0.11 p = 0.52 | Rho = 0.21 p = 0.37 | Rho = 0.24 p = 0.33 |
| IL-18/neutrophils | Rho = −0.03 p = 0.84 | Rho = −0.05 p = 0.80 | Rho = 0.57 p = 0.01 |
| IL-18/lymphocytes | Rho = −0.10 p = 0.54 | Rho = −0.20 p = 0.40 | Rho = −0.25 p = 0.32 |
| IL-18/eosinophils | Rho = −0.06 p = 0.71 | Rho = −0.10 p = 0.68 | Rho = 0.02 p = 0.92 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramos-Martinez, E.; Vega-Sánchez, A.E.; Pérez-Rubio, G.; Mejia, M.; Buendía-Roldán, I.; González-Pérez, M.I.; Mateos-Toledo, H.N.; Andrade, W.A.; Falfán-Valencia, R.; Rojas-Serrano, J. Enhanced Activity of NLRP3 Inflammasome in the Lung of Patients with Anti-Synthetase Syndrome. Cells 2023, 12, 60. https://doi.org/10.3390/cells12010060
Ramos-Martinez E, Vega-Sánchez AE, Pérez-Rubio G, Mejia M, Buendía-Roldán I, González-Pérez MI, Mateos-Toledo HN, Andrade WA, Falfán-Valencia R, Rojas-Serrano J. Enhanced Activity of NLRP3 Inflammasome in the Lung of Patients with Anti-Synthetase Syndrome. Cells. 2023; 12(1):60. https://doi.org/10.3390/cells12010060
Chicago/Turabian StyleRamos-Martinez, Espiridión, Angel E. Vega-Sánchez, Gloria Pérez-Rubio, Mayra Mejia, Ivette Buendía-Roldán, Montserrat I. González-Pérez, Heidegger N. Mateos-Toledo, Warrison A. Andrade, Ramcés Falfán-Valencia, and Jorge Rojas-Serrano. 2023. "Enhanced Activity of NLRP3 Inflammasome in the Lung of Patients with Anti-Synthetase Syndrome" Cells 12, no. 1: 60. https://doi.org/10.3390/cells12010060