Cardiac Ischemia On-a-Chip: Antiarrhythmic Effect of Levosimendan on Ischemic Human-Induced Pluripotent Stem Cell-Derived Cardiomyocytes
Abstract
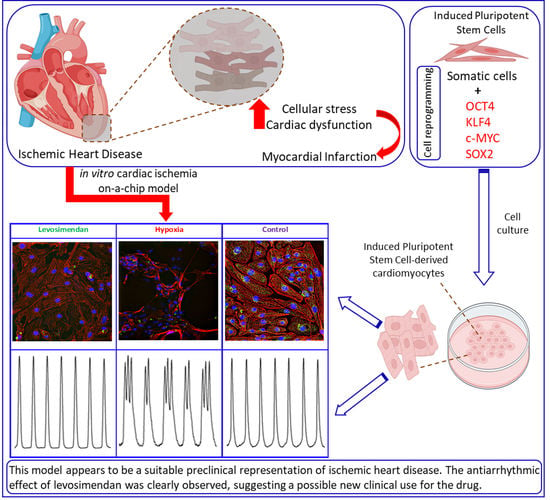
:1. Introduction
2. Materials and Methods
2.1. Generation and Differentiation of hiPSC Lines
2.2. Hypoxia Setup
2.3. Experimental Setup
2.4. Ca2+ Imaging
2.5. Video Microscopy Imaging
2.6. Immunocytochemistry
2.7. Reverse Transcription Polymerase Chain Reaction (RT-PCR)
2.8. Statistical Analysis
3. Results
3.1. Ca2+ Handling of hiPSC-CMs and the Effect of Levosimendan
3.2. Beating Characteristics of hiPSC-CMs with Video Imaging
3.3. Immunocytochemistry
3.4. Gene Expression Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Study Limitations
References
- Nowbar, A.N.; Gitto, M.; Howard, J.P.; Francis, D.P.; Al-Lamee, R. Mortality from Ischemic Heart Disease. Circ. Cardiovasc. Qual. Outcomes 2019, 12, e005375. [Google Scholar] [CrossRef] [PubMed]
- Frangogiannis, N.G. The extracellular matrix in myocardial injury, repair, and remodeling. J. Clin. Investig. 2017, 127, 1600–1612. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Talman, V.; Ruskoaho, H. Cardiac fibrosis in myocardial infarction—from repair and remodeling to regeneration. Cell Tissue Res. 2016, 365, 563–581. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hafez, P.; Chowdhury, S.; Jose, S.; Law, J.; Ruszymah, B.; Ramzisham, A.M.; Ng, M. Development of an In Vitro Cardiac Ischemic Model Using Primary Human Cardiomyocytes. Cardiovasc. Eng. Technol. 2018, 9, 529–538. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.G.; Ishikawa, K. Experimental models of cardiovascular diseases: Overview. In Experimental Models of Cardiovascular Diseases; Springer: New York, NY, USA, 2018; pp. 3–14. [Google Scholar]
- Tamargo, J.; Caballero, R.; Núñez, L.; Gómez, R.; Vaquero, M.; Deleon, E. Genetically engineered mice as a model for studying cardiac arrhythmias. Front. Biosci. 2007, 12, 22–38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ohnuki, M.; Takahashi, K.; Yamanaka, S. Generation and Characterization of Human Induced Pluripotent Stem Cells. Curr. Protoc. Stem Cell Biol. 2009, 9, 4A.2.1–4A.2.25. [Google Scholar] [CrossRef]
- Davis, J.; Chouman, A.; Creech, J.; da Rocha, A.M.; Ponce-Balbuena, D.; Vazquez, E.N.J.; Nichols, R.; Lozhkin, A.; Madamanchi, N.R.; Campbell, K.F.; et al. In vitro model of ischemic heart failure using human induced pluripotent stem cell–derived cardiomyocytes. JCI Insight 2021, 6, e134368. [Google Scholar] [CrossRef]
- Wei, H.; Wang, C.; Guo, R.; Takahashi, K.; Naruse, K. Development of a model of ischemic heart disease using cardiomyocytes differentiated from human induced pluripotent stem cells. Biochem. Biophys. Res. Commun. 2019, 520, 600–605. [Google Scholar] [CrossRef]
- Penttinen, K.; Swan, H.; Vanninen, S.; Paavola, J.; Lahtinen, A.M.; Kontula, K.; Aalto-Setälä, K. Antiarrhythmic effects of dantrolene in patients with catecholaminergic polymorphic ventricular tachycardia and replication of the responses using iPSC models. PLoS ONE 2015, 10, e0125366. [Google Scholar]
- Pölönen, R.P.; Penttinen, K.; Swan, H.; Aalto-Setälä, K. Antiarrhythmic Effects of Carvedilol and Flecainide in Cardiomyocytes Derived from Catecholaminergic Polymorphic Ventricular Tachycardia Patients. Stem Cells Int. 2018, 2018, 9109503. [Google Scholar] [CrossRef]
- Häkli, M.; Kreutzer, J.; Mäki, A.; Välimäki, H.; Lappi, H.; Huhtala, H.; Kallio, P.; Aalto-Setälä, K.; Pekkanen-Mattila, M. Human induced pluripotent stem cell-based platform for modeling cardiac ischemia. Sci. Rep. 2021, 11, 4153. [Google Scholar] [CrossRef] [PubMed]
- Duan, M.; Holland, D.; Duan, B. Concise review: Harnessing iPSC-derived cells for ischemic heart disease treatment. J. Transl. Intern. Med. 2020, 8, 20. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Liu, Y.; Zhang, Q.; Wang, Y.; Zhang, X.; Zhang, H. Danshen-Enhanced Cardioprotective Effect of Cardioplegia on Ischemia Reperfusion Injury in a Human-Induced Pluripotent Stem Cell-Derived Cardiomyocytes Model. Artif. Organs 2017, 41, 452–460. [Google Scholar] [CrossRef] [PubMed]
- Kurt, I.H. Use of levosimendan in patients with ischemic heart disease following mechanical reperfusion. Surg. Today 2009, 39, 381–386. [Google Scholar] [CrossRef] [PubMed]
- Haikala, H.; Kaheinen, P.; Levijoki, J.; Linden, I. The role of cAMP- and cGMP-dependent protein kinases in the cardiac actions of the new calcium sensitizer, levosimendan. Cardiovasc. Res. 1997, 34, 536–546. [Google Scholar] [CrossRef] [Green Version]
- Parissis, J.T.; Andreadou, I.; Bistola, V.; Paraskevaidis, I.; Filippatos, G.; Kremastinos, D.T. Novel biologic mechanisms of levosimendan and its effect on the failing heart. Expert Opin. Investig. Drugs 2008, 17, 1143–1150. [Google Scholar] [CrossRef]
- Pathak, A.; Lebrin, M.; Vaccaro, A.; Senard, J.M.; Despas, F. Pharmacology of levosimendan: Inotropic, vasodilatory and cardioprotective effects. J. Clin. Pharm. Ther. 2013, 38, 341–349. [Google Scholar] [CrossRef]
- Ojala, M.; Rajala, K.; Pekkanen-Mattila, M.; Miettinen, M.; Huhtala, H.; Aalto-Setälä, K. Culture Conditions Affect Cardiac Differentiation Potential of Human Pluripotent Stem Cells. PLoS ONE 2012, 7, e48659. [Google Scholar] [CrossRef] [Green Version]
- Mummery, C.; Zhang, J.; Ng, E.; Elliott, D.; Elefanty, A.; Kamp, T. Differentiation of Human Embryonic Stem Cells and Induced Pluripotent Stem Cells to Cardiomyocytes: A Methods Overview. Circ. Res. 2012, 111, 344–358. [Google Scholar] [CrossRef]
- Lian, X.; Zhang, J.; Azarin, S.M.; Zhu, K.; Hazeltine, L.B.; Bao, X.; Hsiao, C.; Kamp, T.J.; Palecek, S.P. Directed cardiomyocyte differentiation from human pluripotent stem cells by modulating Wnt/β-catenin signaling under fully defined conditions. Nat. Protoc. 2013, 8, 162–175. [Google Scholar] [CrossRef] [Green Version]
- Mummery, C.; Oostwaard, D.W.; Doevendans, P.; Spijker, R.; van den Brink, S.; Hassink, R.; van der Heyden, M.; Opthof, T.; Pera, M.; de la Riviere, S.; et al. Differentiation of Human Embryonic Stem Cells to Cardiomyocytes: Role of Coculture with Visceral Endoderm-Like Cells. Circulation 2003, 107, 2733–2740. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kreutzer, J.; Ylä-Outinen, L.; Mäki, A.; Ristola, M.; Narkilahti, S.; Kallio, P. Cell culture chamber with gas supply for prolonged recording of human neuronal cells on microelectrode array. J. Neurosci. Methods 2017, 280, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Metsälä, O.; Kreutzer, J.; Högel, H.; Miikkulainen, P.; Kallio, P.; Jaakkola, P.M. Transportable system enabling multiple irradiation studies under simultaneous hypoxia in vitro. Radiat. Oncol. 2018, 13, 220. [Google Scholar] [CrossRef] [PubMed]
- Sala, L.; van Meer, B.J.; Tertoolen, L.T.; Bakkers, J.; Bellin, M.; Davis, R.P.; Denning, C.N.; Dieben, M.A.; Eschenhagen, T.; Giacomelli, E.; et al. MUSCLEMOTION: A Versatile Open Software Tool to Quantify Cardiomyocyte and Cardiac Muscle Contraction In Vitro and In Vivo. Circ. Res. 2018, 122, e5–e16. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔC(T) method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Grossini, E.; Caimmi, P.P.; Platini, F.; Molinari, C.; Uberti, F.; Cattaneo, M.; Valente, G.; Mary, D.A.S.G.; Vacca, G.; Tessitore, L. Modulation of Programmed Forms of Cell Death by Intracoronary Levosimendan During Regional Myocardial Ischemia in Anesthetized Pigs. Cardiovasc. Drugs Ther. 2010, 24, 5–15. [Google Scholar] [CrossRef]
- Li, P.; Yang, Y.; Hwang, G.; Kao, L.; Lin, C. Inhibition of reverse-mode sodium-calcium exchanger activity and apoptosis by levosimendan in human cardiomyocyte progenitor cell-derived cardiomyocytes after anoxia and reoxygenation. PLoS ONE 2014, 9, e85909. [Google Scholar] [CrossRef]
- Gonzalez, M.J.G.; Rodriguez, A.D. Pharmacologic Treatment of Heart Failure due to Ventricular Dysfunction by Myocardial Stunning: Potential Role of Levosimendan. Am. J. Cardiovasc. Drugs 2006, 6, 69–75. [Google Scholar]
- McBride, B.F.; White, C.M. Levosimendan: Implications for Clinicians. J. Clin. Pharmacol. 2003, 43, 1071–1081. [Google Scholar] [CrossRef]
- Sedmera, D.; Kucera, P.; Raddatz, E. Developmental changes in cardiac recovery from anoxia-reoxygenation. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2002, 283, 379–388. [Google Scholar] [CrossRef]
- Yeung, C.; Sommerhage, F.; Wrobel, G.; Law, J.K.; Offenhäusser, A.; Rudd, J.A.; Ingebrandt, S.; Chan, M. To establish a pharmacological experimental platform for the study of cardiac hypoxia using the microelectrode array. J. Pharmacol. Toxicol. Methods 2009, 59, 146–152. [Google Scholar] [CrossRef] [PubMed]
- Saint, D.A. The cardiac persistent sodium current: An appealing therapeutic target? Br. J. Pharmacol. 2008, 153, 1133–1142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saint, D.A. The Role of the Persistent Na+ Current During Cardiac Ischemia and Hypoxia. J. Cardiovasc. Electrophysiol. 2006, 17 (Suppl. S1), S96–S103. [Google Scholar] [CrossRef] [PubMed]
- Barry, W.H. Na+Fuzzy Space: Does It Exist, and Is It Important in Ischemic Injury? J. Cardiovasc. Electrophysiol. 2006, 17 (Suppl S1), S43–S46. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Ma, J.; Zhang, P.; Luo, A. Redox reaction modulates transient and persistent sodium current during hypoxia in guinea pig ventricular myocytes. Pflügers Arch.-Eur. J. Physiol. 2007, 454, 461–475. [Google Scholar] [CrossRef] [PubMed]
- Belardinelli, L.; Shryock, J.C.; Fraser, H. Inhibition of the late sodium current as a potential cardioprotective principle: Effects of the late sodium current inhibitor ranolazine. Heart 2006, 92 (Suppl. S4), iv6–iv14. [Google Scholar] [CrossRef] [Green Version]
- Fernández-Morales, J.; Hua, W.; Yao, Y.; Morad, M. Regulation of Ca2+ signaling by acute hypoxia and acidosis in cardiomyocytes derived from human induced pluripotent stem cells. Cell Calcium 2019, 78, 1–14. [Google Scholar] [CrossRef]
- Sebastião, M.J.; Serra, M.; Pereira, R.; Palacios, I.; Gomes-Alves, P.; Alves, P.M. Human cardiac progenitor cell activation and regeneration mechanisms: Exploring a novel myocardial ischemia/reperfusion in vitro model. Stem Cell Res. Ther. 2019, 10, 77. [Google Scholar] [CrossRef] [Green Version]
- Hidalgo, A.; Glass, N.; Ovchinnikov, D.; Yang, S.; Zhang, X.; Mazzone, S.; Chen, C.; Wolvetang, E.; Cooper-White, J. Modelling ischemia-reperfusion injury (IRI) in vitro using metabolically matured induced pluripotent stem cell-derived cardiomyocytes. APL Bioeng. 2018, 2, 026102. [Google Scholar] [CrossRef] [Green Version]
- Yamasaki, Y.; Matsuura, K.; Sasaki, D.; Shimizu, T. Assessment of human bioengineered cardiac tissue function in hypoxic and re-oxygenized environments to understand functional recovery in heart failure. Regen. Ther. 2021, 18, 66–75. [Google Scholar] [CrossRef]
- Patterson, G.H.; Piston, D.W. Photobleaching in Two-Photon Excitation Microscopy. Biophys. J. 2000, 78, 2159–2162. [Google Scholar] [CrossRef] [Green Version]
- Wokosin, D.L.; Loughrey, C.M.; Smith, G.L. Characterization of a Range of Fura Dyes with Two-Photon Excitation. 2004. Available online: http://eprints.gla.ac.uk/19911 (accessed on 20 December 2021).
- Alfonso, F.S.; Zhou, Y.; Liu, E.; Mcguire, A.F.; Yang, Y.; Kantarci, H.; Li, D.; Copenhaver, E.; Zuchero, J.B.; Müller, H.; et al. Label-free optical detection of bioelectric potentials using electrochromic thin films. Proc. Natl. Acad. Sci. USA 2020, 117, 17260–17268. [Google Scholar] [CrossRef] [PubMed]
- Kopustinskiene, D.M.; Pollesello, P.; Saris, N.L. Potassium-specific effects of levosimendan on heart mitochondria. Biochem. Pharmacol. 2004, 68, 807–812. [Google Scholar] [CrossRef]
- Yokoshiki, H.; Katsube, Y.; Sunagawa, M.; Sperelakis, N. Levosimendan, a novel Ca 2q sensitizer, activates the glibenclamide-sensitive K q channel in rat arterial myocytes. Eur. J. Pharmacol. 1997, 333, 249. [Google Scholar] [CrossRef]
- Yokoshiki, H.; Katsube, Y.; Sunagawa, M.; Sperelakis, N. The Novel Calcium Sensitizer Levosimendan Activates the ATP-Sensitive K+ Channel in Rat Ventricular Cells. J. Pharmacol. Exp. Ther. 1997, 283, 375–383. [Google Scholar] [PubMed]
- Parissis, J.T.; Andreadou, I.; Markantonis, S.L.; Bistola, V.; Louka, A.; Pyriochou, A.; Paraskevaidis, I.; Filippatos, G.; Iliodromitis, E.K.; Kremastinos, D.T. Effects of Levosimendan on circulating markers of oxidative and nitrosative stress in patients with advanced heart failure. Atherosclerosis 2007, 195, e210–e215. [Google Scholar] [CrossRef]
- Imahashi, K.; Pott, C.; Goldhaber, J.I.; Steenbergen, C.; Philipson, K.D.; Murphy, E. Cardiac-Specific Ablation of the Na+-Ca2+ Exchanger Confers Protection Against Ischemia/Reperfusion Injury. Circ. Res. 2005, 97, 916–921. [Google Scholar] [CrossRef] [Green Version]
- Mebazaa, A.; Barraud, D.; Welschbillig, S. Randomized Clinical Trials with Levosimendan. Am. J. Cardiol. 2005, 96, 74–79. [Google Scholar] [CrossRef]
- Antoniades, C.; Tousoulis, D.; Koumallos, N.; Marinou, K.; Stefanadis, C. Levosimendan: Beyond its simple inotropic effect in heart failure. Pharmacol. Ther. 2007, 114, 184–197. [Google Scholar] [CrossRef]
- Lee, J.W.; Ko, J.; Ju, C.; Eltzschig, H.K. Hypoxia signaling in human diseases and therapeutic targets. Exp. Mol. Med. 2019, 51, 1–13. [Google Scholar] [CrossRef]
- Fink, S.L.; Cookson, B.T. Apoptosis, pyroptosis, and necrosis: Mechanistic description of dead and dying eukaryotic cells. Infect. Immun. 2005, 73, 1907–1916. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwon, H.; Lee, J.; Shin, H.; Kim, J.; Choi, S. Structural and functional analysis of cell adhesion and nuclear envelope nano-topography in cell death. Sci. Rep. 2015, 5, 15623. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okada, M.; Yamawaki, H. Levosimendan inhibits interleukin-1β-induced apoptosis through activation of Akt and inhibition of inducible nitric oxide synthase in rat cardiac fibroblasts. Eur. J. Pharmacol. 2015, 769, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Anonymous. Using next-generation RNA sequencing to examine ischemic changes induced by cold blood cardioplegia on the human left ventricular myocardium transcriptome. Anesthesiology 2015, 122, 537–550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pinho, J.; Scrimgeour, N.R.; Stolen, T.; Solvang-Garten, K.; Sharma, A.; Fonseca, D.M. Modeling of cardiac ischaemia and reperfusion injury: A human-based in vitro model using iPS-derived cardiomyocytes. Eur. Heart J. 2017, 38, 229–230. [Google Scholar] [CrossRef] [Green Version]
- Mylonis, I.; Simos, G.; Paraskeva, E. Hypoxia-Inducible Factors and the Regulation of Lipid Metabolism. Cells vol. 2019, 8, 214. [Google Scholar] [CrossRef] [Green Version]
- Schönenberger, M.J.; Kovacs, W.J. Hypoxia signaling pathways: Modulators of oxygen-related organelles. Front. Cell Dev. Biol. 2015, 3, 42. [Google Scholar] [CrossRef]
- Goetzenich, A.; Hatam, N.; Preuss, S.; Moza, A.; Bleilevens, C.; Roehl, A.B.; Autschbach, R.; Bernhagen, J.; Stoppe, C. The role of hypoxia-inducible factor-1α and vascular endothelial growth factor in late-phase preconditioning with xenon, isoflurane and levosimendan in rat cardiomyocytes. Interact. Cardiovasc. Thorac. Surg. 2014, 18, 321–328. [Google Scholar] [CrossRef] [Green Version]
- Revermann, M.; Schloss, M.; Mieth, A.; Babelova, A.; Schröder, K.; Neofitidou, S.; Buerkl, J.; Kirschning, T.; Schermuly, R.T.; Hofstetter, C.; et al. Levosimendan attenuates pulmonary vascular remodeling. Intensive Care Med. 2011, 37, 1368. [Google Scholar] [CrossRef]










Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gaballah, M.; Penttinen, K.; Kreutzer, J.; Mäki, A.-J.; Kallio, P.; Aalto-Setälä, K. Cardiac Ischemia On-a-Chip: Antiarrhythmic Effect of Levosimendan on Ischemic Human-Induced Pluripotent Stem Cell-Derived Cardiomyocytes. Cells 2022, 11, 1045. https://doi.org/10.3390/cells11061045
Gaballah M, Penttinen K, Kreutzer J, Mäki A-J, Kallio P, Aalto-Setälä K. Cardiac Ischemia On-a-Chip: Antiarrhythmic Effect of Levosimendan on Ischemic Human-Induced Pluripotent Stem Cell-Derived Cardiomyocytes. Cells. 2022; 11(6):1045. https://doi.org/10.3390/cells11061045
Chicago/Turabian StyleGaballah, Mahmoud, Kirsi Penttinen, Joose Kreutzer, Antti-Juhana Mäki, Pasi Kallio, and Katriina Aalto-Setälä. 2022. "Cardiac Ischemia On-a-Chip: Antiarrhythmic Effect of Levosimendan on Ischemic Human-Induced Pluripotent Stem Cell-Derived Cardiomyocytes" Cells 11, no. 6: 1045. https://doi.org/10.3390/cells11061045