Do Mast Cells Contribute to the Antifungal Host Defense?
Abstract
:1. Introduction
2. Microscopic Fungi and the Immune System
3. How Do MCs Contribute to the Host Defense?
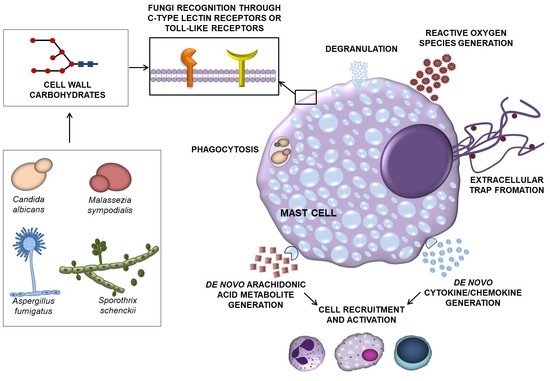
4. Expression of PRRs Involved in Fungus Recognition in MCs
5. Fungi Affect MC Activity
5.1. Effect of Fungal Cells on MCs
5.2. Effect of Fungus-Derived Molecules on MCs
6. MCs and Th17 Cells
7. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rodrigues, M.L.; Nosanchuk, J.D. Fungal diseases as neglected pathogens: A wake-up call to public health officials. PLoS Negl. Trop. Dis. 2020, 14, e0007964. [Google Scholar] [CrossRef] [Green Version]
- Von Lilienfeld-Toal, M.; Wagener, J.; Einsele, H.; Cornely, O.A.; Kurzai, O. Invasive fungal infection. Deutsches Ärzteblatt International 2019, 116, 271–278. [Google Scholar] [CrossRef]
- Chin, V.K.; Yong, V.C.; Chong, P.P.; Nordin, S.A.; Basir, R.; Abdullah, M. Mycobiome in the gut: A multiperspective review. Mediat. Inflamm. 2020, 2020, 9560684. [Google Scholar] [CrossRef] [Green Version]
- Gow, N.A.R.; Latge, J.-P.; Munro, C.A. The fungal cell wall: Structure, biosynthesis, and function. Microbiol. Spectr. 2017, 5, 1–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williams, P.B.; Barnes, C.S.; Portnoy, J.M.; Baxi, S.; Grimes, C.; Horner, W.E.; Kennedy, K.; Linnemann, D.L.; Levetin, E.; Miller, J.D.; et al. Innate and adaptive immune response to fungal products and allergens. J. Allergy Clin. Immunol. Pr. 2016, 4, 386–395. [Google Scholar] [CrossRef] [PubMed]
- Netea, M.G.; Joosten, L.A.B.; Van Der Meer, J.W.M.; Kullberg, B.-J.; Van De Veerdonk, F.L. Immune defence against Candida fungal infections. Nat. Rev. Immunol. 2015, 15, 630–642. [Google Scholar] [CrossRef]
- Blanco, J.L.; Garcia, M. Immune response to fungal infections. Veter. Immunol. Immunopathol. 2008, 125, 47–70. [Google Scholar] [CrossRef] [PubMed]
- Żelechowska, P.; Agier, J.; Brzezińska-Błaszczyk, E. Endogenous antimicrobial factors in the treatment of infectious diseases. Cent. Eur. J. Immunol. 2016, 4, 419–425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chai, L.Y.A.; Netea, M.G.; Vonk, A.G.; Kullberg, B.-J. Fungal strategies for overcoming host innate immune response. Med. Mycol. 2009, 47, 227–236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Latgé, J.-P.; Beauvais, A.; Chamilos, G. The cell wall of the human fungal pathogen Aspergillus fumigatus: Biosynthesis, organization, immune response, and virulence. Annu. Rev. Microbiol. 2017, 71, 99–116. [Google Scholar] [CrossRef]
- Sałkowska, A.; Karaś, K.; Walczak-Drzewiecka, A.; Dastych, J.; Ratajewski, M. Differentiation stage-specific effect of histone deacetylase inhibitors on the expression of RORγT in human lymphocytes. J. Leukoc. Biol. 2017, 102, 1487–1495. [Google Scholar] [CrossRef]
- Leibovici, V.; Evron, R.; Axelrod, O.; Westerman, M.; Shalit, M.; Barak, V.; Frankenburg, S. Imbalance of immune responses in patients with chronic and widespread fungal skin infection. Clin. Exp. Dermatol. 1995, 20, 390–394. [Google Scholar] [CrossRef] [PubMed]
- Netea, M.G.; Van Der Graaf, C.; Van Der Meer, J.W.M.; Kullberg, B.J. Recognition of fungal pathogens by toll-like receptors. Eur. J. Clin. Microbiol. Infect. Dis. 2004, 23, 672–676. [Google Scholar] [CrossRef]
- Drummond, R.A.; Franco, L.M.; Lionakis, M.S. Human CARD9: A critical molecule of fungal immune surveillance. Front. Immunol. 2018, 9, 1836. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bruno, M.; Kersten, S.; Bain, J.M.; Jaeger, M.; Rosati, D.; Kruppa, M.D.; Lowman, D.W.; Rice, P.J.; Graves, B.; Ma, Z.; et al. Transcriptional and functional insights into the host immune response against the emerging fungal pathogen Candida auris. Nat. Microbiol. 2020, 5, 1516–1531. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Chávez, M.J.; Pérez-García, L.A.; Niño-Vega, G.A.; Mora-Montes, H.M. Fungal strategies to evade the host immune recognition. J. Fungi 2017, 3, 51. [Google Scholar] [CrossRef] [Green Version]
- Pal, S.; Leger, R.J.S.; Wu, L. Fungal peptide destruxin a plays a specific role in suppressing the innate immune response in drosophila melanogaster. J. Biol. Chem. 2007, 282, 8969–8977. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Urb, M.; Sheppard, D.C. The role of mast cells in the defence against pathogens. PLoS Pathog. 2012, 8, e1002619. [Google Scholar] [CrossRef]
- Marshall, J.S. Mast-cell responses to pathogens. Nat. Rev. Immunol. 2004, 4, 787–799. [Google Scholar] [CrossRef]
- Jiménez, M.; Cervantes-García, D.; Córdova-Dávalos, L.E.; Pérez-Rodríguez, M.J.; Gonzalez-Espinosa, C.; Salinas, E. Responses of mast cells to pathogens: Beneficial and detrimental roles. Front. Immunol. 2021, 12, 685865. [Google Scholar] [CrossRef] [PubMed]
- Mukai, K.; Tsai, M.; Saito, H.; Galli, S.J. Mast cells as sources of cytokines, chemokines, and growth factors. Immunol. Rev. 2018, 282, 121–150. [Google Scholar] [CrossRef]
- Moon, T.C.; Befus, A.D.; Kulka, M. Mast cell mediators: Their differential release and the secretory pathways involved. Front. Immunol. 2014, 5, 569. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Atiakshin, D.; Buchwalow, I.; Tiemann, M. Mast cell chymase: Morphofunctional characteristics. Histochem. Cell Biol. 2019, 152, 253–269. [Google Scholar] [CrossRef]
- Atiakshin, D.; Buchwalow, I.; Samoilova, V.; Tiemann, M. Tryptase as a polyfunctional component of mast cells. Histochem. Cell Biol. 2018, 149, 461–477. [Google Scholar] [CrossRef] [PubMed]
- Thangam, E.B.; Jemima, E.A.; Singh, H.; Baig, M.S.; Khan, M.; Mathias, C.B.; Church, M.K.; Saluja, R. The role of histamine and histamine receptors in mast cell-mediated allergy and inflammation: The hunt for new therapeutic targets. Front. Immunol. 2018, 9, 1873. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Möllerherm, H.; Von Köckritz-Blickwede, M.; Branitzki-Heinemann, K. Antimicrobial activity of mast cells: Role and relevance of extracellular DNA traps. Front. Immunol. 2016, 7, 265. [Google Scholar] [CrossRef] [Green Version]
- Komi, D.E.A.; Kuebler, W.M. Significance of mast cell formed extracellular traps in microbial defense. Clin. Rev. Allergy Immunol. 2021, 22, 1–20. [Google Scholar] [CrossRef]
- Garcia-Rodriguez, K.M.; Bahri, R.; Sattentau, C.; Roberts, I.S.; Goenka, A.; Bulfone-Paus, S. Human mast cells exhibit an individualized pattern of antimicrobial responses. Immun. Inflamm. Dis. 2020, 8, 198–210. [Google Scholar] [CrossRef]
- Katsoulis-Dimitriou, K.; Kotrba, J.; Voss, M.; Dudeck, J.; Dudeck, A. Mast cell functions linking innate sensing to adaptive immunity. Cells 2020, 9, 2538. [Google Scholar] [CrossRef] [PubMed]
- Agier, J.; Pastwińska, J.; Brzezińska-Błaszczyk, E. An overview of mast cell pattern recognition receptors. Inflamm. Res. 2018, 67, 737–746. [Google Scholar] [CrossRef] [Green Version]
- Yu, M.; Song, X.-T.; Liu, B.; Luan, T.-T.; Liao, S.-L.; Zhao, Z.-T. The emerging role of mast cells in response to fungal infection. Front. Immunol. 2021, 12, 688659. [Google Scholar] [CrossRef] [PubMed]
- Saluja, R.; Metz, M.; Maurer, M. Role and relevance of mast cells in fungal infections. Front. Immunol. 2012, 3, 146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patin, E.C.; Thompson, A.; Orr, S.J. Pattern recognition receptors in fungal immunity. Semin. Cell Dev. Biol. 2019, 89, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Marshall, J.S. Zymosan treatment of mouse mast cells enhances dectin-1 expression and induces dectin-1-dependent reactive oxygen species (ROS) generation. Immunobiology 2009, 214, 321–330. [Google Scholar] [CrossRef]
- Olynych, T.J.; Jakeman, D.L.; Marshall, J.S. Fungal zymosan induces leukotriene production by human mast cells through a dectin-1–dependent mechanism. J. Allergy Clin. Immunol. 2006, 118, 837–843. [Google Scholar] [CrossRef] [PubMed]
- Kimura, Y.; Chihara, K.; Honjoh, C.; Takeuchi, K.; Yamauchi, S.; Yoshiki, H.; Fujieda, S.; Sada, K. Dectin-1-mediated signaling leads to characteristic gene expressions and cytokine secretion via. spleen tyrosine kinase (Syk) in rat mast cells. J. Biol. Chem. 2014, 289, 31565–31575. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ribbing, C.; Engblom, C.; Lappalainen, J.; Lindstedt, K.; Kovanen, P.T.; Karlsson, M.A.; Lundeberg, L.; Johansson, C.; Nilsson, G.; Lunderius-Andersson, C.; et al. Mast cells generated from patients with atopic eczema have enhanced levels of granule mediators and an impaired Dectin-1 expression. Allergy 2010, 66, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Żelechowska, P.; Różalska, S.; Wiktorska, M.; Brzezińska-Błaszczyk, E.; Agier, J. Curdlan stimulates tissue mast cells to synthesize pro-inflammatory mediators, generate ROS, and migrate via. dectin-1 receptor. Cell. Immunol. 2020, 351, 104079. [Google Scholar] [CrossRef]
- Agier, J.; Brzezińska-Błaszczyk, E.; Różalska, S.; Wiktorska, M.; Kozłowska, E.; Żelechowska, P. Mast cell phenotypic plasticity and their activity under the influence of cathelicidin-related antimicrobial peptide (CRAMP) and IL-33 alarmins. Cell. Immunol. 2021, 369, 104424. [Google Scholar] [CrossRef]
- Honjoh, C.; Chihara, K.; Yoshiki, H.; Yamauchi, S.; Takeuchi, K.; Kato, Y.; Hida, Y.; Ishizuka, T.; Sada, K. Association of C-type lectin mincle with FcεRIβγ subunits leads to functional activation of RBL-2H3 cells through Syk. Sci. Rep. 2017, 7, 46064. [Google Scholar] [CrossRef] [Green Version]
- Vukman, K.V.; Ravida, A.; Aldridge, A.M.; O’Neill, S.M. Mannose receptor and macrophage galactose-type lectin are involved in Bordetella pertussis mast cell interaction. J. Leukoc. Biol. 2013, 94, 439–448. [Google Scholar] [CrossRef]
- McCurdy, J.D.; Lin, T.J.; Marshall, J.S. Toll-like receptor 4-mediated activation of murine mast cells. J. Leukoc. Biol. 2001, 70, 977–984. [Google Scholar] [PubMed]
- Kubo, Y.; Fukuishi, N.; Yoshioka, M.; Kawasoe, Y.; Iriguchi, S.; Imajo, N.; Yasui, Y.; Matsui, N.; Akagi, M. Bacterial components regulate the expression of toll-like receptor 4 on human mast cells. Inflamm. Res. 2007, 56, 70–75. [Google Scholar] [CrossRef]
- Matsushima, H.; Yamada, N.; Matsue, H.; Shimada, S. TLR3-, TLR7-, and TLR9-mediated production of proinflammatory cytokines and chemokines from murine connective tissue type skin-derived mast cells but not from bone marrow-derived mast cells. J. Immunol. 2004, 173, 531–541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Inomata, N.; Tomita, H.; Ikezawa, Z.; Saito, H. Differential gene expression profile between cord blood progenitor-derived and adult progenitor-derived human mast cells. Immunol. Lett. 2005, 98, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Agier, J.; Żelechowska, P.; Kozłowska, E.; Brzezińska-Błaszczyk, E. Expression of surface and intracellular toll-like receptors by mature mast cells. Cent. Eur. J. Immunol. 2016, 41, 333–338. [Google Scholar] [CrossRef] [Green Version]
- Yoshioka, M.; Fukuishi, N.; Iriguchi, S.; Ohsaki, K.; Yamanobe, H.; Inukai, A.; Kurihara, D.; Imajo, N.; Yasui, Y.; Matsui, N.; et al. Lipoteichoic acid downregulates FcεRI expression on human mast cells through toll-like receptor 2. J. Allergy Clin. Immunol. 2007, 120, 452–461. [Google Scholar] [CrossRef] [PubMed]
- Mrabet-Dahbi, S.; Metz, M.; Dudeck, A.; Zuberbier, T.; Maurer, M. Murine mast cells secrete a unique profile of cytokines and prostaglandins in response to distinct TLR2 ligands. Exp. Dermatol. 2009, 18, 437–444. [Google Scholar] [CrossRef] [Green Version]
- Kulka, M.; Alexopoulou, L.; Flavell, R.A.; Metcalfe, D.D. Activation of mast cells by double-stranded RNA: Evidence for activation through toll-like receptor 3. J. Allergy Clin. Immunol. 2004, 114, 174–182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pietrzak, A.; Wierzbicki, M.; Wiktorska, M.; Brzezińska-Błaszczyk, E. Surface TLR2 and TLR4 expression on mature rat mast cells can be affected by some bacterial components and proinflammatory cytokines. Mediat. Inflamm. 2011, 2011, 427473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okumura, S.; Kashiwakura, J.-I.; Tomita, H.; Matsumoto, K.; Nakajima, T.; Saito, H.; Okayama, Y. Identification of specific gene expression profiles in human mast cells mediated by toll-like receptor 4 and FcϵRI. Blood 2003, 102, 2547–2554. [Google Scholar] [CrossRef] [Green Version]
- Yang, H.; Wei, J.; Zhang, H.; Song, W.; Wei, W.; Zhang, L.; Qian, K.; He, S. Upregulation of toll-like receptor (TLR) expression and release of cytokines from mast cells by IL-12. Cell. Physiol. Biochem. 2010, 26, 337–346. [Google Scholar] [CrossRef]
- Agier, J.; Brzezińska-Błaszczyk, E.; Żelechowska, P.; Wiktorska, M.; Pietrzak, J.; Różalska, S. Cathelicidin LL-37 affects surface and intracellular toll-like receptor expression in tissue mast cells. J. Immunol. Res. 2018, 2018, 7357162. [Google Scholar] [CrossRef] [Green Version]
- Yoshioka, M.; Fukuishi, N.; Kubo, Y.; Yamanobe, H.; Ohsaki, K.; Kawasoe, Y.; Murata, M.; Ishizumi, A.; Nishii, Y.; Matsui, N.; et al. Human cathelicidin CAP18/LL-37 changes mast cell function toward innate immunity. Biol. Pharm. Bull. 2008, 31, 212–216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nieto-Patlán, A.; Campillo-Navarro, M.; Rodríguez-Cortés, O.; Muñoz-Cruz, S.; Wong-Baeza, I.; Estrada-Parra, S.; Estrada-García, I.; Serafín-López, J.; Chacón-Salinas, R. Recognition of Candida albicans by dectin-1 induces mast cell activation. Immunobiol. 2015, 220, 1093–1100. [Google Scholar] [CrossRef]
- Sakurai, A.; Yamaguchi, N.; Sonoyama, K. Cell wall polysaccharides of Candida albicans induce mast cell degranulation in the gut. Biosci. Microbiota Food Health 2012, 31, 67–70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lopes, J.P.; Stylianou, M.; Nilsson, G.; Urban, C.F. Opportunistic pathogen Candida albicans elicits a temporal response in primary human mast cells. Sci. Rep. 2015, 5, 12287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Urb, M.; Pouliot, P.; Gravelat, F.N.; Olivier, M.; Sheppard, D.C. Aspergillus fumigatus induces immunoglobulin e–independent mast cell degranulation. J. Infect. Dis. 2009, 200, 464–472. [Google Scholar] [CrossRef] [Green Version]
- Renga, G.; Moretti, S.; Oikonomou, V.; Borghi, M.; Zelante, T.; Paolicelli, G.; Costantini, C.; De Zuani, M.; Villella, V.R.; Raia, V.; et al. IL-9 and mast cells are key players of Candida albicans commensalism and pathogenesis in the gut. Cell Rep. 2018, 23, 1767–1778. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Zuani, M.; Paolicelli, G.; Zelante, T.; Renga, G.; Romani, L.; Arzese, A.; Pucillo, C.E.M.; Frossi, B. Mast cells respond to Candida albicans infections and modulate macrophages phagocytosis of the fungus. Front. Immunol. 2018, 9, 2829. [Google Scholar] [CrossRef] [PubMed]
- Lopes, J.P.; Stylianou, M.; Backman, E.; Holmberg, S.; Ekoff, M.; Nilsson, G.; Urban, C.F. Cryptococcus neoformans induces MCP-1 release and delays the death of human mast cells. Front. Cell. Infect. Microbiol. 2019, 9, 289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Selander, C.; Engblom, C.; Nilsson, G.; Scheynius, A.; Andersson, C.L. TLR2/MyD88-dependent and -independent activation of mast cell IgE responses by the skin commensal yeast Malassezia sympodialis. J. Immunol. 2009, 182, 4208–4216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valim, C.X.R.; Da Silva, E.Z.M.; Assis, M.A.; Fernandes, F.F.; Coelho, P.S.R.; Oliver, C.; Jamur, M.C. rPbPga1 from Paracoccidioides brasiliensis activates mast cells and macrophages via. NFkB. PLoS Negl. Trop. Dis. 2015, 9, e0004032. [Google Scholar] [CrossRef] [Green Version]
- Romo-Lozano, Y.; Hernández-Hernández, F.; Salinas, E. Sporothrix schenckii yeasts induce ERK pathway activation and secretion of IL-6 and TNF-α in rat mast cells, but no degranulation. Med. Mycol. 2014, 52, 862–868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Romo-Lozano, Y.; Hernández-Hernández, F.; Salinas, E.; Salinas, E. Mast cell activation by conidia of Sporothrix schenckii: Role in the severity of infection. Scand. J. Immunol. 2012, 76, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Q.; Luo, Y.; Scheffel, J.; Geng, P.; Wang, Y.; Frischbutter, S.; Li, R.; Maurer, M.; Zhao, Z. Skin mast cells contribute to Sporothrix schenckii infection. Front. Immunol. 2020, 11, 469. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gomez, C.; Carsin, A.; Gouitaa, M.; Reynaud-Gaubert, M.; Dubus, J.-C.; Mège, J.-L.; Ranque, S.; Vitte, J. Mast cell tryptase changes with Aspergillus fumigatus—host crosstalk in cystic fibrosis patients. J. Cyst. Fibros. 2018, 17, 631–635. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Atiakshin, D.; Buchwalow, I.; Tiemann, M. Mast cells and collagen fibrillogenesis. Histochem. Cell Biol. 2020, 154, 21–40. [Google Scholar] [CrossRef] [PubMed]
- Alshahrani, M.Y.; Alfaifi, M.; Al Shahrani, M.; Alshahrani, A.S.; Alkhathami, A.G.; Dera, A.A.; Ahmad, I.; Wahab, S.; Beg, M.M.A.; Hakamy, A.; et al. Increased mRNA expression of key cytokines among suspected cases of Pneumocystis jirovecii infection. BMC Infect. Dis. 2021, 21, 28. [Google Scholar] [CrossRef] [PubMed]
- Khodavaisy, S.; Mortaz, E.; Mohammadi, F.; Aliyali, M.; Fakhim, H.; Badali, H. Pneumocystis jirovecii colonization in chronic obstructive pulmonary disease (COPD). Curr. Med. Mycol. 2015, 1, 42–48. [Google Scholar] [CrossRef] [Green Version]
- Aponte-López, A.; Muñoz-Cruz, S. Mast cells in the tumor microenvironment. Adv. Exp. Med. Biol. 2020, 1273, 159–173. [Google Scholar] [CrossRef]
- Komi, D.E.A.; Redegeld, F.A. Role of mast cells in shaping the tumor microenvironment. Clin. Rev. Allergy Immunol. 2019, 58, 313–325. [Google Scholar] [CrossRef] [Green Version]
- Di Cosola, M.; Cazzolla, A.; Charitos, I.; Ballini, A.; Inchingolo, F.; Santacroce, L. Candida albicans and oral carcinogenesis. A brief review. J. Fungi 2021, 7, 476. [Google Scholar] [CrossRef] [PubMed]
- Trevisan, E.; Vita, F.; Medic, N.; Soranzo, M.R.; Zabucchi, G.; Borelli, V. Mast cells kill Candida albicans in the extracellular environment but spare ingested fungi from death. Inflammation 2014, 37, 2174–2189. [Google Scholar] [CrossRef]
- Yan, Y.; Zhao, Z.; Wan, H.; Wu, R.; Fang, J.; Liu, H. A novel fungus concentration-dependent rat model for acute invasive fungal rhinosinusitis: An experimental study. BMC Infect. Dis. 2014, 14, 3856. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, Y.; Zhang, H.; Liu, S.; Chen, G.; He, S.; Li, Z.; Wang, L. Mast cell activation protects cornea by promoting neutrophil infiltration via. stimulating ICAM-1 and vascular dilation in fungal keratitis. Sci. Rep. 2018, 8, 8365. [Google Scholar] [CrossRef] [PubMed]
- Marangon, A.V.; Svidzinski, T.I.E.; Salci, T.P.; Meurer, R.; Fernandes, M.D.C.; Hernandes, L. Metabolic extract of Fusarium oxysporum induces histopathologic alterations and apoptosis in the skin of wistar rats. Int. J. Dermatol. 2009, 48, 697–703. [Google Scholar] [CrossRef] [PubMed]
- Pinke, K.H.; de Lima, H.G.; Cunha, F.Q.; Lara, V.S. Mast cells phagocyte Candida albicans and produce nitric oxide by mechanisms involving TLR2 and Dectin-1. Immunobiology 2016, 221, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Padawer, J.; Fruhman, G.J. Phagocytosis of zymosan particles by mast cells. Cell. Mol. Life Sci. 1968, 24, 471–472. [Google Scholar] [CrossRef] [PubMed]
- Nosál, R.; Novotný, J.; Sikl, D. The effect of glycoprotein from Candida albicans on isolated rat mast cells. Toxicon 1974, 12, 103–106. [Google Scholar] [CrossRef]
- Yamaki, K.; Yoshino, S. Aspergillus oryzae lectin induces anaphylactoid oedema and mast cell activation through its interaction with fucose of mast cell-bound non-specific IgE. Scand. J. Immunol. 2011, 74, 445–453. [Google Scholar] [CrossRef] [PubMed]
- Żelechowska, P.; Brzezińska-Błaszczyk, E.; Różalska, S.; Agier, J.; Kozłowska, E. Mannan activates tissue native and IgE-sensitized mast cells to proinflammatory response and chemotaxis in TLR4-dependent manner. J. Leukoc. Biol. 2021, 109, 931–942. [Google Scholar] [CrossRef]
- Żelechowska, P.; Brzezińska-Błaszczyk, E.; Różalska, S.; Agier, J.; Kozłowska, E. Native and IgE-primed rat peritoneal mast cells exert pro-inflammatory activity and migrate in response to yeast zymosan upon dectin-1 engagement. Immunol. Res. 2021, 69, 176–188. [Google Scholar] [CrossRef] [PubMed]
- McCurdy, J.D.; Olynych, T.J.; Maher, L.H.; Marshall, J.S. Cutting edge: Distinct toll-like receptor 2 activators selectively induce different classes of mediator production from human mast cells. J. Immunol. 2003, 170, 1625–1629. [Google Scholar] [CrossRef] [PubMed]
- Sato, H.; Kobayashi, Y.; Hattori, A.; Suzuki, T.; Shigekawa, M.; Jippo, T. Inhibitory effects of water-soluble low-molecular-weight β-(1,3-1,6)D-glucan isolated from Aureobasidium pullulans 1A1 strain black yeast on mast cell degranulation and passive cutaneous anaphylaxis. Biosci. Biotechnol. Biochem. 2012, 76, 84–88. [Google Scholar] [CrossRef] [Green Version]
- Niide, O.; Suzuki, Y.; Yoshimaru, T.; Inoue, T.; Takayama, T.; Ra, C. Fungal metabolite gliotoxin blocks mast cell activation by a calcium- and superoxide-dependent mechanism: Implications for immunosuppressive activities. Clin. Immunol. 2006, 118, 108–116. [Google Scholar] [CrossRef]
- Barbosa-Lorenzi, V.C.; Peyda, S.; Scheynius, A.; Nilsson, G.; Lunderius-Andersson, C. Curdlan induces selective mast cell degranulation without concomitant release of LTC 4, IL-6 or CCL2. Immunobiology 2017, 222, 647–650. [Google Scholar] [CrossRef]
- Tung, H.-Y.; Plunkett, B.; Huang, S.-K.; Zhou, Y. Murine mast cells secrete and respond to interleukin-33. J. Interf. Cytokine Res. 2014, 34, 141–147. [Google Scholar] [CrossRef] [Green Version]
- Zelante, T.; De Luca, A.; Angelo, C.D.; Moretti, S.; Romani, L. IL-17/Th17 in anti-fungal immunity: What’s new? Eur. J. Immunol. 2009, 39, 645–648. [Google Scholar] [CrossRef]
- Sparber, F.; Leibund Gut-Landmann, S. Interleukin-17 in antifungal immunity. Pathogens 2019, 8, 54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suurmond, J.; Habets, K.L.L.; Dorjée, A.L.; Huizinga, T.W.; Toes, R.E.M. Expansion of Th17 cells by human mast cells is driven by inflammasome-independent IL-1β. J. Immunol. 2016, 197, 4473–4481. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakae, S.; Suto, H.; Berry, G.J.; Galli, S.J. Mast cell-derived TNF can promote Th17 cell–dependent neutrophil recruitment in ovalbumin-challenged OTII mice. Blood 2006, 109, 3640–3648. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cho, K.-A.; Suh, J.W.; Sohn, J.H.; Park, J.W.; Lee, H.; Kang, J.L.; Woo, S.-Y.; Cho, Y.J. IL-33 induces Th17-mediated airway inflammation via. mast cells in ovalbumin-challenged mice. Am. J. Physiol. Cell. Mol. Physiol. 2012, 302, L429–L440. [Google Scholar] [CrossRef] [PubMed]
- Mommert, S.; Gschwandtner, M.; Koether, B.; Gutzmer, R.; Werfel, T. Human memory Th17 cells express a functional histamine H4 receptor. Am. J. Pathol. 2012, 180, 177–185. [Google Scholar] [CrossRef]
- Lee, W.; Kim, H.S.; Lee, G.R. Leukotrienes induce the migration of Th17 cells. Immunol. Cell Biol. 2014, 93, 472–479. [Google Scholar] [CrossRef]
- Wang, A.; Wang, Z.; Cao, Y.; Cheng, S.; Chen, H.; Bunjhoo, H.; Xie, J.; Wang, C.; Xu, Y.; Xiong, W. CCL2/CCR2-dependent recruitment of Th17 cells but not Tc17 cells to the lung in a murine asthma model. Int. Arch. Allergy Immunol. 2015, 166, 52–62. [Google Scholar] [CrossRef]
- Dudeck, A.; Suender, C.A.; Kostka, S.L.; von Stebut, E.; Maurer, M. Mast cells promote Th1 and Th17 responses by modulating dendritic cell maturation and function. Eur. J. Immunol. 2011, 41, 1883–1893. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.-A.; Park, M.; Kim, Y.-H.; Woo, S.-Y. Th17 cell-mediated immune responses promote mast cell proliferation by triggering stem cell factor in keratinocytes. Biochem. Biophys. Res. Commun. 2017, 487, 856–861. [Google Scholar] [CrossRef]
- Kolaczkowska, E.; Seljelid, R.; Plytycz, B. Role of mast cells in zymosan-induced peritoneal inflammation in Balb/c and mast cell-deficient WBB6F1 mice. J. Leukoc. Biol. 2001, 69, 33–42. [Google Scholar]
- Wypasek, E.; Natorska, J.; Stankiewicz, E.; Kolaczkowska, E. Morphine-modulated mast cell migration and proliferation during early stages of zymosan-induced peritonitis in CBA mice. Folia Biol. 2011, 59, 99–106. [Google Scholar] [CrossRef]



| Fungi | MC Types | Mediators | References |
|---|---|---|---|
| Aspergillus fumigatus (mature hyphae) | RBL-2H3 | histamine, β-hexosaminidase | [58] |
| Candida albicans | HMC-1 | IL-1Ra, IL-16, MIF, CXCL8 | [57] |
| Candida albicans (hyphae) | SMCs | TGF-β and IL-10 | [59] |
| Candida albicans (yeasts) | BMMCs | IL-1β | [55] |
| Candida albicans (hyphae, yeasts) | BMMCs | TNF, IL-6, IL-10, IL-13, CCL3, CCL4 | [55,60] |
| Candida albicans (cell wall, hyphae, yeasts) | BMMCs, HMC-1, PMCs, RBL-2H3 | histamine, β-hexosaminidase | [55,56,57] |
| Cryptococcus neoformans | CBMCs, HMC-1 | β-hexosaminidase, tryptase | [61] |
| Malasezzia sympodialis | BMMCs, PMCs IgE-sensitized BMMCs PBMCs | cysLTs, TNF, IL-6 histamine, cysLTs, CCL2 IL-6 | [37,62] |
| Paracoccidioides brasiliensis | RBL-2H3 | histamine, β-hexosaminidase | [63] |
| Sporothrix schenckii | BMMCs, PMCs | cysLTs, TNF, IL-6 | [64,65,66] |
| Fungus-Derived Molecules | MC Types | Mediators | References |
|---|---|---|---|
| curdlan (Alcaligensis faecalis) | PMCs | histamine, cysLTs, TNF, IFN-α, IFN-γ CCL3, GM-CSF, ROS | [38,87] |
| BMMCs | histamine, β-hexosaminidase | ||
| glycoprotein (Candida albicans) | PMCs | histamine | [80] |
| lectin (Aspergillus oryzae) | IgE-sensitized RBL-2H3 | β-hexosaminidase | [81] |
| mannan (Candida albicans) | RBL-2H3 | histamine, β-hexosaminidase | [56,82,83,84] |
| mannan (Saccharomyces cerevisiae) | PMCs | histamine, cysLTs, TNF, CCL2, CCL3, IFN-γ, GM-CSF, ROS | |
| PbPga1 (Paracoccidioides brasiliensis) | RBL-2H3 | IL-6 | [63] |
| zymosan (Saccharomyces cerevisiae) | BMMCs CBMCs PMCs | ROS, NO LTB4, LTC4, IL-1β, GM-CSF histamine, cysLTs, TNF, IFNs, GM-CSF, CCL2, ROS | [35,82,83,84,88] |
| β-glucan (Candida albicans) | RBL-2H3 | histamine, β-hexosaminidase | [56] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Żelechowska, P.; Pastwińska, J.; Brzezińska-Błaszczyk, E.; Agier, J. Do Mast Cells Contribute to the Antifungal Host Defense? Cells 2021, 10, 2510. https://doi.org/10.3390/cells10102510
Żelechowska P, Pastwińska J, Brzezińska-Błaszczyk E, Agier J. Do Mast Cells Contribute to the Antifungal Host Defense? Cells. 2021; 10(10):2510. https://doi.org/10.3390/cells10102510
Chicago/Turabian StyleŻelechowska, Paulina, Joanna Pastwińska, Ewa Brzezińska-Błaszczyk, and Justyna Agier. 2021. "Do Mast Cells Contribute to the Antifungal Host Defense?" Cells 10, no. 10: 2510. https://doi.org/10.3390/cells10102510