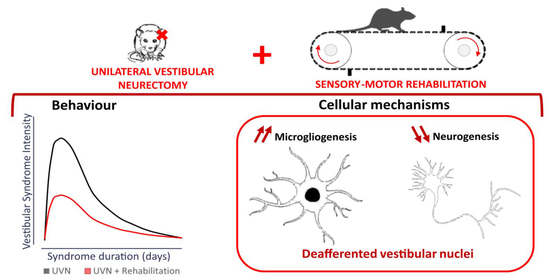
Sensorimotor Rehabilitation Promotes Vestibular Compensation in a Rodent Model of Acute Peripheral Vestibulopathy by Promoting Microgliogenesis in the Deafferented Vestibular Nuclei
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Study Design
2.3. Surgery
2.4. Sensory-Motor Rehabilitation
2.5. Qualitative Evaluation of the Vestibular Syndrome
2.6. Quantitative Evaluation of the Vestibular Syndrome
2.6.1. Analysis Device
2.6.2. Data Analysis
- -
- The weight distribution on the left paws (static vs. dynamic), which represents the percentage of weight the animal applies on its left paws. In healthy animals, this percentage is equally distributed between right and left.
- -
- The time spent on the abdomen, in which we add the time of each support located between the four paws of the animal.
- -
- The circling, where each complete and fast lap made by the animal is counted.
- -
- The distance travelled and the average speed of the animal, calculated when the animal is dynamic.
- -
- The body sway, which is the average of the area of the confidence ellipse at 90% of the points of the barycenter. It is a classical index of postural stability in posturology.
- -
- The amount of energy spent for postural stabilization, which is a ratio between the Speed of displacement of the barycenter and the body sway.
2.7. Cellular Investigation
2.7.1. Tissue Preparation
2.7.2. Immunohistochemistry
2.7.3. Cells Count
2.8. Statistical Analysis
3. Results
3.1. Qualitative Evaluation of the Vestibular Syndrome
3.2. Quantitative Evaluation of the Vestibular Syndrome
3.2.1. Lateral Weight Distribution
3.2.2. Distance Travelled
3.2.3. Average Speed
3.2.4. Time Spent in the Abdomen
3.2.5. Left Circling
3.2.6. Posturological Parameters
3.3. Influence of the Rehabilitation Protocol on Cell Populations of the Ipsilateral Vestibular Nucleus
3.3.1. Cell Population Counts
3.3.2. New Cells Differentiation
4. Discussion
4.1. Recovery Kinetics of Postural and Locomotor Functions
4.1.1. Immediate Restoration of Static Deficits
4.1.2. Later Dynamic Recovery
4.2. Neurobiological Correlates of Sensorimotor Rehabilitation in the VN
4.3. Clinical Relevance
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cullen, K.E. The vestibular system: Multimodal integration and encoding of self-motion for motor control. Trends Neurosci. 2012, 35, 185–196. [Google Scholar] [CrossRef] [Green Version]
- Dieterich, M.; Brandt, T. The bilateral central vestibular system: Its pathways, functions, and disorders. Ann. N. Y. Acad. Sci. 2015, 1343, 10–26. [Google Scholar] [CrossRef]
- Lacour, M.; Barthelemy, J.; Borel, L.; Magnan, J.; Xerri, C.; Chays, A.; Ouaknine, M. Sensory strategies in human postural control before and after unilateral vestibular neurotomy. Exp. Brain Res. 1997, 115, 300–310. [Google Scholar] [CrossRef]
- Borel, L.; Harlay, F.; Lopez, C.; Magnan, J.; Chays, A.; Lacour, M. Walking performance of vestibular-defective patients before and after unilateral vestibular neurotomy. Behav. Brain Res. 2004, 150, 191–200. [Google Scholar] [CrossRef]
- Borel, L.; Harlay, F.; Magnan, J.; Chays, A.; Lacour, M. Deficits and recovery of head and trunk orientation and stabilization after unilateral vestibular loss. Brain 2002, 125, 880–894. [Google Scholar] [CrossRef] [Green Version]
- Smith, P.F.; Curthoys, I.S. Mechanisms of recovery following unilateral labyrinthectomy: A review. Brain Res. Rev. 1989, 14, 155–180. [Google Scholar] [CrossRef]
- Darlington, C.L.; Dutia, M.B.; Smith, P.F. The contribution of the intrinsic excitability of vestibular nucleus neurons to recovery from vestibular damage. Eur. J. Neurosci. 2002, 15, 1719–1727. [Google Scholar] [CrossRef]
- Tighilet, B.; Chabbert, C. Adult neurogenesis promotes balance recovery after vestibular loss. Prog. Neurobiol. 2019, 174, 28–35. [Google Scholar] [CrossRef]
- Rastoldo, G.; El Mahmoudi, N.; Marouane, E.; Pericat, D.; Watabe, I.; Toneto, A.; López-Juárez, A.; Chabbert, C.; Tighilet, B. Adult and endemic neurogenesis in the vestibular nuclei after unilateral vestibular neurectomy. Prog. Neurobiol. 2021, 196. [Google Scholar] [CrossRef]
- Dutheil, S.; Lacour, M.; Tighilet, B. Neurogenic potential of the vestibular nuclei and behavioural recovery time course in the adult cat are governed by the nature of the vestibular damage. PLoS ONE 2011, 6, e22262. [Google Scholar] [CrossRef] [Green Version]
- Dutheil, S.; Watabe, I.; Sadlaoud, K.; Tonetto, A.; Tighilet, B. BDNF signaling promotes vestibular compensation by increasing neurogenesis and remodeling the expression of potassium-chloride cotransporter KCC2 and GABAA receptor in the vestibular nuclei. J. Neurosci. 2016, 36, 6199–6212. [Google Scholar] [CrossRef] [Green Version]
- Tighilet, B.; Dutheil, S.; Siponen, M.I.; Noreña, A.J. Reactive neurogenesis and down-regulation of the potassium-chloride cotransporter KCC2 in the cochlear nuclei after cochlear deafferentation. Front. Pharmacol. 2016, 7, 281. [Google Scholar] [CrossRef] [Green Version]
- Dutheil, S.; Brezun, J.M.; Leonard, J.; Lacour, M.; Tighilet, B. Neurogenesis and astrogenesis contribution to recovery of vestibular functions in the adult cat following unilateral vestibular neurectomy: Cellular and behavioral evidence. Neuroscience 2009, 164, 1444–1456. [Google Scholar] [CrossRef]
- Liberge, M.; Manrique, C.; Bernard-Demanze, L.; Lacour, M. Changes in TNFα, NFκB and MnSOD protein in the vestibular nuclei after unilateral vestibular deafferentation. J. Neuroinflamm. 2010, 7, 91. [Google Scholar] [CrossRef] [Green Version]
- Chabbert, C. New insights into vestibular neuropharmacology: From bench to bedside. J. Vestib. Res. Equilib. Orientat. 2013, 23, 107–111. [Google Scholar] [CrossRef]
- Weckel, A.; Fraysse, M.J.E.; Marx, M.; Fraysse, B.; Gallois, Y.; Chabbert, C. Vestibular disorders: Clinician ENT perspective on the need for research and innovation. J. Neurol. 2020, 267, 36–44. [Google Scholar] [CrossRef]
- Soto, E.; Vega, R. Neuropharmacology of Vestibular System Disorders. Curr. Neuropharmacol. 2010, 8, 26–40. [Google Scholar] [CrossRef] [Green Version]
- Sulway, S.; Whitney, S.L. Advances in vestibular rehabilitation. Adv. Otorhinolaryngol. 2019, 82, 164–169. [Google Scholar] [CrossRef]
- Herdman, S.J.; Blatt, P.J.; Schubert, M.C. Vestibular rehabilitation of patients with vestibular hypofunction or with benign paroxysmal positional vertigo. Curr. Opin. Neurobiol. 2000, 13, 39–43. [Google Scholar] [CrossRef]
- Tjernström, F.; Zur, O.; Jahn, K. Current concepts and future approaches to vestibular rehabilitation. J. Neurol. 2016, 263, 65–70. [Google Scholar] [CrossRef] [Green Version]
- McDonnell, M.N.; Hillier, S.L. Vestibular rehabilitation for unilateral peripheral vestibular dysfunction. Cochrane Database Syst. Rev. 2015, 2015, 1–95. [Google Scholar] [CrossRef]
- Balaban, C.D.; Hoffer, M.E.; Gottshall, K.R. Top-down approach to vestibular compensation: Translational lessons from vestibular rehabilitation. Brain Res. 2012, 1482, 101–111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shefer, S.; Gordon, C.; Avraham, K.B.; Mintz, M. Balance deficit enhances anxiety and balance training decreases anxiety in vestibular mutant mice. Behav. Brain Res. 2015, 276, 76–83. [Google Scholar] [CrossRef]
- Battilana, F.; Steurer, S.; Rizzi, G.; Delgado, A.C.; Tan, K.R.; Handschin, C. Exercise-linked improvement in age-associated loss of balance is associated with increased vestibular input to motor neurons. Aging Cell 2020, 19, e13274. [Google Scholar] [CrossRef]
- Stranahan, M.A.; Martin, B.; Maudsley, S. Anti-Inflammatory Effects of Physical Activity in Relationship to Improved Cognitive Status in Humans and Mouse Models of Alzheimers Disease. Curr. Alzheimer Res. 2012, 9, 86–92. [Google Scholar] [CrossRef] [Green Version]
- Nokia, M.S.; Lensu, S.; Ahtiainen, J.P.; Johansson, P.P.; Koch, L.G.; Britton, S.L.; Kainulainen, H. Physical exercise increases adult hippocampal neurogenesis in male rats provided it is aerobic and sustained. J. Physiol. 2016, 594, 1855–1873. [Google Scholar] [CrossRef]
- Van Praag, H.; Christie, B.R.; Sejnowski, T.J.; Gage, F.H. Running enhances neurogenesis, learning, and long-term potentiation in mice. Proc. Natl. Acad. Sci. USA 1999, 96, 13427–13431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marouane, E.; Rastoldo, G.; El Mahmoudi, N.; Péricat, D.; Chabbert, C.; Artzner, V.; Tighilet, B. Identification of New Biomarkers of Posturo-Locomotor Instability in a Rodent Model of Vestibular Pathology. Front. Neurol. 2020, 11, 470. [Google Scholar] [CrossRef] [PubMed]
- Péricat, D.; Farina, A.; Agavnian-Couquiaud, E.; Chabbert, C.; Tighilet, B. Complete and irreversible unilateral vestibular loss: A novel rat model of vestibular pathology. J. Neurosci. Methods 2017, 283, 83–91. [Google Scholar] [CrossRef]
- Lacour, M.; Bernard-Demanze, L. Interaction between vestibular compensation mechanisms and vestibular rehabilitation therapy: 10 recommendations for optimal functional recovery. Front. Neurol. 2015, 5, 285. [Google Scholar] [CrossRef] [Green Version]
- Tighilet, B.; Péricat, D.; Frelat, A.; Cazals, Y.; Rastoldo, G.; Boyer, F.; Dumas, O.; Chabbert, C. Adjustment of the dynamic weight distribution as a sensitive parameter for diagnosis of postural alteration in a rodent model of vestibular deficit. PLoS ONE 2017, 12, e0187472. [Google Scholar] [CrossRef] [Green Version]
- Rastoldo, G.; Marouane, E.; El Mahmoudi, N.; Péricat, D.; Bourdet, A.; Timon-David, E.; Dumas, O.; Chabbert, C.; Tighilet, B. Quantitative Evaluation of a New Posturo-Locomotor Phenotype in a Rodent Model of Acute Unilateral Vestibulopathy. Front. Neurol. 2020, 11, 505. [Google Scholar] [CrossRef] [PubMed]
- Lacour, M.; Tighilet, B. Plastic events in the vestibular nuclei during vestibular compensation: The brain orchestration of a “deafferentation” code. Restor. Neurol. Neurosci. 2010, 28, 19–35. [Google Scholar] [CrossRef]
- Facchini, J.; Rastoldo, G.; Xerri, C.; Péricat, D.; El Ahmadi, A.; Tighilet, B.; Zennou-Azogui, Y. Unilateral vestibular neurectomy induces a remodeling of somatosensory cortical maps. Prog. Neurobiol. 2021, 205, 102119. [Google Scholar] [CrossRef]
- Jendzjowsky, N.; Delorey, D.S. A prospective evaluation of non-interval- and interval-based exercise training progressions in rodents. Appl. Physiol. Nutr. Metab. 2011, 36, 723–729. [Google Scholar] [CrossRef]
- Cassel, R.; Bordiga, P.; Pericat, D.; Hautefort, C.; Tighilet, B.; Chabbert, C. New mouse model for inducing and evaluating unilateral vestibular deafferentation syndrome. J. Neurosci. Methods 2018, 293, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Tighilet, B.; Leonard, J.; Bernard-Demanze, L.; Lacour, M. Comparative analysis of pharmacological treatments with N-acetyl-dl-leucine (Tanganil) and its two isomers (N-acetyl-L-leucine and N-acetyl-D-leucine) on vestibular compensation: Behavioral investigation in the cat. Eur. J. Pharmacol. 2015, 769, 342–349. [Google Scholar] [CrossRef]
- Montardy, Q.; Wei, M.; Liu, X.; Yi, T.; Zhou, Z.; Lai, J.; Zhao, B.; Besnard, S.; Tighilet, B.; Chabbert, C.; et al. Selective optogenetic stimulation of glutamatergic, but not GABAergic, vestibular nuclei neurons induces immediate and reversible postural imbalance in mice. Prog. Neurobiol. 2021, 204, 102085. [Google Scholar] [CrossRef] [PubMed]
- El Mahmoudi, N.; Rastoldo, G.; Marouane, E.; Péricat, D.; Watabe, I.; Tonetto, A.; Hautefort, C.; Chabbert, C.; Sargolini, F.; Tighilet, B. Breaking a dogma: Acute anti-inflammatory treatment alters both post-lesional functional recovery and endogenous adaptive plasticity mechanisms in a rodent model of acute peripheral vestibulopathy. J. Neuroinflamm. 2021, 18, 183. [Google Scholar] [CrossRef]
- Gage, G.J.; Kipke, D.R.; Shain, W. Whole animal perfusion fixation for rodents. J. Vis. Exp. 2012, 65, e3564. [Google Scholar] [CrossRef] [Green Version]
- Tighilet, B.; Brezun, J.M.; Dit Duflo Sylvie, G.; Gaubert, C.; Lacour, M. New neurons in the vestibular nuclei complex after unilateral vestibular neurectomy in the adult cat. Eur. J. Neurosci. 2007, 25, 47–58. [Google Scholar] [CrossRef]
- Highstein, S.M.; Holstein, G.R. The Anatomy of the vestibular nuclei. Prog. Brain Res. 2006, 151, 157–203. [Google Scholar] [CrossRef]
- Ris, L.; Godaux, E. Neuronal activity in the vestibular nuclei after contralateral or bilateral labyrinthectomy in the alert guinea pig. J. Neurophysiol. 1998, 80, 2352–2367. [Google Scholar] [CrossRef] [Green Version]
- McCall, A.A.; Miller, D.M.; Yates, B.J. Descending influences on vestibulospinal and vestibulosympathetic reflexes. Front. Neurol. 2017, 8, 112. [Google Scholar] [CrossRef] [Green Version]
- Gimmon, Y.; Millar, J.; Pak, R.; Liu, E.; Schubert, M.C. Central not peripheral vestibular processing impairs gait coordination, Springer Berlin Heidelberg. Exp. Brain Res. 2017, 235, 3345–3355. [Google Scholar] [CrossRef] [PubMed]
- Maitre, J.; Paillard, T. Postural effects of vestibular manipulation depend on the physical activity status. PLoS ONE 2016, 11, e0162966. [Google Scholar] [CrossRef] [PubMed]
- Stiles, L.; Smith, P.F. The vestibular-basal ganglia connection: Balancing motor control. Brain Res. 2015, 1597, 180–188. [Google Scholar] [CrossRef]
- Löscher, W. Abnormal circling behavior in rat mutants and its relevance to model specific brain dysfunctions. Neurosci. Biobehav. Rev. 2010, 34, 31–49. [Google Scholar] [CrossRef] [PubMed]
- Lacour, M.; Helmchen, C.; Vidal, P.P. Vestibular compensation: The neuro-otologist’s best friend. J. Neurol. 2016, 263, 54–64. [Google Scholar] [CrossRef] [Green Version]
- Van Praag, H.; Kempermann, G.; Gage, F.H. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat. Neurosci. 1999, 2, 266–270. [Google Scholar] [CrossRef]
- Brandt, M.D.; Maass, A.; Kempermann, G.; Storch, A. Physical exercise increases Notch activity, proliferation and cell cycle exit of type-3 progenitor cells in adult hippocampal neurogenesis. Eur. J. Neurosci. 2010, 32, 1256–1264. [Google Scholar] [CrossRef]
- Cotman, C.W.; Berchtold, N.C. Exercise: A behavioral intervention to enhance brain health and plasticity. Trends Neurosci. 2002, 25, 295–301. [Google Scholar] [CrossRef]
- Walsh, J.J.; Tschakovsky, M.E. Exercise and circulating bdnf: Mechanisms of release and implications for the design of exercise interventions. Appl. Physiol. Nutr. Metab. 2018, 43, 1095–1104. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, B.K. Physical activity and muscle–brain crosstalk. Nat. Rev. Endocrinol. 2019, 15, 383–392. [Google Scholar] [CrossRef]
- Campos Torres, A.; Vidal, P.P.; De Waele, C. Evidence for a microglial reaction within the vestibular and cochlear nuclei following inner ear lesion in the rat. Neuroscience 1999, 92, 1475–1490. [Google Scholar] [CrossRef]
- Zwergal, A.; Günther, L.; Brendel, M.; Beck, R.; Lindner, S.; Xiong, G.; Eilles, E.; Unterrainer, M.; Albert, N.L.; Becker-Bense, S.; et al. In vivo imaging of glial activation after unilateral labyrinthectomy in the rat: A [18F]GE180-PET study. Front. Neurol. 2017, 8, 665. [Google Scholar] [CrossRef] [Green Version]
- Rivera, C.; Voipio, J.; Thomas-Crusells, J.; Li, H.; Emri, Z.; Sipilä, S.; Payne, J.A.; Minichiello, L.; Saarma, M.; Kaila, K. Mechanism of activity-dependent downregulation of the neuron-specific K-Cl cotransporter KCC2. J. Neurosci. 2004, 24, 4683–4691. [Google Scholar] [CrossRef] [Green Version]
- Rivera, C.; Li, H.; Thomas-Crusells, J.; Lahtinen, H.; Viitanen, T.; Nanobashvili, A.; Kokaia, Z.; Airaksinen, M.S.; Voipio, J.; Kaila, K.; et al. BDNF-induced TrkB activation down-regulates the K+-Cl- cotransporter KCC2 and impairs neuronal Cl- extrusion. J. Cell Biol. 2002, 159, 747–752. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferrini, F.; De Koninck, Y. Microglia Control Neuronal Network Excitability via BDNF Signalling. Neural Plast. 2013, 2013, 1–11. [Google Scholar] [CrossRef]
- Jamali, M.; Mitchell, D.E.; Dale, A.; Carriot, J.; Sadeghi, S.G.; Cullen, K.E. Neuronal detection thresholds during vestibular compensation: Contributions of response variability and sensory substitution. J. Physiol. 2014, 592, 1565–1580. [Google Scholar] [CrossRef]
- Sadeghi, S.G.; Minor, L.B.; Cullen, K.E. Multimodal integration after unilateral labyrinthine lesion: Single vestibular nuclei neuron responses and implications for postural compensation. J. Neurophysiol. 2011, 105, 661–673. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clark, A.K.; Gruber-Schoffnegger, D.; Drdla-Schutting, R.; Gerhold, K.J.; Malcangio, M.; Sandkühler, J. Selective activation of microglia facilitates synaptic strength. J. Neurosci. 2015, 35, 4552–4570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strupp, M.; Arbusow, V.; Maag, K.P.; Gall, C.; Brandt, T. Vestibular exercises improve central vestibulespinal compensation after vestibular neuritis. Neurology 1998, 51, 838–844. [Google Scholar] [CrossRef]
- Dieterich, M.; Brandt, T. Perception of verticality and vestibular disorders of balance and falls. Front. Neurol. 2019, 10, 172. [Google Scholar] [CrossRef] [PubMed] [Green Version]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marouane, E.; El Mahmoudi, N.; Rastoldo, G.; Péricat, D.; Watabe, I.; Lapôtre, A.; Tonetto, A.; Xavier, F.; Dumas, O.; Chabbert, C.; et al. Sensorimotor Rehabilitation Promotes Vestibular Compensation in a Rodent Model of Acute Peripheral Vestibulopathy by Promoting Microgliogenesis in the Deafferented Vestibular Nuclei. Cells 2021, 10, 3377. https://doi.org/10.3390/cells10123377
Marouane E, El Mahmoudi N, Rastoldo G, Péricat D, Watabe I, Lapôtre A, Tonetto A, Xavier F, Dumas O, Chabbert C, et al. Sensorimotor Rehabilitation Promotes Vestibular Compensation in a Rodent Model of Acute Peripheral Vestibulopathy by Promoting Microgliogenesis in the Deafferented Vestibular Nuclei. Cells. 2021; 10(12):3377. https://doi.org/10.3390/cells10123377
Chicago/Turabian StyleMarouane, Emna, Nada El Mahmoudi, Guillaume Rastoldo, David Péricat, Isabelle Watabe, Agnès Lapôtre, Alain Tonetto, Frédéric Xavier, Olivier Dumas, Christian Chabbert, and et al. 2021. "Sensorimotor Rehabilitation Promotes Vestibular Compensation in a Rodent Model of Acute Peripheral Vestibulopathy by Promoting Microgliogenesis in the Deafferented Vestibular Nuclei" Cells 10, no. 12: 3377. https://doi.org/10.3390/cells10123377