Obesity: The Fat Tissue Disease Version of Cancer
Abstract
:1. Introduction
2. Obesity Is a Disease
3. The Obesity–Cancer Connection: Exemplification of Potential Disease Amplification
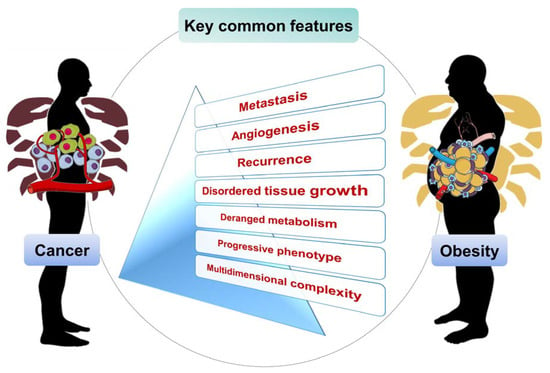
4. Obesity and Cancer Common Features
4.1. Multifactorial Grounds and Deranged Metabolism
4.2. Disordered Cell Growth and Proliferation
4.3. Metastasis
4.4. Aggravation by Angiogenesis
4.5. Progressive Development and Stages
4.6. Recurrence
4.7. Multidimensional Complexity from the Gut Microbiome
5. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Condon, C. The fat bomb exploded but no one heard the bang. Eur. J. Cardiovasc. Nurs. 2006, 5, 99–101. [Google Scholar] [CrossRef]
- Montgomery, K.; Chester, J.; Nixon, L.; Levy, L.; Dorfman, L. Big Data and the transformation of food and beverage marketing: Undermining efforts to reduce obesity? Crit. Public Health 2019, 29, 110–117. [Google Scholar] [CrossRef]
- Strong, D.R.; Pierce, J.P.; Pulvers, K.; Stone, M.D.; Villaseñor, A.; Pu, M.; Dimofte, C.V.; Leas, E.C.; Oratowski, J.; Brighton, E.; et al. Effect of Graphic Warning Labels on Cigarette Packs on US Smokers’ Cognitions and Smoking Behavior After 3 Months: A Randomized Clinical Trial. JAMA Netw. Open 2021, 4, e2121387. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.J.; Thrasher, J.F.; Davis, R.; Kim, S.-H.; Hardin, J.; Popova, L. Effective package warning label systems for communicating relative risks of cigarettes, heated tobacco products, and e-cigarettes: An experimental study with Korean adults. Int. J. Drug Policy 2022, 99, 103468. [Google Scholar] [CrossRef] [PubMed]
- Stanford, F.C.; Tauqeer, Z.; Kyle, T.K. Media and Its Influence on Obesity. Curr. Obes. Rep. 2018, 7, 186–192. [Google Scholar] [CrossRef]
- The Lancet Gastroenterology & Hepatology. Obesity: Another ongoing pandemic. Lancet Gastroenterol. Hepatol. 2021, 6, 411. [Google Scholar] [CrossRef]
- Smith, K.B.; Smith, M.S. Obesity Statistics. Prim. Care Clin. Off. Pract. 2016, 43, 121–135. [Google Scholar] [CrossRef]
- Kulkarni, K.; Karssiens, T.; Kumar, V.; Pandit, H. Obesity and osteoarthritis. Maturitas 2016, 89, 22–28. [Google Scholar] [CrossRef]
- Polyzos, S.A.; Kountouras, J.; Mantzoros, C.S. Obesity and nonalcoholic fatty liver disease: From pathophysiology to therapeutics. Metabolism 2019, 92, 82–97. [Google Scholar] [CrossRef]
- Mariani, S.; Fiore, D.; Varone, L.; Basciani, S.; Persichetti, A.; Watanabe, M.; Saponara, M.; Spera, G.; Moretti, C.; Gnessi, L. Obstructive sleep apnea and bone mineral density in obese patients. Diabetes Metab. Syndr. Obes. Targets Ther. 2012, 5, 395–401. [Google Scholar] [CrossRef] [Green Version]
- Duflou, J.; Virmani, R.; Rabin, I.; Burke, A.; Farb, A.; Smialek, J. Sudden death as a result of heart disease in morbid obesity. Am. Heart J. 1995, 130, 306–313. [Google Scholar] [CrossRef]
- Friedenreich, C.M.; Ryder-Burbidge, C.; McNeil, J. Physical activity, obesity and sedentary behavior in cancer etiology: Epidemiologic evidence and biologic mechanisms. Mol. Oncol. 2021, 15, 790–800. [Google Scholar] [CrossRef] [PubMed]
- Busetto, L.; Bettini, S.; Fabris, R.; Serra, R.; Dal Pra, C.; Maffei, P.; Rossato, M.; Fioretto, P.; Vettor, R. Obesity and COVID-19: An Italian Snapshot. Obesity 2020, 28, 1600–1605. [Google Scholar] [CrossRef] [PubMed]
- Hancková, M.; Betáková, T. Pandemics of the 21st Century: The Risk Factor for Obese People. Viruses 2021, 14, 25. [Google Scholar] [CrossRef] [PubMed]
- Castro, A.M.; Macedo-de la Concha, L.E.; Pantoja-Meléndez, C.A. Low-grade inflammation and its relation to obesity and chronic degenerative diseases. Rev. Médica Hosp. Gen. México 2017, 80, 101–105. [Google Scholar] [CrossRef]
- Katz, D.L. Perspective: Obesity is not a disease. Nature 2014, 508, S57. [Google Scholar] [CrossRef]
- Cornier, M.-A.; Dabelea, D.; Hernandez, T.L.; Lindstrom, R.C.; Steig, A.J.; Stob, N.R.; Van Pelt, R.E.; Wang, H.; Eckel, R.H. The Metabolic Syndrome. Endocr. Rev. 2008, 29, 777–822. [Google Scholar] [CrossRef]
- Engin, A. The Definition and Prevalence of Obesity and Metabolic Syndrome. Adv. Exp. Med. Biol. 2017, 960, 1–17. [Google Scholar] [CrossRef]
- Parikh, R.M.; Joshi, S.R.; Menon, P.S.; Shah, N.S. Index of central obesity—A novel parameter. Med. Hypotheses 2007, 68, 1272–1275. [Google Scholar] [CrossRef]
- Sahakyan, K.R.; Somers, V.K.; Rodriguez-Escudero, J.P.; Hodge, D.O.; Carter, R.E.; Sochor, O.; Coutinho, T.; Jensen, M.D.; Roger, V.L.; Singh, P. Normal-weight central obesity: Implications for total and cardiovascular mortality. Ann. Intern. Med. 2015, 163, 827–835. [Google Scholar] [CrossRef] [Green Version]
- Vrinten, C.; McGregor, L.M.; Heinrich, M.; von Wagner, C.; Waller, J.; Wardle, J.; Black, G.B. What do people fear about cancer? A systematic review and meta-synthesis of cancer fears in the general population. Psychooncology 2017, 26, 1070–1079. [Google Scholar] [CrossRef] [PubMed]
- Scully, J.L. What is a disease? EMBO Rep. 2004, 5, 650–653. [Google Scholar] [CrossRef] [PubMed]
- Rosen, H. Is Obesity A Disease or A Behavior Abnormality? Did the AMA Get It Right? MO Med. 2014, 111, 104–108. [Google Scholar] [PubMed]
- Jung, R.T. Obesity as a disease. Br. Med. Bull. 1997, 53, 307–321. [Google Scholar] [CrossRef]
- Bray, G.A.; Kim, K.K.; Wilding, J.P.H. Obesity: A chronic relapsing progressive disease process. A position statement of the World Obesity Federation. Obes. Rev. 2017, 18, 715–723. [Google Scholar] [CrossRef] [Green Version]
- Tucker, S.; Bramante, C.; Conroy, M.; Fitch, A.; Gilden, A.; Wittleder, S.; Jay, M. The Most Undertreated Chronic Disease: Addressing Obesity in Primary Care Settings. Curr. Obes. Rep. 2021, 10, 396–408. [Google Scholar] [CrossRef]
- Rippe, J.M.; Crossley, S.; Ringer, R. Obesity as a Chronic Disease: Modern Medical and Lifestyle Management. J. Am. Diet. Assoc. 1998, 98, S9–S15. [Google Scholar] [CrossRef]
- Fujioka, K. Management of obesity as a chronic disease: Nonpharmacologic, pharmacologic, and surgical options. Obes. Res. 2002, 10 (Suppl. S2), 116S–123S. [Google Scholar] [CrossRef] [Green Version]
- Obesity. Available online: https://www.who.int/health-topics/obesity#tab=tab_1 (accessed on 22 March 2022).
- Berenbaum, F.; Eymard, F.; Houard, X. Osteoarthritis, inflammation and obesity. Curr. Opin. Rheumatol. 2013, 25, 114–118. [Google Scholar] [CrossRef]
- Guilherme, A.; Virbasius, J.V.; Puri, V.; Czech, M.P. Adipocyte dysfunctions linking obesity to insulin resistance and type 2 diabetes. Nat. Rev. Mol. Cell Biol. 2008, 9, 367–377. [Google Scholar] [CrossRef] [Green Version]
- Skurk, T.; Alberti-Huber, C.; Herder, C.; Hauner, H. Relationship between Adipocyte Size and Adipokine Expression and Secretion. J. Clin. Endocrinol. Metab. 2007, 92, 1023–1033. [Google Scholar] [CrossRef] [PubMed]
- Kong, Y.; Zhang, S.; Wu, R.; Su, X.; Peng, D.; Zhao, M.; Su, Y. New insights into different adipokines in linking the pathophysiology of obesity and psoriasis. Lipids Health Dis. 2019, 18, 171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rothman, K.J. BMI-related errors in the measurement of obesity. Int. J. Obes. 2008, 32 (Suppl. S3), S56–S59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanchez-Ramirez, D.C.; van der Leeden, M.; van der Esch, M.; Roorda, L.D.; Verschueren, S.; van Dieën, J.; Lems, W.F.; Dekker, J. Increased knee muscle strength is associated with decreased activity limitations in established knee osteoarthritis: Two-year follow-up study in the Amsterdam osteoarthritis cohort. J. Rehabil. Med. 2015, 47, 647–654. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nishizawa, T.; Akaoka, I.; Nishida, Y.; Kawaguchi, Y.; Hayashi, E.; Yoshimura, T. Some factors related to obesity in the Japanese sumo wrestler. Am. J. Clin. Nutr. 1976, 29, 1167–1174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Cuevillas, B.; Alvarez-Alvarez, I.; Riezu-Boj, J.I.; Navas-Carretero, S.; Martinez, J.A. The hypertriglyceridemic-waist phenotype as a valuable and integrative mirror of metabolic syndrome traits. Sci. Rep. 2021, 11, 21859. [Google Scholar] [CrossRef] [PubMed]
- Haga, B.M.; Furnes, B.; Dysvik, E.; Ueland, V. Putting life on hold: Lived experiences of people with obesity. Scand J. Caring Sci. 2020, 34, 514–523. [Google Scholar] [CrossRef] [Green Version]
- Syed-Abdul, S.; Fernandez-Luque, L.; Jian, W.S.; Li, Y.C.; Crain, S.; Hsu, M.H.; Wang, Y.C.; Khandregzen, D.; Chuluunbaatar, E.; Nguyen, P.A.; et al. Misleading health-related information promoted through video-based social media: Anorexia on YouTube. J. Med. Internet Res. 2013, 15, e30. [Google Scholar] [CrossRef]
- Nobile, M. The who definition of health: A critical reading. Med. Law 2014, 33, 33–40. [Google Scholar]
- De Pergola, G.; Silvestris, F. Obesity as a major risk factor for cancer. J. Obes. 2013, 2013, 291546. [Google Scholar] [CrossRef] [Green Version]
- Berger, N.A. Obesity and cancer pathogenesis. Ann. N. Y. Acad. Sci. 2014, 1311, 57–76. [Google Scholar] [CrossRef] [PubMed]
- Ungefroren, H.; Gieseler, F.; Lehnert, H. Obesity and cancer. Internist 2015, 56, 127–128, 130–136. [Google Scholar] [CrossRef] [PubMed]
- The, L. The link between cancer and obesity. Lancet 2017, 390, 1716. [Google Scholar] [CrossRef]
- Ottaiano, A.; De Divitiis, C.; Capozzi, M.; Avallone, A.; Pisano, C.; Pignata, S.; Tafuto, S. Obesity and Cancer: Biological Links and Treatment Implications. Curr. Cancer Drug Targets 2018, 18, 231–238. [Google Scholar] [CrossRef]
- Allott, E.H.; Hursting, S.D. Obesity and cancer: Mechanistic insights from transdisciplinary studies. Endocr. Relat. Cancer 2015, 22, R365–R386. [Google Scholar] [CrossRef] [Green Version]
- Gutiérrez-Salmerón, M.; Chocarro-Calvo, A.; García-Martínez, J.M.; de la Vieja, A.; García-Jiménez, C. Epidemiological bases and molecular mechanisms linking obesity, diabetes, and cancer. Endocrinol. Diabetes Nutr. 2017, 64, 109–117. [Google Scholar] [CrossRef]
- Fernandez, C.J.; George, A.S.; Subrahmanyan, N.A.; Pappachan, J.M. Epidemiological link between obesity, type 2 diabetes mellitus and cancer. World J. Methodol. 2021, 11, 23–45. [Google Scholar] [CrossRef]
- Zhao, X.; Yang, Y.; Sun, B.F.; Zhao, Y.L.; Yang, Y.G. FTO and obesity: Mechanisms of association. Curr. Diabetes Rep. 2014, 14, 486. [Google Scholar] [CrossRef]
- Lan, N.; Lu, Y.; Zhang, Y.; Pu, S.; Xi, H.; Nie, X.; Liu, J.; Yuan, W. FTO—A Common Genetic Basis for Obesity and Cancer. Front. Genet. 2020, 11, 559138. [Google Scholar] [CrossRef]
- Cavazos, D.A.; deGraffenried, M.J.; Apte, S.A.; Bowers, L.W.; Whelan, K.A.; deGraffenried, L.A. Obesity promotes aerobic glycolysis in prostate cancer cells. Nutr. Cancer 2014, 66, 1179–1186. [Google Scholar] [CrossRef] [Green Version]
- Teslow, E.A.; Mitrea, C.; Bao, B.; Mohammad, R.M.; Polin, L.A.; Dyson, G.; Purrington, K.S.; Bollig-Fischer, A. Obesity-induced MBD2_v2 expression promotes tumor-initiating triple-negative breast cancer stem cells. Mol. Oncol. 2019, 13, 894–908. [Google Scholar] [CrossRef] [PubMed]
- Ringel, A.E.; Drijvers, J.M.; Baker, G.J.; Catozzi, A.; García-Cañaveras, J.C.; Gassaway, B.M.; Miller, B.C.; Juneja, V.R.; Nguyen, T.H.; Joshi, S.; et al. Obesity Shapes Metabolism in the Tumor Microenvironment to Suppress Anti-Tumor Immunity. Cell 2020, 183, 1848–1866.e1826. [Google Scholar] [CrossRef] [PubMed]
- Asante, E.C.; Pallegar, N.K.; Hoffmann, A.J.; Viloria-Petit, A.M.; Christian, S.L. Adipose Tissue from Lean and Obese Mice Induces a Mesenchymal to Epithelial Transition-Like Effect in Triple Negative Breast Cancers Cells Grown in 3-Dimensional Culture. Int. J. Mol. Sci. 2020, 21, 6439. [Google Scholar] [CrossRef] [PubMed]
- Salameh, T.S.; Le, T.T.; Nichols, M.B.; Bauer, E.; Cheng, J.; Camarillo, I.G. An ex vivo co-culture model system to evaluate stromal-epithelial interactions in breast cancer. Int. J. Cancer 2013, 132, 288–296. [Google Scholar] [CrossRef]
- Kim, W.G.; Cheng, S.Y. Mechanisms Linking Obesity and Thyroid Cancer Development and Progression in Mouse Models. Horm. Cancer 2018, 9, 108–116. [Google Scholar] [CrossRef]
- Hursting, S.D.; Nunez, N.P.; Varticovski, L.; Vinson, C. The obesity-cancer link: Lessons learned from a fatless mouse. Cancer Res. 2007, 67, 2391–2393. [Google Scholar] [CrossRef] [Green Version]
- Avgerinos, K.I.; Spyrou, N.; Mantzoros, C.S.; Dalamaga, M. Obesity and cancer risk: Emerging biological mechanisms and perspectives. Metabolism 2019, 92, 121–135. [Google Scholar] [CrossRef]
- Calle, E.E.; Kaaks, R. Overweight, obesity and cancer: Epidemiological evidence and proposed mechanisms. Nat. Rev. Cancer 2004, 4, 579–591. [Google Scholar] [CrossRef]
- Scully, T.; Ettela, A.; LeRoith, D.; Gallagher, E.J. Obesity, Type 2 Diabetes, and Cancer Risk. Front. Oncol. 2020, 10, 615375. [Google Scholar] [CrossRef]
- Nieman, K.M.; Romero, I.L.; Van Houten, B.; Lengyel, E. Adipose tissue and adipocytes support tumorigenesis and metastasis. Biochim. Biophys. Acta 2013, 1831, 1533–1541. [Google Scholar] [CrossRef] [Green Version]
- Snaebjornsson, M.T.; Janaki-Raman, S.; Schulze, A. Greasing the Wheels of the Cancer Machine: The Role of Lipid Metabolism in Cancer. Cell Metab. 2020, 31, 62–76. [Google Scholar] [CrossRef] [PubMed]
- Kolb, R.; Sutterwala, F.S.; Zhang, W. Obesity and cancer: Inflammation bridges the two. Curr. Opin. Pharm. 2016, 29, 77–89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iyengar, N.M.; Gucalp, A.; Dannenberg, A.J.; Hudis, C.A. Obesity and Cancer Mechanisms: Tumor Microenvironment and Inflammation. J. Clin. Oncol. 2016, 34, 4270–4276. [Google Scholar] [CrossRef] [PubMed]
- Cowey, S.; Hardy, R.W. The metabolic syndrome: A high-risk state for cancer? Am. J. Pathol. 2006, 169, 1505–1522. [Google Scholar] [CrossRef]
- Stone, T.W.; McPherson, M.; Gail Darlington, L. Obesity and Cancer: Existing and New Hypotheses for a Causal Connection. EBioMedicine 2018, 30, 14–28. [Google Scholar] [CrossRef] [Green Version]
- Iyengar, N.M.; Zhou, X.K.; Mendieta, H.; El-Hely, O.; Giri, D.D.; Winston, L.; Falcone, D.J.; Wang, H.; Meng, L.; Ha, T.; et al. Effects of obesity on breast aromatase expression and systemic metabo-inflammation in women with BRCA1 or BRCA2 mutations. NPJ Breast Cancer 2021, 7, 18. [Google Scholar] [CrossRef]
- Deng, T.; Lyon, C.J.; Bergin, S.; Caligiuri, M.A.; Hsueh, W.A. Obesity, Inflammation, and Cancer. Ann. Rev. Pathol. 2016, 11, 421–449. [Google Scholar] [CrossRef] [Green Version]
- Pu, X.; Chen, D. Targeting Adipokines in Obesity-Related Tumors. Front. Oncol. 2021, 11, 685923. [Google Scholar] [CrossRef]
- Ackerman, G.E.; Smith, M.E.; Mendelson, C.R.; MacDonald, P.C.; Simpson, E.R. Aromatization of androstenedione by human adipose tissue stromal cells in monolayer culture. J. Clin. Endocrinol. Metab. 1981, 53, 412–417. [Google Scholar] [CrossRef]
- Mair, K.M.; Gaw, R.; MacLean, M.R. Obesity, estrogens and adipose tissue dysfunction—Implications for pulmonary arterial hypertension. Pulm. Circ. 2020, 10, 2045894020952019. [Google Scholar] [CrossRef]
- Baker, J.M.; Al-Nakkash, L.; Herbst-Kralovetz, M.M. Estrogen-gut microbiome axis: Physiological and clinical implications. Maturitas 2017, 103, 45–53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nelson, L.R.; Bulun, S.E. Estrogen production and action. J. Am. Acad. Derm. 2001, 45, S116–S124. [Google Scholar] [CrossRef] [PubMed]
- Bulun, S.E.; Chen, D.; Moy, I.; Brooks, D.C.; Zhao, H. Aromatase, breast cancer and obesity: A complex interaction. Trends Endocrinol. Metab. 2012, 23, 83–89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lewis-Wambi, J.S.; Jordan, V.C. Estrogen regulation of apoptosis: How can one hormone stimulate and inhibit? Breast Cancer Res. 2009, 11, 206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hopkins, B.D.; Goncalves, M.D.; Cantley, L.C. Obesity and Cancer Mechanisms: Cancer Metabolism. J. Clin. Oncol. 2016, 34, 4277–4283. [Google Scholar] [CrossRef] [Green Version]
- Daniele, A.; Divella, R.; Pilato, B.; Tommasi, S.; Pasanisi, P.; Patruno, M.; Digennaro, M.; Minoia, C.; Dellino, M.; Pisconti, S.; et al. Can harmful lifestyle, obesity and weight changes increase the risk of breast cancer in BRCA 1 and BRCA 2 mutation carriers? A Mini review. Hered Cancer Clin. Pract. 2021, 19, 45. [Google Scholar] [CrossRef]
- Hawsawi, Y.M.; Al-Numair, N.S.; Sobahy, T.M.; Al-Ajmi, A.M.; Al-Harbi, R.M.; Baghdadi, M.A.; Oyouni, A.A.; Alamer, O.M. The role of BRCA1/2 in hereditary and familial breast and ovarian cancers. Mol. Genet. Genom. Med. 2019, 7, e879. [Google Scholar] [CrossRef] [Green Version]
- Sefton, P. Testing for BRCA1/2 Mutations. JAMA 2017, 318, 2054. [Google Scholar] [CrossRef]
- Farooqi, I.S.; Keogh, J.M.; Yeo, G.S.; Lank, E.J.; Cheetham, T.; O’Rahilly, S. Clinical spectrum of obesity and mutations in the melanocortin 4 receptor gene. N. Engl. J. Med. 2003, 348, 1085–1095. [Google Scholar] [CrossRef] [Green Version]
- Farooqi, I.S.; Yeo, G.S.; Keogh, J.M.; Aminian, S.; Jebb, S.A.; Butler, G.; Cheetham, T.; O’Rahilly, S. Dominant and recessive inheritance of morbid obesity associated with melanocortin 4 receptor deficiency. J. Clin. Investig. 2000, 106, 271–279. [Google Scholar] [CrossRef] [Green Version]
- Fairbrother, U.; Kidd, E.; Malagamuwa, T.; Walley, A. Genetics of Severe Obesity. Curr. Diabetes Rep. 2018, 18, 85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kompella, P.; Vasquez, K.M. Obesity and cancer: A mechanistic overview of metabolic changes in obesity that impact genetic instability. Mol. Carcinog. 2019, 58, 1531–1550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qin, Y.; Roberts, J.D.; Grimm, S.A.; Lih, F.B.; Deterding, L.J.; Li, R.; Chrysovergis, K.; Wade, P.A. An obesity-associated gut microbiome reprograms the intestinal epigenome and leads to altered colonic gene expression. Genome Biol. 2018, 19, 7. [Google Scholar] [CrossRef] [PubMed]
- Mohr, A.E.; Jäger, R.; Carpenter, K.C.; Kerksick, C.M.; Purpura, M.; Townsend, J.R.; West, N.P.; Black, K.; Gleeson, M.; Pyne, D.B.; et al. The athletic gut microbiota. J. Int. Soc. Sports Nutr. 2020, 17, 24. [Google Scholar] [CrossRef]
- Lynch, B.M. Sedentary behavior and cancer: A systematic review of the literature and proposed biological mechanisms. Cancer Epidemiol. Biomark. Prev. 2010, 19, 2691–2709. [Google Scholar] [CrossRef] [Green Version]
- Kushner, R.F.; Sorensen, K.W. Lifestyle medicine: The future of chronic disease management. Curr. Opin. Endocrinol. Diabetes Obes. 2013, 20, 389–395. [Google Scholar] [CrossRef]
- Onitilo, A.A.; Engel, J.M.; Glurich, I.; Stankowski, R.V.; Williams, G.M.; Doi, S.A. Diabetes and cancer II: Role of diabetes medications and influence of shared risk factors. Cancer Causes Control 2012, 23, 991–1008. [Google Scholar] [CrossRef] [Green Version]
- Leslie, W.S.; Hankey, C.R.; Lean, M.E.J. Weight gain as an adverse effect of some commonly prescribed drugs: A systematic review. QJM Int. J. Med. 2007, 100, 395–404. [Google Scholar] [CrossRef] [Green Version]
- Brown, L.M.; Clegg, D.J. Central effects of estradiol in the regulation of food intake, body weight, and adiposity. J. Steroid Biochem. Mol. Biol. 2010, 122, 65–73. [Google Scholar] [CrossRef] [Green Version]
- Kelly, D.M.; Jones, T.H. Testosterone and obesity. Obes. Rev. 2015, 16, 581–606. [Google Scholar] [CrossRef]
- Zamani, A.R.N.; Avci, Ç.B.; Ahmadi, M.; Pouyafar, A.; Bagheri, H.S.; Fathi, F.; Heidarzadeh, M.; Rezaie, J.; Mirhosseini, Y.; Saberianpour, S.; et al. Estradiol modulated colorectal cancer stem cells bioactivity and interaction with endothelial cells. Life Sci. 2020, 257, 118078. [Google Scholar] [CrossRef] [PubMed]
- Yassin, A.; AlRumaihi, K.; Alzubaidi, R.; Alkadhi, S.; Al Ansari, A. Testosterone, testosterone therapy and prostate cancer. Aging Male 2019, 22, 219–227. [Google Scholar] [CrossRef] [PubMed]
- St-Onge, M.P. Sleep-obesity relation: Underlying mechanisms and consequences for treatment. Obes. Rev. 2017, 18 (Suppl. S1), 34–39. [Google Scholar] [CrossRef] [PubMed]
- Michopoulos, V. Stress-induced alterations in estradiol sensitivity increase risk for obesity in women. Physiol. Behav. 2016, 166, 56–64. [Google Scholar] [CrossRef] [Green Version]
- Iftikhar, A.; Islam, M.; Shepherd, S.; Jones, S.; Ellis, I. Cancer and Stress: Does It Make a Difference to the Patient When These Two Challenges Collide? Cancers 2021, 13, 163. [Google Scholar] [CrossRef]
- Loos, R.J. Recent progress in the genetics of common obesity. Br. J. Clin. Pharm. 2009, 68, 811–829. [Google Scholar] [CrossRef] [Green Version]
- Tremblay, A.; Fogelholm, M.; Jalo, E.; Westerterp-Plantenga, M.S.; Adam, T.C.; Huttunen-Lenz, M.; Stratton, G.; Lam, T.; Handjieva-Darlenska, T.; Handjiev, S.; et al. What Is the Profile of Overweight Individuals Who Are Unsuccessful Responders to a Low-Energy Diet? A Preview Sub-study. Front. Nutr. 2021, 8, 707682. [Google Scholar] [CrossRef]
- Arocha Rodulfo, J.I. Sedentary lifestyle a disease from xxi century. Clin. Investig. Arter. 2019, 31, 233–240. [Google Scholar] [CrossRef]
- Pereira, R.M.; Botezelli, J.D.; da Cruz Rodrigues, K.C.; Mekary, R.A.; Cintra, D.E.; Pauli, J.R.; da Silva, A.S.R.; Ropelle, E.R.; de Moura, L.P. Fructose Consumption in the Development of Obesity and the Effects of Different Protocols of Physical Exercise on the Hepatic Metabolism. Nutrients 2017, 9, 405. [Google Scholar] [CrossRef] [Green Version]
- Nakagawa, T.; Lanaspa, M.A.; Millan, I.S.; Fini, M.; Rivard, C.J.; Sanchez-Lozada, L.G.; Andres-Hernando, A.; Tolan, D.R.; Johnson, R.J. Fructose contributes to the Warburg effect for cancer growth. Cancer Metab. 2020, 8, 16. [Google Scholar] [CrossRef]
- Tappy, L.; Lê, K.A. Metabolic effects of fructose and the worldwide increase in obesity. Physiol. Rev. 2010, 90, 23–46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mortera, R.R.; Bains, Y.; Gugliucci, A. Fructose at the crossroads of the metabolic syndrome and obesity epidemics. Front. Biosci. (Landmark Ed.) 2019, 24, 186–211. [Google Scholar] [CrossRef] [PubMed]
- Tumor Definition: What You Need to Know. Available online: https://blog.dana-farber.org/insight/2018/05/difference-cancer-tumor/#:~:text=Cancer%20is%20a%20disease%20in,the%20blood%20and%20lymph%20systems (accessed on 22 March 2022).
- Fasshauer, M.; Blüher, M. Adipokines in health and disease. Trends Pharm. Sci. 2015, 36, 461–470. [Google Scholar] [CrossRef] [PubMed]
- Ghaben, A.L.; Scherer, P.E. Adipogenesis and metabolic health. Nat. Rev. Mol. Cell Biol. 2019, 20, 242–258. [Google Scholar] [CrossRef]
- Jo, J.; Gavrilova, O.; Pack, S.; Jou, W.; Mullen, S.; Sumner, A.E.; Cushman, S.W.; Periwal, V. Hypertrophy and/or Hyperplasia: Dynamics of Adipose Tissue Growth. PLoS Comput. Biol. 2009, 5, e1000324. [Google Scholar] [CrossRef]
- Longo, M.; Zatterale, F.; Naderi, J.; Parrillo, L.; Formisano, P.; Raciti, G.A.; Beguinot, F.; Miele, C. Adipose Tissue Dysfunction as Determinant of Obesity-Associated Metabolic Complications. Int. J. Mol. Sci. 2019, 20, 2358. [Google Scholar] [CrossRef] [Green Version]
- Hersoug, L.G.; Møller, P.; Loft, S. Role of microbiota-derived lipopolysaccharide in adipose tissue inflammation, adipocyte size and pyroptosis during obesity. Nutr. Res. Rev. 2018, 31, 153–163. [Google Scholar] [CrossRef]
- Borén, J.; Taskinen, M.R.; Olofsson, S.O.; Levin, M. Ectopic lipid storage and insulin resistance: A harmful relationship. J. Intern. Med. 2013, 274, 25–40. [Google Scholar] [CrossRef]
- Pascual, G.; Domínguez, D.; Benitah, S.A. The contributions of cancer cell metabolism to metastasis. Dis. Models Mech. 2018, 11, dmm032920. [Google Scholar] [CrossRef] [Green Version]
- Ibrahim, M.M. Subcutaneous and visceral adipose tissue: Structural and functional differences. Obes. Rev. 2010, 11, 11–18. [Google Scholar] [CrossRef]
- Opazo-Ríos, L.; Mas, S.; Marín-Royo, G.; Mezzano, S.; Gómez-Guerrero, C.; Moreno, J.A.; Egido, J. Lipotoxicity and Diabetic Nephropathy: Novel Mechanistic Insights and Therapeutic Opportunities. Int. J. Mol. Sci. 2020, 21, 2632. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, M.H.; Cao, Y.X.; Wu, L.G.; Guo, N.; Hou, B.J.; Sun, L.J.; Guo, Y.L.; Wu, N.Q.; Dong, Q.; Li, J.J. Association of plasma free fatty acids levels with the presence and severity of coronary and carotid atherosclerotic plaque in patients with type 2 diabetes mellitus. BMC Endocr. Disord. 2020, 20, 156. [Google Scholar] [CrossRef] [PubMed]
- Lowe, G.D. Common risk factors for both arterial and venous thrombosis. Br. J. Haematol. 2008, 140, 488–495. [Google Scholar] [CrossRef] [PubMed]
- Daryabor, G.; Atashzar, M.R.; Kabelitz, D.; Meri, S.; Kalantar, K. The Effects of Type 2 Diabetes Mellitus on Organ Metabolism and the Immune System. Front. Immunol. 2020, 11, 1582. [Google Scholar] [CrossRef] [PubMed]
- Valcarcel-Ares, M.N.; Tucsek, Z.; Kiss, T.; Giles, C.B.; Tarantini, S.; Yabluchanskiy, A.; Balasubramanian, P.; Gautam, T.; Galvan, V.; Ballabh, P.; et al. Obesity in Aging Exacerbates Neuroinflammation, Dysregulating Synaptic Function-Related Genes and Altering Eicosanoid Synthesis in the Mouse Hippocampus: Potential Role in Impaired Synaptic Plasticity and Cognitive Decline. J. Gerontol. Ser. A 2019, 74, 290–298. [Google Scholar] [CrossRef]
- Wang, Z.; Ge, Q.; Wu, Y.; Zhang, J.; Gu, Q.; Han, J. Impairment of long-term memory by a short-term high-fat diet via hippocampal oxidative stress and alterations in synaptic plasticity. Neuroscience 2020, 424, 24–33. [Google Scholar] [CrossRef]
- Folkman, J. Angiogenesis. In Biology of Endothelial Cells; Jaffe, E.A., Ed.; Springer: Boston, MA, USA, 1984; pp. 412–428. [Google Scholar] [CrossRef]
- Carmeliet, P. Angiogenesis in health and disease. Nat. Med. 2003, 9, 653–660. [Google Scholar] [CrossRef]
- Hamming, L.C.; Slotman, B.J.; Verheul, H.M.W.; Thijssen, V.L. The clinical application of angiostatic therapy in combination with radiotherapy: Past, present, future. Angiogenesis 2017, 20, 217–232. [Google Scholar] [CrossRef] [Green Version]
- Weiss, A.; Bonvin, D.; Berndsen, R.H.; Scherrer, E.; Wong, T.J.; Dyson, P.J.; Griffioen, A.W.; Nowak-Sliwinska, P. Angiostatic treatment prior to chemo- or photodynamic therapy improves anti-tumor efficacy. Sci. Rep. 2015, 5, 8990. [Google Scholar] [CrossRef] [Green Version]
- Hafidi, M.E.; Buelna-Chontal, M.; Sánchez-Muñoz, F.; Carbó, R. Adipogenesis: A Necessary but Harmful Strategy. Int. J. Mol. Sci. 2019, 20, 3657. [Google Scholar] [CrossRef] [Green Version]
- Herold, J.; Kalucka, J. Angiogenesis in Adipose Tissue: The Interplay Between Adipose and Endothelial Cells. Front. Physiol. 2021, 11, 1861. [Google Scholar] [CrossRef] [PubMed]
- Crewe, C.; An, Y.A.; Scherer, P.E. The ominous triad of adipose tissue dysfunction: Inflammation, fibrosis, and impaired angiogenesis. J. Clin. Investig. 2017, 127, 74–82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ribatti, D.; Crivellato, E. Immune cells and angiogenesis. J. Cell Mol. Med. 2009, 13, 2822–2833. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sunderkötter, C.; Goebeler, M.; Schulze-Osthoff, K.; Bhardwaj, R.; Sorg, C. Macrophage-derived angiogenesis factors. Pharmacol. Ther. 1991, 51, 195–216. [Google Scholar] [CrossRef]
- Catalan, V.; Gomez-Ambrosi, J.; Rodríguez, A.; Frühbeck, G. Adipose tissue immunity and cancer. Front. Physiol. 2013, 4, 275. [Google Scholar] [CrossRef] [Green Version]
- Nishida, N.; Yano, H.; Nishida, T.; Kamura, T.; Kojiro, M. Angiogenesis in cancer. Vasc. Health Risk Manag. 2006, 2, 213–219. [Google Scholar] [CrossRef]
- Aggarwal, B.B.; Shishodia, S.; Sandur, S.K.; Pandey, M.K.; Sethi, G. Inflammation and cancer: How hot is the link? Biochem. Pharmacol. 2006, 72, 1605–1621. [Google Scholar] [CrossRef]
- Nijhawans, P.; Behl, T.; Bhardwaj, S. Angiogenesis in obesity. Biomed. Pharm. 2020, 126, 110103. [Google Scholar] [CrossRef]
- Muz, B.; de la Puente, P.; Azab, F.; Azab, A.K. The role of hypoxia in cancer progression, angiogenesis, metastasis, and resistance to therapy. Hypoxia 2015, 3, 83–92. [Google Scholar] [CrossRef] [Green Version]
- Pogodziński, D.; Ostrowska, L.; Smarkusz-Zarzecka, J.; Zyśk, B. Secretome of Adipose Tissue as the Key to Understanding the Endocrine Function of Adipose Tissue. Int. J. Mol. Sci. 2022, 23, 2309. [Google Scholar] [CrossRef]
- Wang, H.; Chen, Y.; Lu, X.A.; Liu, G.; Fu, Y.; Luo, Y. Endostatin Prevents Dietary-Induced Obesity by Inhibiting Adipogenesis and Angiogenesis. Diabetes 2015, 64, 2442–2456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, Y.; Xue, L. Angiostatin. In Seminars in Thrombosis and Hemostasis; Thieme Medical Publishers, Inc.: New York, NY, USA, 2004; Volume 30, pp. 83–93. [Google Scholar] [CrossRef]
- Zhang, C.; Deng, W.-Y.; Li, N.; Luo, S.-X. Clinical observation and therapeutic evaluation of intravenous pump of recombinant human endostatin combined with TP regimen in treating patients with advanced ovarian cancer. Chronic Dis. Transl. Med. 2015, 1, 158–162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Phillips, L.H.; Whisnant, J.P.; Reagan, T.J. Sudden death from stroke. Stroke 1977, 8, 392–395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riezzo, I.; Zamparese, R.; Neri, M.; De Stefano, F.; Parente, R.; Pomara, C.; Turillazzi, E.; Ventura, F.; Fineschi, V. Sudden, unexpected death due to glioblastoma: Report of three fatal cases and review of the literature. Diagn. Pathol. 2013, 8, 73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weir, C.B.; Jan, A. BMI Classification Percentile and Cut off Points. In StatPearls; StatPearls Publishing: Tampa Island, FL, USA, 2022. [Google Scholar]
- Canning, K.L.; Brown, R.E.; Wharton, S.; Sharma, A.M.; Kuk, J.L. Edmonton Obesity Staging System Prevalence and Association with Weight Loss in a Publicly Funded Referral-Based Obesity Clinic. J. Obes. 2015, 2015, 619734. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clark, W.H. Tumour progression and the nature of cancer. Br. J. Cancer 1991, 64, 631–644. [Google Scholar] [CrossRef] [Green Version]
- Telloni, S.M. Tumor Staging and Grading: A Primer. Methods Mol. Biol. 2017, 1606, 1–17. [Google Scholar] [CrossRef]
- Smith, A.B.; Costa, D.; Galica, J.; Lebel, S.; Tauber, N.; van Helmondt, S.J.; Zachariae, R. Spotlight on the Fear of Cancer Recurrence Inventory (FCRI). Psychol. Res. Behav. Manag. 2020, 13, 1257–1268. [Google Scholar] [CrossRef]
- Mehta, T.; Smith, D.L., Jr.; Muhammad, J.; Casazza, K. Impact of weight cycling on risk of morbidity and mortality. Obes. Rev. 2014, 15, 870–881. [Google Scholar] [CrossRef]
- Bacon, J.G.; Scheltema, K.E.; Robinson, B.E. Fat phobia scale revisited: The short form. Int. J. Obes. 2001, 25, 252–257. [Google Scholar] [CrossRef] [Green Version]
- Stein, J.; Luppa, M.; Ruzanska, U.; Sikorski, C.; König, H.-H.; Riedel-Heller, S.G. Measuring Negative Attitudes towards Overweight and Obesity in the German Population—Psychometric Properties and Reference Values for the German Short Version of the Fat Phobia Scale (FPS). PLoS ONE 2014, 9, e114641. [Google Scholar] [CrossRef] [PubMed]
- Gomaa, E.Z. Human gut microbiota/microbiome in health and diseases: A review. Antonie Van Leeuwenhoek 2020, 113, 2019–2040. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.J.; Sears, C.L.; Maruthur, N. Gut microbiome and its role in obesity and insulin resistance. Ann. N. Y. Acad. Sci. 2020, 1461, 37–52. [Google Scholar] [CrossRef] [PubMed]
- Rajagopala, S.V.; Vashee, S.; Oldfield, L.M.; Suzuki, Y.; Venter, J.C.; Telenti, A.; Nelson, K.E. The Human Microbiome and Cancer. Cancer Prev. Res. 2017, 10, 226–234. [Google Scholar] [CrossRef] [Green Version]
- Adak, A.; Khan, M.R. An insight into gut microbiota and its functionalities. Cell Mol. Life Sci. 2019, 76, 473–493. [Google Scholar] [CrossRef]
- Chen, D.; Wu, J.; Jin, D.; Wang, B.; Cao, H. Fecal microbiota transplantation in cancer management: Current status and perspectives. Int. J. Cancer 2019, 145, 2021–2031. [Google Scholar] [CrossRef] [Green Version]
- Kang, Y.; Cai, Y. Gut microbiota and obesity: Implications for fecal microbiota transplantation therapy. Hormones 2017, 16, 223–234. [Google Scholar] [CrossRef]
- Bultman, S.J. The microbiome and its potential as a cancer preventive intervention. In Seminars in Oncology; Elsevier: Amsterdam, The Netherlands, 2016; pp. 97–106. [Google Scholar]
- Delzenne, N.M.; Neyrinck, A.M.; Cani, P.D. Modulation of the gut microbiota by nutrients with prebiotic properties: Consequences for host health in the context of obesity and metabolic syndrome. Microb. Cell Factories 2011, 10, S10. [Google Scholar] [CrossRef] [Green Version]
- Yang, M.; Fukui, H.; Eda, H.; Xu, X.; Kitayama, Y.; Hara, K.; Kodani, M.; Tomita, T.; Oshima, T.; Watari, J.; et al. Involvement of gut microbiota in association between GLP-1/GLP-1 receptor expression and gastrointestinal motility. Am. J. Physiol. Gastrointest Liver Physiol. 2017, 312, G367–G373. [Google Scholar] [CrossRef] [Green Version]
- Thompson, N.A.; Stewart, G.D.; Welsh, S.J.; Doherty, G.J.; Robinson, M.J.; Neville, B.A.; Vervier, K.; Harris, S.R.; Adams, D.J.; Dalchau, K.; et al. The MITRE trial protocol: A study to evaluate the microbiome as a biomarker of efficacy and toxicity in cancer patients receiving immune checkpoint inhibitor therapy. BMC Cancer 2022, 22, 99. [Google Scholar] [CrossRef]
- Cuevas-Sierra, A.; Ramos-Lopez, O.; Riezu-Boj, J.I.; Milagro, F.I.; Martinez, J.A. Diet, Gut Microbiota, and Obesity: Links with Host Genetics and Epigenetics and Potential Applications. Adv. Nutr. 2019, 10, S17–S30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miro-Blanch, J.; Yanes, O. Epigenetic regulation at the interplay between gut microbiota and host metabolism. Front. Genet. 2019, 10, 638. [Google Scholar] [CrossRef] [PubMed]
- Tabibnia, G.; Lieberman, M.D.; Craske, M.G. The lasting effect of words on feelings: Words may facilitate exposure effects to threatening images. Emotion 2008, 8, 307–317. [Google Scholar] [CrossRef] [PubMed]
- Darlow, B.; Dowell, A.; Baxter, G.D.; Mathieson, F.; Perry, M.; Dean, S. The enduring impact of what clinicians say to people with low back pain. Ann. Fam. Med. 2013, 11, 527–534. [Google Scholar] [CrossRef] [Green Version]
- Wood, M.L. Naming the illness: The power of words. Fam. Med. 1991, 23, 534–538. [Google Scholar]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boubertakh, B.; Silvestri, C.; Di Marzo, V. Obesity: The Fat Tissue Disease Version of Cancer. Cells 2022, 11, 1872. https://doi.org/10.3390/cells11121872
Boubertakh B, Silvestri C, Di Marzo V. Obesity: The Fat Tissue Disease Version of Cancer. Cells. 2022; 11(12):1872. https://doi.org/10.3390/cells11121872
Chicago/Turabian StyleBoubertakh, Besma, Cristoforo Silvestri, and Vincenzo Di Marzo. 2022. "Obesity: The Fat Tissue Disease Version of Cancer" Cells 11, no. 12: 1872. https://doi.org/10.3390/cells11121872