When Electrospun Fiber Support Matters: In Vitro Ovine Long-Term Folliculogenesis on Poly (Epsilon Caprolactone) (PCL)-Patterned Fibers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Scaffold Fabrication
Poly(Epsilon-Caprolactone) (PCL) Patterned Electrospun Scaffolds/(PCL) Randomic Electrospun Scaffolds
2.3. Isolation, Morphological Evaluation, and In Vitro Culture of PA Follicles
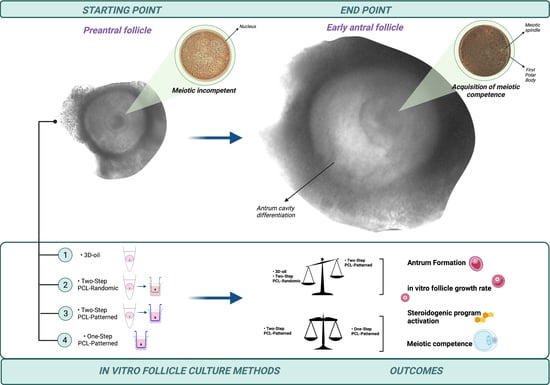
2.4. In Vitro PA Follicles Culture Protocols Comparison
2.5. In Vitro PA Follicles Culture Outcomes
2.5.1. Morphological and Functional Analysis of In Vitro Follicle Development
2.5.2. Meiotic Competence Acquisition of ivF Grown Oocytes
2.6. Statistical Analysis
3. Results
3.1. Patterned PCL Topology Enabling Long Term In Vitro Follicle PA Development in Ovine Model
3.1.1. Two-Step PCL Patterned In Vitro Follicles Culture Protocols Was Able to Support PA Follicle Growth and Antrum Formation
3.1.2. Follicle Gene Expression Profile Was Positively Regulated by Two-Step PCL-Patterned In Vitro Follicles Culture-Based Protocols
3.1.3. PCL-Patterned Scaffolds Support a Synergic Follicle and Oocytes Development
3.2. Two and One-Step PCL-Patterned-Based Protocols Showed the Same Ability to Support the Synergic In Vitro Growth of Follicle and Oocyte Compartments
Similar Morphological Performances of In Vitro Grown PA Follicles Cultured under Two- and One-Step PCL-Patterned In Vitro Follicles Culture Protocols
3.3. Steroidogenic and Somatic Specific Gene Expression on In Vitro Follicles Culture: Two-Step vs. One-Step PCL-Patterned System
3.4. Both Two and One-Step-Derived Oocytes Achieved on Fibrous Patterned Scaffolds a Complete Meiotic Competence
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dolmans, M.M.; Amorim, C.A. Construction and Use of Artificial Ovaries. Reproduction 2019, 158, F15–F25. [Google Scholar] [CrossRef] [Green Version]
- Gougeon, A. Regulation of Ovarian Follicular Development in Primates: Facts and Hypotheses. Endocr. Rev. 1996, 17, 121–155. [Google Scholar] [CrossRef] [PubMed]
- Scaramuzzi, R.J.; Baird, D.T.; Campbell, B.K.; Driancourt, M.A.; Dupont, J.; Fortune, J.E.; Gilchrist, R.B.; Martin, G.B.; McNatty, K.P.; McNeilly, A.S.; et al. Regulation of Folliculogenesis and the Determination of Ovulation Rate in Ruminants. Reprod. Fertil. Dev. 2011, 23, 444–467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adamson, G.D.; de Mouzon, J.; Chambers, G.M.; Zegers-Hochschild, F.; Mansour, R.; Ishihara, O.; Banker, M.; Dyer, S. International Committee for Monitoring Assisted Reproductive Technology: World Report on Assisted Reproductive Technology, 2011. Fertil. Steril. 2018, 110, 1067–1080. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ori, S. International Federation of Fertility Societies’ Surveillance International Federation of Fertility Societies’ Surveillance (IFFS). In Global Trends in Reproductive Policy and Practice, 8th ed.; IFFS: Royal, NJ, USA, 2019; Volume 4. [Google Scholar] [CrossRef]
- Ovarian, C.; Society, A.; Medicine, R.; Lancet, T. Live Birth Rate After Otc and Reimplantation in a Series of 111 Women Gnrh-a: Questions Remain. Well 2015, 104, 1097–1098. [Google Scholar]
- Meirow, D.; Ra’anani, H.; Shapira, M.; Brenghausen, M.; Derech Chaim, S.; Aviel-Ronen, S.; Amariglio, N.; Schiff, E.; Orvieto, R.; Dor, J. Transplantations of Frozen-Thawed Ovarian Tissue Demonstrate High Reproductive Performance and the Need to Revise Restrictive Criteria. Fertil. Steril. 2016, 106, 467–474. [Google Scholar] [CrossRef] [Green Version]
- Van DerVen, H.; Liebenthron, J.; Beckmann, M.; Toth, B.; Korell, M.; Krüssel, J.; Frambach, T.; Kupka, M.; Hohl, M.K.; Winkler-Crepaz, K.; et al. Ninety-Five Orthotopic Transplantations in 74 Women of Ovarian Tissue after Cytotoxic Treatment in a Fertility Preservation Network: Tissue Activity, Pregnancy and Delivery Rates. Hum. Reprod. 2016, 31, 2031–2041. [Google Scholar] [CrossRef]
- Donnez, J.; Dolmans, M.-M. Fertility Preservation in Women. N. Engl. J. Med. 2017, 377, 1657–1665. [Google Scholar] [CrossRef]
- Jensen, A.K.; Macklon, K.T.; Fedder, J.; Ernst, E.; Humaidan, P.; Andersen, C.Y. 86 Successful Births and 9 Ongoing Pregnancies Worldwide in Women Transplanted with Frozen-Thawed Ovarian Tissue: Focus on Birth and Perinatal Outcome in 40 of These Children. J. Assist. Reprod. Genet. 2017, 34, 325–336. [Google Scholar] [CrossRef] [Green Version]
- Diaz-Garcia, C.; Domingo, J.; Garcia-Velasco, J.A.; Herraiz, S.; Mirabet, V.; Iniesta, I.; Cobo, A.; Remohí, J.; Pellicer, A. Oocyte Vitrification versus Ovarian Cortex Transplantation in Fertility Preservation for Adult Women Undergoing Gonadotoxic Treatments: A Prospective Cohort Study. Fertil. Steril. 2018, 109, 478–485.e2. [Google Scholar] [CrossRef] [Green Version]
- Silber, S.J.; DeRosa, M.; Goldsmith, S.; Fan, Y.; Castleman, L.; Melnick, J. Cryopreservation and Transplantation of Ovarian Tissue: Results from One Center in the USA. J. Assist. Reprod. Genet. 2018, 35, 2205–2213. [Google Scholar] [CrossRef] [Green Version]
- Soares, M.; Sahrari, K.; Amorim, C.A.; Saussoy, P.; Donnez, J.; Dolmans, M.M. Evaluation of a Human Ovarian Follicle Isolation Technique to Obtain Disease-Free Follicle Suspensions before Safely Grafting to Cancer Patients. Fertil. Steril. 2015, 104, 672–680.e2. [Google Scholar] [CrossRef] [PubMed]
- Barboni, B.; Russo, V.; Cecconi, S.; Curini, V.; Colosimo, A.; Garofalo, M.L.A.; Capacchietti, G.; Giacinto, O.; Mattioli, M. In Vitro Grown Sheep Preantral Follicles Yield Oocytes with Normal Nuclear-Epigenetic Maturation. PLoS ONE 2011, 6, e27550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McLaughlin, M.; Albertini, D.F.; Wallace, W.H.B.; Anderson, R.A.; Telfer, E.E. Metaphase II Oocytes from Human Unilaminar Follicles Grown in a Multistep Culture System. Mol. Hum. Reprod. 2018, 24, 135–142. [Google Scholar] [CrossRef] [Green Version]
- Costa, S.L.; Costa, E.P.; Pereira, E.C.M.; Benjamin, L.A.; Rodrigues, M.T.; Mendes, V.R.A.; Silva, T.F. Influence of Insulin-like Growth Factor I (IGF-I) on the Survival and the In Vitro Development of Caprine Preantral Follicles. Pesqui. Vet. Bras. 2014, 34, 1037–1044. [Google Scholar] [CrossRef] [Green Version]
- Telfer, E.E.; McLaughlin, M.; Ding, C.; Thong, K.J. A Two-Step Serum-Free Culture System Supports Development of Human Oocytes from Primordial Follicles in the Presence of Activin. Hum. Reprod. 2008, 23, 1151–1158. [Google Scholar] [CrossRef] [Green Version]
- Newton, H.; Picton, H.; Gosden, R.G. From Cryopreserved Ovine Tissue. Science 1997, 115, 141–150. [Google Scholar]
- Sun, J.; Li, X. Growth and Antrum Formation of Bovine Primary Follicles in Long-Term Culture In Vitro. Reprod. Biol. 2013, 13, 221–228. [Google Scholar] [CrossRef]
- Da Silva, G.M.; Rossetto, R.; Chaves, R.N.; Duarte, A.B.G.; Araújo, V.R.; Feltrin, C.; Bernuci, M.P.; Anselmo-Franci, J.A.; Xu, M.; Woodruff, T.K.; et al. In Vitro Development of Secondary Follicles from Pre-Pubertal and Adult Goats Cultured in Two-Dimensional or Three-Dimensional Systems. Zygote 2015, 23, 475–484. [Google Scholar] [CrossRef] [Green Version]
- Cecconi, S.; Barboni, B.; Coccia, M.; Mattioli, M. In Vitro Development of Sheep Preantral Follicles. Biol. Reprod. 1999, 60, 594–601. [Google Scholar] [CrossRef] [Green Version]
- Brito, I.R.; Lima, I.M.T.; Xu, M.; Shea, L.D.; Woodruff, T.K.; Figueiredo, J.R. Three-Dimensional Systems for In Vitro Follicular Culture: Overview of Alginate-Based Matrices. Reprod. Fertil. Dev. 2014, 26, 915–930. [Google Scholar] [CrossRef] [PubMed]
- Quan, N.; Mara, J.N.; Grover, A.R.; Pavone, M.E.; Duncan, F.E. Spatial Analysis of Growing Follicles in the Human Ovary to Inform Tissue Engineering Strategies. Tissue Eng. Part A 2020, 26, 733–746. [Google Scholar] [CrossRef] [PubMed]
- Pors, S.E.; Ramløse, M.; Nikiforov, D.; Lundsgaard, K.; Cheng, J.; Andersen, C.Y.; Kristensen, S.G. Initial Steps in Reconstruction of the Human Ovary: Survival of Pre-Antral Stage Follicles in a Decellularized Human Ovarian Scaffold. Hum. Reprod. 2019, 34, 1523–1535. [Google Scholar] [CrossRef] [PubMed]
- Laronda, M.M.; Rutz, A.L.; Xiao, S.; Whelan, K.A.; Duncan, F.E.; Roth, E.W.; Woodruff, T.K.; Shah, R.N. A Bioprosthetic Ovary Created Using 3D Printed Microporous Scaffolds Restores Ovarian Function in Sterilized Mice. Nat. Commun. 2017, 8, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Bakhshandeh, B.; Zarrintaj, P.; Oftadeh, M.O.; Keramati, F.; Fouladiha, H.; Sohrabi-Jahromi, S.; Ziraksaz, Z. Tissue Engineering; Strategies, Tissues, and Biomaterials. Biotechnol. Genet. Eng. Rev. 2017, 33, 144–172. [Google Scholar] [CrossRef]
- Sharma, P.; Kumar, P.; Sharma, R.; Bhatt, V.D.; Dhot, P.S. Tissue Engineering; Current Status & Futuristic Scope. J. Med. Life 2019, 12, 225–229. [Google Scholar] [CrossRef]
- Amorim, C.A. Special Issue Devoted to a New Field of Regenerative Medicine: Reproductive Tissue Engineering. Ann. Biomed. Eng. 2017, 45, 1589–1591. [Google Scholar] [CrossRef] [Green Version]
- Liverani, L.; Guarino, V.; La Carrubba, V.; Boccaccini, A.R. Porous Biomaterials and Scaffolds for Tissue Engineering. In Encyclopedia of Biomedical Engineering; Elsevier: Amsterdam, The Netherlands, 2019; pp. 188–202. ISBN 978-0-12-805144-3. [Google Scholar]
- Shikanov, A.; Zhang, Z.; Xu, M.; Smith, R.M.; Rajan, A.; Woodruff, T.K.; Shea, L.D. Fibrin Encapsulation and Vascular Endothelial Growth Factor Delivery Promotes Ovarian Graft Survival in Mice. Tissue Eng. Part A 2011, 17, 3095–3104. [Google Scholar] [CrossRef] [Green Version]
- Kniazeva, E.; Hardy, A.N.; Boukaidi, S.A.; Woodruff, T.K.; Jeruss, J.S.; Shea, L.D. Primordial Follicle Transplantation within Designer Biomaterial Grafts Produce Live Births in a Mouse Infertility Model. Sci. Rep. 2015, 5, 17709. [Google Scholar] [CrossRef] [Green Version]
- Carroll, J.; Gosden, R.G. Transplantation of Frozen-Thawed Mouse Primordial Follicles. Hum. Reprod. 1993, 8, 1163–1167. [Google Scholar] [CrossRef]
- Liverani, L.; Raffel, N.; Fattahi, A.; Preis, A.; Hoffmann, I.; Boccaccini, A.R.; Beckmann, M.W.; Dittrich, R. Electrospun Patterned Porous Scaffolds for the Support of Ovarian Follicles Growth: A Feasibility Study. Sci. Rep. 2019, 9, 1150. [Google Scholar] [CrossRef] [Green Version]
- Raffel, N.; Dittrich, R.; Bäuerle, T.; Seyler, L.; Fattahi, A.; Hoffmann, I.; Leal-Egaña, A.; Beckmann, M.W.; Boccaccini, A.R.; Liverani, L. Novel Approach for the Assessment of Ovarian Follicles Infiltration in Polymeric Electrospun Patterned Scaffolds. PLoS ONE 2019, 14, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Tamadon, A.; Park, K.-H.; Kim, Y.Y.; Kang, B.-C.; Ku, S.-Y. Efficient Biomaterials for Tissue Engineering of Female Reproductive Organs. Tissue Eng. Regen. Med. 2016, 13, 447–454. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, N.; Asawa, S.; Birru, B.; Baadhe, R.; Rao, S. PCL-Based Composite Scaffold Matrices for Tissue Engineering Applications. Mol. Biotechnol. 2018, 60, 506–532. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.B.; Kim, G.H. PCL/Alginate Composite Scaffolds for Hard Tissue Engineering: Fabrication, Characterization, and Cellular Activities. ACS Comb. Sci. 2015, 17, 87–99. [Google Scholar] [CrossRef]
- Rumiński, S.; Ostrowska, B.; Jaroszewicz, J.; Skirecki, T.; Włodarski, K.; Święszkowski, W.; Lewandowska-Szumieł, M. Three-Dimensional Printed Polycaprolactone-Based Scaffolds Provide an Advantageous Environment for Osteogenic Differentiation of Human Adipose-Derived Stem Cells: ADSC Osteogenesis in 3D Scaffolds. J. Tissue Eng. Regen. Med. 2018, 12, e473–e485. [Google Scholar] [CrossRef]
- Ronca, D.; Langella, F.; Chierchia, M.; D’Amora, U.; Russo, T.; Domingos, M.; Gloria, A.; Bartolo, P.; Ambrosio, L. Bone Tissue Engineering: 3D PCL-Based Nanocomposite Scaffolds with Tailored Properties. Procedia CIRP 2016, 49, 51–54. [Google Scholar] [CrossRef]
- Van Rie, J.; Declercq, H.; Van Hoorick, J.; Dierick, M.; Van Hoorebeke, L.; Cornelissen, R.; Thienpont, H.; Dubruel, P.; Van Vlierberghe, S. Cryogel-PCL Combination Scaffolds for Bone Tissue Repair. J. Mater. Sci. Mater. Med. 2015, 26, 123. [Google Scholar] [CrossRef]
- Yoshimoto, H.; Shin, Y.M.; Terai, H.; Vacanti, J.P. A Biodegradable Nanofiber Scaffold by Electrospinning and Its Potential for Bone Tissue Engineering. Biomaterials 2003, 24, 2077–2082. [Google Scholar] [CrossRef]
- Zhu, Y.; Wan, Y.; Zhang, J.; Yin, D.; Cheng, W. Manufacture of Layered Collagen/Chitosan-Polycaprolactone Scaffolds with Biomimetic Microarchitecture. Colloids Surf. B Biointerfaces 2014, 113, 352–360. [Google Scholar] [CrossRef]
- Lee, L.-W.; Hsiao, S.-H.; Hung, W.-C.; Lin, Y.-H.; Chen, P.-Y.; Chiang, C.-P. Clinical Outcomes for Teeth Treated with Electrospun Poly(ε-Caprolactone) Fiber Meshes/Mineral Trioxide Aggregate Direct Pulp Capping. J. Endod. 2015, 41, 628–636. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.-Y.; Hung, L.-H.; Chu, I.-M.; Ko, C.-S.; Lee, Y.-D. The Application of Type II Collagen and Chondroitin Sulfate Grafted PCL Porous Scaffold in Cartilage Tissue Engineering. J. Biomed. Mater. Res. 2010, 92, 712–723. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-J.; Lee, J.-H.; Im, G.-I. Chondrogenesis Using Mesenchymal Stem Cells and PCL Scaffolds. J. Biomed. Mater. Res. 2010, 92, 659–666. [Google Scholar] [CrossRef]
- Valonen, P.K.; Moutos, F.T.; Kusanagi, A.; Moretti, M.G.; Diekman, B.O.; Welter, J.F.; Caplan, A.I.; Guilak, F.; Freed, L.E. In Vitro Generation of Mechanically Functional Cartilage Grafts Based on Adult Human Stem Cells and 3D-Woven Poly(ɛ-Caprolactone) Scaffolds. Biomaterials 2010, 31, 2193–2200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cui, Z.; Wright, L.D.; Guzzo, R.; Freeman, J.W.; Drissi, H.; Nair, L.S. Poly( d -Lactide)/Poly(Caprolactone) Nanofiber-Thermogelling Chitosan Gel Composite Scaffolds for Osteochondral Tissue Regeneration in a Rat Model. J. Bioact. Compat. Polym. 2013, 28, 115–125. [Google Scholar] [CrossRef]
- Swieszkowski, W.; Tuan, B.H.S.; Kurzydlowski, K.J.; Hutmacher, D.W. Repair and Regeneration of Osteochondral Defects in the Articular Joints. Biomol. Eng. 2007, 24, 489–495. [Google Scholar] [CrossRef]
- Du, Y.; Liu, H.; Yang, Q.; Wang, S.; Wang, J.; Ma, J.; Noh, I.; Mikos, A.G.; Zhang, S. Selective Laser Sintering Scaffold with Hierarchical Architecture and Gradient Composition for Osteochondral Repair in Rabbits. Biomaterials 2017, 137, 37–48. [Google Scholar] [CrossRef]
- Mellor, L.F.; Huebner, P.; Cai, S.; Mohiti-Asli, M.; Taylor, M.A.; Spang, J.; Shirwaiker, R.A.; Loboa, E.G. Fabrication and Evaluation of Electrospun, 3D-Bioplotted, and Combination of Electrospun/3D-Bioplotted Scaffolds for Tissue Engineering Applications. BioMed Res. Int. 2017, 2017, 1–9. [Google Scholar] [CrossRef]
- Yeo, M.; Kim, G. Cell-Printed Hierarchical Scaffolds Consisting of Micro-Sized Polycaprolactone (PCL) and Electrospun PCL Nanofibers/Cell-Laden Alginate Struts for Tissue Regeneration. J. Mater. Chem. B 2014, 2, 314–324. [Google Scholar] [CrossRef]
- Hsieh, Y.-H.; Hsieh, M.-F.; Fang, C.-H.; Jiang, C.-P.; Lin, B.; Lee, H.-M. Osteochondral Regeneration Induced by TGF-β Loaded Photo Cross-Linked Hyaluronic Acid Hydrogel Infiltrated in Fused Deposition-Manufactured Composite Scaffold of Hydroxyapatite and Poly (Ethylene Glycol)-Block-Poly(ε-Caprolactone). Polymers 2017, 9, 182. [Google Scholar] [CrossRef] [Green Version]
- Firoozi, N.; Rezayan, A.H.; Tabatabaei Rezaei, S.J.; Mir-Derikvand, M.; Nabid, M.R.; Nourmohammadi, J.; Mohammadnejad Arough, J. Synthesis of Poly(ε-Caprolactone)-Based Polyurethane Semi-Interpenetrating Polymer Networks as Scaffolds for Skin Tissue Regeneration. Int. J. Polym. Mater. Polym. Biomater. 2017, 66, 805–811. [Google Scholar] [CrossRef]
- Ghosal, K.; Manakhov, A.; Zajíčková, L.; Thomas, S. Structural and Surface Compatibility Study of Modified Electrospun Poly(ε-Caprolactone) (PCL) Composites for Skin Tissue Engineering. AAPS PharmSciTech 2017, 18, 72–81. [Google Scholar] [CrossRef] [Green Version]
- Powell, H.M.; Boyce, S.T. Engineered Human Skin Fabricated Using Electrospun Collagen–PCL Blends: Morphogenesis and Mechanical Properties. Tissue Eng. Part A 2009, 15, 2177–2187. [Google Scholar] [CrossRef] [PubMed]
- Gautam, S.; Chou, C.-F.; Dinda, A.K.; Potdar, P.D.; Mishra, N.C. Surface Modification of Nanofibrous Polycaprolactone/Gelatin Composite Scaffold by Collagen Type I Grafting for Skin Tissue Engineering. Mater. Sci. Eng. C 2014, 34, 402–409. [Google Scholar] [CrossRef] [PubMed]
- Lou, T.; Leung, M.; Wang, X.; Chang, J.Y.F.; Tsao, C.T.; Sham, J.G.C.; Edmondson, D.; Zhang, M. Bi-Layer Scaffold of Chitosan/PCL-Nanofibrous Mat and PLLA-Microporous Disc for Skin Tissue Engineering. J. Biomed. Nanotechnol. 2014, 10, 1105–1113. [Google Scholar] [CrossRef] [PubMed]
- Bolaina-Lorenzo, E.; Martínez-Ramos, C.; Monleón-Pradas, M.; Herrera-Kao, W.; Cauich-Rodríguez, J.V.; Cervantes-Uc, J.M. Electrospun Polycaprolactone/Chitosan Scaffolds for Nerve Tissue Engineering: Physicochemical Characterization and Schwann Cell Biocompatibility. Biomed. Mater. 2016, 12, 015008. [Google Scholar] [CrossRef]
- Ghasemi-Mobarakeh, L.; Prabhakaran, M.P.; Morshed, M.; Nasr-Esfahani, M.H.; Ramakrishna, S. Bio-Functionalized PCL Nanofibrous Scaffolds for Nerve Tissue Engineering. Mater. Sci. Eng. C 2010, 30, 1129–1136. [Google Scholar] [CrossRef]
- Mohammadi, S.; Shafiei, S.S.; Asadi-Eydivand, M.; Ardeshir, M.; Solati-Hashjin, M. Graphene Oxide-Enriched Poly(ε-Caprolactone) Electrospun Nanocomposite Scaffold for Bone Tissue Engineering Applications. J. Bioact. Compat. Polym. 2017, 32, 325–342. [Google Scholar] [CrossRef]
- Niu, Y.; Chen, K.C.; He, T.; Yu, W.; Huang, S.; Xu, K. Scaffolds from Block Polyurethanes Based on Poly(ε-Caprolactone) (PCL) and Poly(Ethylene Glycol) (PEG) for Peripheral Nerve Regeneration. Biomaterials 2014, 35, 4266–4277. [Google Scholar] [CrossRef]
- Ahmed, L.A. Stem Cells and Cardiac Repair: Alternative and Multifactorial Approaches. J. Regen. Med. Tissue Eng. 2013, 2, 8. [Google Scholar] [CrossRef] [Green Version]
- Baheiraei, N.; Yeganeh, H.; Ai, J.; Gharibi, R.; Ebrahimi-Barough, S.; Azami, M.; Vahdat, S.; Baharvand, H. Preparation of a Porous Conductive Scaffold from Aniline Pentamer-Modified Polyurethane/PCL Blend for Cardiac Tissue Engineering: Preparation of a porous conductive scaffold from polyurethane/pcl blend. J. Biomed. Mater. Res. 2015, 103, 3179–3187. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.-C.; Sun, Y.-C.; Chen, Y.-H. Electrically Conductive Nanofibers with Highly Oriented Structures and Their Potential Application in Skeletal Muscle Tissue Engineering. Acta Biomater. 2013, 9, 5562–5572. [Google Scholar] [CrossRef] [PubMed]
- Chaudhuri, B.; Bhadra, D.; Moroni, L.; Pramanik, K. Myoblast Differentiation of Human Mesenchymal Stem Cells on Graphene Oxide and Electrospun Graphene Oxide–Polymer Composite Fibrous Meshes: Importance of Graphene Oxide Conductivity and Dielectric Constant on Their Biocompatibility. Biofabrication 2015, 7, 015009. [Google Scholar] [CrossRef] [PubMed]
- Fasolino, I.; Guarino, V.; Cirillo, V.; Ambrosio, L. 5-Azacytidine-Mediated HMSC Behavior on Electrospun Scaffolds for Skeletal Muscle Regeneration. J. Biomed. Mater. Res. A 2017, 105, 2551–2561. [Google Scholar] [CrossRef]
- Semnani, D.; Naghashzargar, E.; Hadjianfar, M.; Dehghan Manshadi, F.; Mohammadi, S.; Karbasi, S.; Effaty, F. Evaluation of PCL/Chitosan Electrospun Nanofibers for Liver Tissue Engineering. Int. J. Polym. Mater. Polym. Biomater. 2017, 66, 149–157. [Google Scholar] [CrossRef]
- Huang, H.; Oizumi, S.; Kojima, N.; Niino, T.; Sakai, Y. Avidin–Biotin Binding-Based Cell Seeding and Perfusion Culture of Liver-Derived Cells in a Porous Scaffold with a Three-Dimensional Interconnected Flow-Channel Network. Biomaterials 2007, 28, 3815–3823. [Google Scholar] [CrossRef]
- Grant, R.; Hay, D.C.; Callanan, A. A Drug-Induced Hybrid Electrospun Poly-Capro-Lactone: Cell-Derived Extracellular Matrix Scaffold for Liver Tissue Engineering. Tissue Eng. Part A 2017, 23, 650–662. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Morsi, Y.; Wang, Y.; Li, Y.; Ramakrishna, S. Review Scaffold Design and Stem Cells for Tooth Regeneration. Jpn. Dent. Sci. Rev. 2013, 49, 14–26. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.; Yang, F.; Walboomers, X.F.; Bian, Z.; Fan, M.; Jansen, J.A. The Performance of Dental Pulp Stem Cells on Nanofibrous PCL/Gelatin/NHA Scaffolds. J. Biomed. Mater. Res. 2010, 93, 247–257. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.; Azmi, D.F.B.; Rosa, V.; Fawzy, A.S.; Fuh, J.Y.H.; Wong, Y.S.; Lu, W.F. Fabrication of Dentin-like Scaffolds through Combined 3D Printing and Bio-Mineralisation. Cogent Eng. 2016, 3, 1222777. [Google Scholar] [CrossRef]
- Jensen, J.; Kraft, D.C.E.; Lysdahl, H.; Foldager, C.B.; Chen, M.; Kristiansen, A.A.; Rölfing, J.H.D.; Bünger, C.E. Functionalization of Polycaprolactone Scaffolds with Hyaluronic Acid and β-TCP Facilitates Migration and Osteogenic Differentiation of Human Dental Pulp Stem Cells In Vitro. Tissue Eng. Part A 2015, 21, 729–739. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flores-Cedillo, M.L.; Alvarado-Estrada, K.N.; Pozos-Guillén, A.J.; Murguía-Ibarra, J.S.; Vidal, M.A.; Cervantes-Uc, J.M.; Rosales-Ibáñez, R.; Cauich-Rodríguez, J.V. Multiwall Carbon Nanotubes/Polycaprolactone Scaffolds Seeded with Human Dental Pulp Stem Cells for Bone Tissue Regeneration. J. Mater. Sci: Mater. Med. 2016, 27, 35. [Google Scholar] [CrossRef] [PubMed]
- Wurth, J.J.; Blumenthal, N.R.; Shastri, V.P. Hydrophilization of Poly(Caprolactone) Copolymers through Introduction of Oligo(Ethylene Glycol) Moieties. PLoS ONE 2014, 9, e99157. [Google Scholar] [CrossRef] [PubMed]
- Khodaverdi, E.; Gharechahi, M.; Alibolandi, M.; Tekie, F.M.; Khashyarmanesh, B.; Hadizadeh, F. Self-Assembled Supramolecular Hydrogel Based on PCL-PEG-PCL Triblock Copolymer and γ-Cyclodextrin Inclusion Complex for Sustained Delivery of Dexamethasone. Int. J. Pharm. Investig. 2016, 6, 78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fattahi, A.; Liverani, L.; Dittrich, R.; Hoffmann, I.; Boccaccini, A.R.; Beckmann, M.W.; Bleisinger, N. Optimization of Porcine Ovarian Follicle Isolation Methods for Better Developmental Potential. Tissue Eng. Part A 2020, 26, 712–719. [Google Scholar] [CrossRef]
- Liverani, L.; Boccaccini, A.R. Versatile Production of Poly(Epsilon-Caprolactone) Fibers by Electrospinning Using Benign Solvents. Nanomaterials 2016, 6, 75. [Google Scholar] [CrossRef]
- Cecconi, S.; Capacchietti, G.; Russo, V.; Berardinelli, P.; Mattioli, M.; Barboni, B. In Vitro Growth of Preantral Follicles Isolated from Cryopreserved Ovine Ovarian Tissue. Biol. Reprod. 2004, 70, 12–17. [Google Scholar] [CrossRef] [Green Version]
- Magalhães, D.M.; Duarte, A.B.G.; Araújo, V.R.; Brito, I.R.; Soares, T.G.; Lima, I.M.T.; Lopes, C.A.P.; Campello, C.C.; Rodrigues, A.P.R.; Figueiredo, J.R. In Vitro Production of a Caprine Embryo from a Preantral Follicle Cultured in Media Supplemented with Growth Hormone. Theriogenology 2011, 75, 182–188. [Google Scholar] [CrossRef]
- Chaves, R.N.; Duarte, A.B.G.; Rodrigues, G.Q.; Celestino, J.J.H.; Silva, G.M.; Lopes, C.A.P.; Almeida, A.P.; Donato, M.A.M.; Peixoto, C.A.; Moura, A.A.A.; et al. The Effects of Insulin and Follicle-Simulating Hormone (FSH) During In Vitro Development of Ovarian Goat Preantral Follicles and the Relative MRNA Expression for Insulin and FSH Receptors and Cytochrome P450 Aromatase in Cultured Follicles1. Biol. Reprod. 2012, 87, 1–11. [Google Scholar] [CrossRef]
- Adams, G.P.; Singh, J.; Baerwald, A.R. Large Animal Models for the Study of Ovarian Follicular Dynamics in Women. Theriogenology 2012, 78, 1733–1748. [Google Scholar] [CrossRef]
- De Figueiredo, J.R.; Cadenas, J.; de Lima, L.F.; Santos, R.R. Advances in in Vitro Folliculogenesis in Domestic Ruminants. Anim. Reprod. 2018, 16, 52–65. [Google Scholar] [CrossRef]
- Barros, V.R.P.; Monte, A.P.O.; Lins, T.L.B.G.; Santos, J.M.; Menezes, V.G.; Cavalcante, A.Y.P.; Araújo, V.R.; Gouveia, B.B.; Matos, M.H.T. In Vitro Survival, Growth, and Maturation of Sheep Oocytes from Secondary Follicles Cultured in Serum-Free Conditions: Impact of a Constant or a Sequential Medium Containing Recombinant Human FSH. Domest. Anim. Endocrinol. 2019, 67, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Cadoret, V.; Frapsauce, C.; Jarrier, P.; Maillard, V.; Bonnet, A.; Locatelli, Y.; Royère, D.; Monniaux, D.; Guérif, F.; Monget, P. Molecular Evidence That Follicle Development Is Accelerated In Vitro Compared to In Vivo. Reproduction 2017, 153, 493–508. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Berardino, C.; Peserico, A.; Capacchietti, G.; Crociati, M.; Monaci, M.; Tosi, U.; Mauro, A.; Russo, V.; Bernabò, N.; Barboni, B. Equine Chorionic Gonadotropin as an Effective Fsh Replacement for In Vitro Ovine Follicle and Oocyte Development. Int. J. Mol. Sci. 2021, 22, 12422. [Google Scholar] [CrossRef]
- Kuo, C.-Y.; Baker, H.; Fries, M.H.; Yoo, J.J.; Kim, P.C.W.; Fisher, J.P. Bioengineering Strategies to Treat Female Infertility. Tissue Eng. Part B Rev. 2017, 23, 294–306. [Google Scholar] [CrossRef]
- Kim, B.S.; Mooney, D.J. Development of Biocompatible Synthetic Extracellular Matrices for Tissue Engineering. Trends Biotechnol. 1998, 16, 224–230. [Google Scholar] [CrossRef]
- Peng, G.; Liu, H.; Fan, Y. Biomaterial Scaffolds for Reproductive Tissue Engineering. Ann. Biomed. Eng. 2017, 45, 1592–1607. [Google Scholar] [CrossRef]
- Eppig, J.J.; Schroeder, A.C. Capacity of Mouse Oocytes from Preantral Follicles to Undergo Embryogenesis and Development to Live Young after Growth, Maturation, and Fertilization In Vitro. Biol. Reprod. 1989, 41, 268–276. [Google Scholar] [CrossRef] [Green Version]
- Silva, J.R.V.; van den Hurk, R.; Figueiredo, J.R. Ovarian Follicle Development In Vitro and Oocyte Competence: Advances and Challenges for Farm Animals. Domest. Anim. Endocrinol. 2016, 55, 123–135. [Google Scholar] [CrossRef]
- Ouni, E.; Peaucelle, A.; Haas, K.T.; Van Kerk, O.; Dolmans, M.-M.; Tuuri, T.; Otala, M.; Amorim, C.A. A Blueprint of the Topology and Mechanics of the Human Ovary for Next-Generation Bioengineering and Diagnosis. Nat. Commun. 2021, 12, 5603. [Google Scholar] [CrossRef]
- Nuttinck, F.; Peynot, N.; Humblot, P.; Massip, A.; Dessy, F.; Fléchon, J.E. Comparative Immunohistochemical Distribution of Connexin 37 and Connexin 43 throughout Folliculogenesis in the Bovine Ovary. Mol. Reprod. Dev. 2000, 57, 60–66. [Google Scholar] [CrossRef]
- Kawai, T.; Mihara, T.; Kawashima, I.; Fujita, Y.; Ikeda, C.; Negishi, H.; Richards, J.S.; Shimada, M. Endogenous Acetaldehyde Toxicity during Antral Follicular Development in the Mouse Ovary. Reprod. Toxicol. 2012, 33, 322–330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kakuta, H.; Iguchi, T.; Sato, T. The Involvement of Granulosa Cells in the Regulation by Gonadotropins of Cyp17a1 in Theca Cells. Vivo 2018, 32, 1387–1401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Katz, Y.; Armstrong, D.T. Inhibition of Ovarian Estradiol-17beta Secretion by Luteinizing Hormone in Prepubertal, Pregnant Mare Serum-Treated Rats. Endocrinology 1976, 99, 1442–1447. [Google Scholar] [CrossRef] [PubMed]
- McGee, E.A.; Hsueh, A.J. Initial and Cyclic Recruitment of Ovarian Follicles. Endocr. Rev. 2000, 21, 200–214. [Google Scholar] [CrossRef] [Green Version]
- Heidarzadehpilehrood, R.; Pirhoushiaran, M.; Abdollahzadeh, R.; Binti Osman, M.; Sakinah, M.; Nordin, N.; Abdul Hamid, H. A Review on CYP11A1, CYP17A1, and CYP19A1 Polymorphism Studies: Candidate Susceptibility Genes for Polycystic Ovary Syndrome (PCOS) and Infertility. Genes 2022, 13, 302. [Google Scholar] [CrossRef]
- Jin, S.Y.; Lei, L.; Shikanov, A.; Shea, L.D.; Woodruff, T.K. A Novel Two-Step Strategy for in Vitro Culture of Early-Stage Ovarian Follicles in the Mouse. Fertil. Steril. 2010, 93, 2633–2639. [Google Scholar] [CrossRef] [Green Version]
- Woodruff, T.K.; Shea, L.D. The Role of the Extracellular Matrix in Ovarian Follicle Development. Reprod. Sci. 2007, 14, 6–10. [Google Scholar] [CrossRef] [Green Version]
- Albertini, D.F.; Akkoyunlu, G. Ovarian Follicle Culture Systems for Mammals. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 2010; Volume 476, pp. 107–121. ISBN 978-0-12-374775-4. [Google Scholar]
- Araújo, V.R.; Gastal, M.O.; Wischral, A.; Figueiredo, J.R.; Gastal, E.L. In Vitro Development of Bovine Secondary Follicles in Two- and Three-Dimensional Culture Systems Using Vascular Endothelial Growth Factor, Insulin-like Growth Factor-1, and Growth Hormone. Theriogenology 2014, 82, 1246–1253. [Google Scholar] [CrossRef]
- Dadashzadeh, A.; Moghassemi, S.; Shavandi, A.; Amorim, C.A. A Review on Biomaterials for Ovarian Tissue Engineering. Acta Biomater. 2021, 135, 48–63. [Google Scholar] [CrossRef]
- Shikanov, A.; Xu, M.; Woodruff, T.K.; Shea, L.D. Interpenetrating Fibrin–Alginate Matrices for In Vitro Ovarian Follicle Development. Biomaterials 2009, 30, 5476–5485. [Google Scholar] [CrossRef] [Green Version]
- Drummond, A.E. The Role of Steroids in Follicular Growth. Reprod. Biol. Endocrinol. 2006, 4, 16. [Google Scholar] [CrossRef] [Green Version]
- Chiti, M.C.; Dolmans, M.M.; Donnez, J.; Amorim, C.A. Fibrin in Reproductive Tissue Engineering: A Review on Its Application as a Biomaterial for Fertility Preservation. Ann. Biomed. Eng. 2017, 45, 1650–1663. [Google Scholar] [CrossRef] [PubMed]
- Amorim, C.A.; Shikanov, A. The Artificial Ovary: Current Status and Future Perspectives. Future Oncol. 2016, 12, 2323–2332. [Google Scholar] [CrossRef] [PubMed]
- Luyckx, V.; Dolmans, M.-M.; Vanacker, J.; Legat, C.; Fortuño Moya, C.; Donnez, J.; Amorim, C.A. A New Step toward the Artificial Ovary: Survival and Proliferation of Isolated Murine Follicles after Autologous Transplantation in a Fibrin Scaffold. Fertil. Steril. 2014, 101, 1149–1156. [Google Scholar] [CrossRef] [PubMed]
- Wycherley, G.; Downey, D.; Kane, M.T.; Hynes, A.C. A Novel Follicle Culture System Markedly Increases Follicle Volume, Cell Number and Oestradiol Secretion. Reproduction 2004, 127, 669–677. [Google Scholar] [CrossRef] [Green Version]













| ivF Groups | N° PA | PA Diameter | N° EA | Mean Diameter of EA Follicles and ∆ Growth (%) | N° No Antrum | Mean Diameters of No Antrum Follicles and ∆ Growth (%) |
|---|---|---|---|---|---|---|
| (n) | (μm ± SD) | (n) | (%) | (n) | (μm ± SD) | |
| 3D-oil | 120 | 249 ± 5 | 75 | 421.3 ± 12 ** (70%) | 21 | 393 ± 16 ** (67.4%) |
| Two-Step PCL-Randomic | 125 | 251 ± 4 | 39 | 404.3 ± 12 ** (62%) b | 42 | 421.5 ± 16 ** (58%) ## |
| Two-step PCL-Patterned | 122 | 253 ± 6 | 96 | 438.5 ± 11 ** (75%) a | 16 | 418 ± 10 ** (67.3%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Berardino, C.; Liverani, L.; Peserico, A.; Capacchietti, G.; Russo, V.; Bernabò, N.; Tosi, U.; Boccaccini, A.R.; Barboni, B. When Electrospun Fiber Support Matters: In Vitro Ovine Long-Term Folliculogenesis on Poly (Epsilon Caprolactone) (PCL)-Patterned Fibers. Cells 2022, 11, 1968. https://doi.org/10.3390/cells11121968
Di Berardino C, Liverani L, Peserico A, Capacchietti G, Russo V, Bernabò N, Tosi U, Boccaccini AR, Barboni B. When Electrospun Fiber Support Matters: In Vitro Ovine Long-Term Folliculogenesis on Poly (Epsilon Caprolactone) (PCL)-Patterned Fibers. Cells. 2022; 11(12):1968. https://doi.org/10.3390/cells11121968
Chicago/Turabian StyleDi Berardino, Chiara, Liliana Liverani, Alessia Peserico, Giulia Capacchietti, Valentina Russo, Nicola Bernabò, Umberto Tosi, Aldo Roberto Boccaccini, and Barbara Barboni. 2022. "When Electrospun Fiber Support Matters: In Vitro Ovine Long-Term Folliculogenesis on Poly (Epsilon Caprolactone) (PCL)-Patterned Fibers" Cells 11, no. 12: 1968. https://doi.org/10.3390/cells11121968