Temporal Profiling of the Cortical Synaptic Mitochondrial Proteome Identifies Ageing Associated Regulators of Stability
Abstract
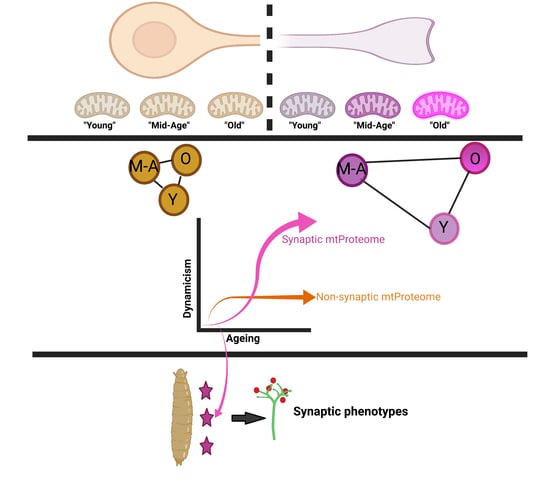
:1. Introduction
2. Methods
2.1. Ethics
2.2. Animals
2.3. Mitochondrial Isolations
2.4. Label-Free Proteomics
2.5. BioLayout Express3D
2.6. Ingenuity Pathway Analysis
2.7. Drosophila Stocks
2.8. Immunohistochemistry
2.9. Statistical Analyses
3. Results
3.1. Spatiotemporal Characterisation of Discrete Mitochondrial Proteomes
3.2. Mitochondrial Ageing Demonstrates Subcellular Heterogeneity
3.3. Temporal Protein Profiling of Discrete Mitochondrial Populations Reveals Protein Expression Trends Correlating with Synaptic Vulnerability
3.4. Temporal Regulation of the Synaptic Mitochondrial Proteome Can Modulate Synaptic Morphology
4. Discussion
Mitochondrial Candidates Modulating Synaptic Morphology
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bratic, A.; Larsson, N.-G. The role of mitochondria in aging. J. Clin. Investig. 2013, 123, 951–957. [Google Scholar] [CrossRef] [Green Version]
- Rossmann, M.P.; Dubois, S.M.; Agarwal, S.; Zon, L.I. Mitochondrial function in development and disease. Dis. Model. Mech. 2021, 14, dmm048912. [Google Scholar] [CrossRef]
- Ingram, T.; Chakrabarti, L. Proteomic profiling of mitochondria: What does it tell us about the ageing brain? Aging 2016, 8, 3161–3179. [Google Scholar] [CrossRef] [Green Version]
- Butsch, T.J.; Ghosh, B.; Bohnert, K.A. Organelle-Specific Autophagy in Cellular Aging and Rejuvenation. Adv. Geriatr. Med. Res. 2021, 3, e210010. [Google Scholar] [CrossRef] [PubMed]
- Forster, M.J.; Dubey, A.; Dawson, K.M.; Stutts, W.A.; Lal, H.; Sohal, R.S. Age-related losses of cognitive function and motor skills in mice are associated with oxidative protein damage in the brain. Proc. Natl. Acad. Sci. USA 1996, 93, 4765–4769. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.M.; He, W.; Liou, Y.-C. The redox language in neurodegenerative diseases: Oxidative post-translational modifications by hydrogen peroxide. Cell Death Dis. 2021, 12, 1–13. [Google Scholar] [CrossRef]
- Grimm, A.; Eckert, A. Brain aging and neurodegeneration: From a mitochondrial point of view. J. Neurochem. 2017, 143, 418–431. [Google Scholar] [CrossRef] [Green Version]
- Kandlur, A.; Satyamoorthy, K.; Gangadharan, G. Oxidative Stress in Cognitive and Epigenetic Aging: A Retrospective Glance. Front. Mol. Neurosci. 2020, 13, 41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Foley, T.D. Reductive Reprogramming: A Not-So-Radical Hypothesis of Neurodegeneration Linking Redox Perturbations to Neuroinflammation and Excitotoxicity. Cell. Mol. Neurobiol. 2019, 39, 577–590. [Google Scholar] [CrossRef] [PubMed]
- Gilmer, L.K.; Ansari, M.A.; Roberts, K.N.; Scheff, S.W. Age-related changes in mitochondrial respiration and oxidative damage in the cerebral cortex of the Fischer 344 rat. Mech. Ageing Dev. 2010, 131, 133–143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tatarkova, Z.; Kovalska, M.; Timkova, V.; Racay, P.; Lehotsky, J.; Kaplan, P. The Effect of Aging on Mitochondrial Complex I and the Extent of Oxidative Stress in the Rat Brain Cortex. Neurochem. Res. 2016, 41, 2160–2172. [Google Scholar] [CrossRef] [PubMed]
- Lores-Arnaiz, S.; Bustamante, J. Age-related alterations in mitochondrial physiological parameters and nitric oxide production in synaptic and non-synaptic brain cortex mitochondria. Neuroscience 2011, 188, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Lores-Arnaiz, S.; Lombardi, P.; Karadayian, A.G.; Orgambide, F.; Cicerchia, D.; Bustamante, J. Brain cortex mitochondrial bioenergetics in synaptosomes and non-synaptic mitochondria during aging. Neurochem. Res. 2016, 41, 353–363. [Google Scholar] [CrossRef]
- Brown, M.R.; Geddes, J.W.; Sullivan, P.G. Brain Region-Specific, Age-Related, Alterations in Mitochondrial Responses to Elevated Calcium. J. Bioenerg. Biomembr. 2004, 36, 401–406. [Google Scholar] [CrossRef] [PubMed]
- Pandya, V.A.; Patani, R. Region-specific vulnerability in neurodegeneration: Lessons from normal ageing. Ageing Res. Rev. 2021, 67, 101311. [Google Scholar] [CrossRef] [PubMed]
- Cortopassi, G.A.; Arnheim, N. Detection of a specific mitochondrial DNA deletion in tissues of older humans. Nucleic Acids Res. 1990, 18, 6927–6933. [Google Scholar] [CrossRef] [Green Version]
- Arnheim, N.; Cortopassi, G. Deleterious mitochondrial DNA mutations accumulate in aging human tissues. Mutat. Res. 1992, 275, 157–167. [Google Scholar] [CrossRef]
- Bua, E.; Johnson, J.; Herbst, A.; Delong, B.; McKenzie, D.; Salamat, S.; Aiken, J.M. Mitochondrial DNA–Deletion Mutations Accumulate Intracellularly to Detrimental Levels in Aged Human Skeletal Muscle Fibers. Am. J. Hum. Genet. 2006, 79, 469–480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kauppila, T.E.S.; Bratic, A.; Jensen, M.B.; Baggio, F.; Partridge, L.; Jasper, H.; Grönke, S.; Larsson, N.-G. Mutations of mitochondrial DNA are not major contributors to aging of fruit flies. Proc. Natl. Acad. Sci. USA 2018, 115, E9620–E9629. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hara, Y.; Yuk, F.; Puri, R.; Janssen, W.G.M.; Rapp, P.R.; Morrison, J.H. Presynaptic mitochondrial morphology in monkey prefrontal cortex correlates with working memory and is improved with estrogen treatment. Proc. Natl. Acad. Sci. USA 2014, 111, 486–491. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.J.; McIntyre, R.; Janssens, G.E.; Houtkooper, R.H. Mitochondrial fission and fusion: A dynamic role in aging and potential target for age-related disease. Mech. Ageing Dev. 2020, 186, 111212. [Google Scholar] [CrossRef]
- Faitg, J.; Lacefield, C.; Davey, T.; White, K.; Laws, R.; Kosmidis, S.; Reeve, A.K.; Kandel, E.R.; Vincent, A.E.; Picard, M. 3D neuronal mitochondrial morphology in axons, dendrites, and somata of the aging mouse hippocampus. Cell Rep. 2021, 36, 109509. [Google Scholar] [CrossRef] [PubMed]
- Picard, M.; McEwen, B.S. Mitochondria impact brain function and cognition. Proc. Natl. Acad. Sci. USA 2014, 111, 7–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, T.; Qiao, H.; Pan, P.-Y.; Chen, Y.; Sheng, Z.-H. Motile Axonal Mitochondria Contribute to the Variability of Presynaptic Strength. Cell Rep. 2013, 4, 413–419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwon, S.-K.; Sando, R.; Lewis, T.L.; Hirabayashi, Y.; Maximov, A.; Polleux, F. LKB1 Regulates Mitochondria-Dependent Presynaptic Calcium Clearance and Neurotransmitter Release Properties at Excitatory Synapses along Cortical Axons. PLoS Biol. 2016, 14, e1002516. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferrándiz, M.L.; Martínez, M.; De Juan, E.; Díez, A.; Bustos, G.; Miquel, J. Impairment of mitochondrial oxidative phosphorylation in the brain of aged mice. Brain Res. 1994, 644, 335–338. [Google Scholar] [CrossRef]
- Stauch, K.L.; Purnell, P.; Fox, H.S. Quantitative Proteomics of Synaptic and Nonsynaptic Mitochondria: Insights for Synaptic Mitochondrial Vulnerability. J. Proteome Res. 2014, 13, 2620–2636. [Google Scholar] [CrossRef]
- Stauch, K.L.; Purnell, P.; Fox, H.S. Aging synaptic mitochondria exhibit dynamic proteomic changes while maintaining bioenergetic function. Aging 2014, 6, 320–334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, A.; Hirabayashi, Y.; Kwon, S.-K.; Lewis, T.L.; Polleux, F. Emerging roles of mitochondria in synaptic transmission and neurodegeneration. Curr. Opin. Physiol. 2018, 3, 82–93. [Google Scholar] [CrossRef]
- Tavallaie, M.; Voshtani, R.; Deng, X.; Qiao, Y.; Jiang, F.; Collman, J.P.; Fu, L. Moderation of mitochondrial respiration mitigates metabolic syndrome of aging. Proc. Natl. Acad. Sci. USA 2020, 117, 9840–9850. [Google Scholar] [CrossRef]
- Graham, L.C.; Eaton, S.L.; Brunton, P.; Atrih, A.; Smith, C.; Lamont, D.J.; Gillingwater, T.; Pennetta, G.; Skehel, P.A.; Wishart, T.M. Proteomic profiling of neuronal mitochondria reveals modulators of synaptic architecture. Mol. Neurodegener. 2017, 12, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Graham, L.C.; Naldrett, M.J.; Kohama, S.G.; Smith, C.; Lamont, D.J.; McColl, B.W.; Gillingwater, T.; Skehel, P.; Urbanski, H.F.; Wishart, T.M. Regional Molecular Mapping of Primate Synapses during Normal Healthy Aging. Cell Rep. 2019, 27, 1018–1026.e4. [Google Scholar] [CrossRef] [Green Version]
- Hardy, J.A.; Dodd, P.R.; Oakley, A.E.; Perry, R.H.; Edwardson, J.A.; Kidd, A.M. Metabolically Active Synaptosomes Can Be Prepared from Frozen Rat and Human Brain. J. Neurochem. 1983, 40, 608–614. [Google Scholar] [CrossRef]
- Lai, J.C.K.; Walsh, J.M.; Dennis, S.C.; Clark, J.B. SYNAPTIC AND NON-SYNAPTIC MITOCHONDRIA FROM RAT BRAIN: ISOLATION AND CHARACTERIZATION. J. Neurochem. 1977, 28, 625–631. [Google Scholar] [CrossRef]
- Theocharidis, A.; Van Dongen, S.; Enright, A.; Freeman, T. Network visualization and analysis of gene expression data using BioLayout Express3D. Nat. Protoc. 2009, 4, 1535–1550. [Google Scholar] [CrossRef] [PubMed]
- Hurtado, M.L.; Fuller, H.R.; Wong, A.M.S.; Eaton, S.L.; Gillingwater, T.H.; Pennetta, G.; Cooper, J.D.; Wishart, T.M. Proteomic mapping of differentially vulnerable pre-synaptic populations identifies regulators of neuronal stability in vivo. Sci. Rep. 2017, 7, 12412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kline, R.A.; Wishart, T.M.; Mills, K.; Heywood, W.E. Applying modern Omic technologies to the Neuronal Ceroid Lipofuscinoses. Biochim. Et Biophys. Acta (BBA) Mol. Basis Dis. 2020, 1866, 165498. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Flockhart, I.; Vinayagam, A.; Bergwitz, C.; Berger, B.; Perrimon, N.; Mohr, S.E. An integrative approach to ortholog prediction for disease-focused and other functional studies. BMC Bioinform. 2011, 12, 357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andlauer, T.F.; Sigrist, S.J. Quantitative Analysis of Drosophila Larval Neuromuscular Junction Morphology. Cold Spring Harb. Protoc. 2012, 2012, 490–493. [Google Scholar] [CrossRef] [Green Version]
- Toescu, E.; Myronova, N.; Verkhratsky, A. Age-related structural and functional changes of brain mitochondria. Cell Calcium 2000, 28, 329–338. [Google Scholar] [CrossRef]
- Xiong, J.; Verkhratsky, A.; Toescu, E.C. Changes in Mitochondrial Status Associated with Altered Ca2+Homeostasis in Aged Cerebellar Granule Neurons in Brain Slices. J. Neurosci. 2002, 22, 10761–10771. [Google Scholar] [CrossRef] [PubMed]
- Todorova, V. Mitochondria and Synaptic Plasticity in the Mature and Aging Nervous System. Curr. Neuropharmacol. 2016, 15, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Kollins, K.M.; Davenport, R.W. Branching Morphogenesis in Vertebrate Neurons. In Madame Curie Bioscience Database [Internet]; Landes Bioscience: Austin, TX, USA, 2000–2013. [Google Scholar]
- Bell, C.G.; Lowe, R.; Adams, P.D.; Baccarelli, A.A.; Beck, S.; Bell, J.T.; Christensen, B.C.; Gladyshev, V.N.; Heijmans, B.T.; Horvath, S.; et al. DNA methylation aging clocks: Challenges and recommendations. Genome Biol. 2019, 20, 1–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bacalini, M.G.; D’Aquila, P.; Marasco, E.; Nardini, C.; Montesanto, A.; Franceschi, C.; Passarino, G.; Garagnani, P.; Bellizzi, D. The methylation of nuclear and mitochondrial DNA in ageing phenotypes and longevity. Mech. Ageing Dev. 2017, 165, 156–161. [Google Scholar] [CrossRef] [PubMed]
- Devall, M.; Smith, R.G.; Jeffries, A.; Hannon, E.; Davies, M.N.; Schalkwyk, L.; Mill, J.; Weedon, M.; Lunnon, K. Regional differences in mitochondrial DNA methylation in human post-mortem brain tissue. Clin. Epigenetics 2017, 9, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zinovkina, L.A.; Zinovkin, R.A. DNA methylation, mitochondria, and programmed aging. Biochemistry (Moscow) 2015, 80, 1571–1577. [Google Scholar] [CrossRef]
- Rots, M.G. Regulation of mitochondrial gene expression the epigenetic enigma. Front. Biosci. 2017, 22, 1099–1113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matkovic, T.; Siebert, M.; Knoche, E.; Depner, H.; Mertel, S.; Owald, D.; Schmidt, M.; Thomas, U.; Sickmann, A.; Kamin, D.; et al. The Bruchpilot cytomatrix determines the size of the readily releasable pool of synaptic vesicles. J. Cell Biol. 2013, 202, 667–683. [Google Scholar] [CrossRef] [Green Version]
- Bao, X.; Faris, A.E.; Jang, E.K.; Haslam, R.J. Molecular cloning, bacterial expression and properties of Rab31and Rab32. JBIC J. Biol. Inorg. Chem. 2002, 269, 259–271. [Google Scholar] [CrossRef]
- Chua, C.E.L.; Tang, B.L. Engagement of the Small GTPase Rab31 Protein and Its Effector, Early Endosome Antigen 1, Is Important for Trafficking of the Ligand-bound Epidermal Growth Factor Receptor from the Early to the Late Endosome. J. Biol. Chem. 2014, 289, 12375–12389. [Google Scholar] [CrossRef] [Green Version]
- Wei, D.; Zhan, W.; Gao, Y.; Huang, L.; Gong, R.; Wang, W.; Zhang, R.; Wu, Y.; Gao, S.; Kang, T. RAB31 marks and controls an ESCRT-independent exosome pathway. Cell Res. 2021, 31, 157–177. [Google Scholar] [CrossRef] [PubMed]
- Star, E.N.; Newton, A.J.; Murthy, V.N. Real-time imaging of Rab3a and Rab5a reveals differential roles in presynaptic function. J. Physiol. 2005, 569, 103–117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aspenström, P.; Fransson, Å.; Saras, J. Rho GTPases have diverse effects on the organization of the actin filament system. Biochem. J. 2004, 377, 327–337. [Google Scholar] [CrossRef] [PubMed]
- Franke, K.; Otto, W.; Johannes, S.; Baumgart, J.; Nitsch, R.; Schumacher, S. miR-124-regulated RhoG reduces neuronal process complexity via ELMO/Dock180/Rac1 and Cdc42 signalling. EMBO J. 2012, 31, 2908–2921. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marland, J.; Hasel, P.; Bonnycastle, K.; Cousin, M.A. Mitochondrial Calcium Uptake Modulates Synaptic Vesicle Endocytosis in Central Nerve Terminals. J. Biol. Chem. 2016, 291, 2080–2086. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qiu, J.; Tan, Y.-W.; Hagenston, A.M.; Martel, M.-A.; Kneisel, N.; Skehel, P.A.; Wyllie, D.; Bading, H.; Hardingham, G.E. Mitochondrial calcium uniporter Mcu controls excitotoxicity and is transcriptionally repressed by neuroprotective nuclear calcium signals. Nat. Commun. 2013, 4, 2034. [Google Scholar] [CrossRef] [Green Version]







| Gene | Source | Annotation Symbol | Drosophila Stock ID | DIOPT Score |
|---|---|---|---|---|
| Mcu | BDSC | CG18769 | 42,580 | 9 |
| Rab31 | BDSC | CG3870 | 9837 | 2 |
| Rhog | BDSC | CG8556 | 26,650 | 3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Graham, L.C.; Kline, R.A.; Lamont, D.J.; Gillingwater, T.H.; Mabbott, N.A.; Skehel, P.A.; Wishart, T.M. Temporal Profiling of the Cortical Synaptic Mitochondrial Proteome Identifies Ageing Associated Regulators of Stability. Cells 2021, 10, 3403. https://doi.org/10.3390/cells10123403
Graham LC, Kline RA, Lamont DJ, Gillingwater TH, Mabbott NA, Skehel PA, Wishart TM. Temporal Profiling of the Cortical Synaptic Mitochondrial Proteome Identifies Ageing Associated Regulators of Stability. Cells. 2021; 10(12):3403. https://doi.org/10.3390/cells10123403
Chicago/Turabian StyleGraham, Laura C., Rachel A. Kline, Douglas J. Lamont, Thomas H. Gillingwater, Neil A. Mabbott, Paul A. Skehel, and Thomas M. Wishart. 2021. "Temporal Profiling of the Cortical Synaptic Mitochondrial Proteome Identifies Ageing Associated Regulators of Stability" Cells 10, no. 12: 3403. https://doi.org/10.3390/cells10123403