Facile Synthesis of Bi2MoO6 Microspheres Decorated by CdS Nanoparticles with Efficient Photocatalytic Removal of Levfloxacin Antibiotic
Abstract
:1. Introduction
2. Results
2.1. Preparation and Characterization
2.2. Photocatalytic Performance
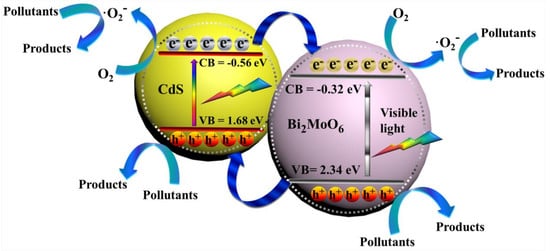
2.3. Photocatalytic Reaction Mechanism
3. Materials and Methods
3.1. Chemicals
3.2. Chemicals Synthesis of Catalysts
3.3. Characterization
3.4. Photocatalytic Performance Tests
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Du, J.; Guo, W.; Wang, H.; Yin, R.; Zheng, H.; Feng, X.; Che, D.; Ren, N. Hydroxyl radical dominated degradation of aquatic sulfamethoxazole by Fe0/bisulfite/O2: Kinetics, mechanisms, and pathways. Water Res. 2018, 138, 323–332. [Google Scholar] [CrossRef]
- Hong, Y.Z.; Li, C.S.; Zhang, G.Y.; Meng, Y.D.; Yin, B.X.; Zhao, Y.; Shi, W.D. Efficient and stable Nb2O5 modified g-C3N4 photocatalyst for removal of antibiotic pollutant. Chem. Eng. J. 2016, 299, 74–84. [Google Scholar] [CrossRef]
- Wang, F.; Li, Q.; Xu, D. Recent progress in semiconductor-based nanocomposite photocatalysts for solar-to-chemical energy conversion. Adv. Energy Mater. 2017, 7, 1700529. [Google Scholar] [CrossRef]
- Cates, E.L. Photocatalytic water treatment: So where are we going with this? Environ. Sci. Technol. 2017, 51, 757–758. [Google Scholar] [CrossRef] [PubMed]
- Pirhashemi, M.; Habibi-Yangjeh, A.; Pouran, S.R. Review on the criteria anticipated for the fabrication of highly efficient ZnO-based visible-light-driven photocatalysts. J. Ind. Eng. Chem. 2018, 62, 1–25. [Google Scholar] [CrossRef]
- Zhu, S.S.; Wang, D.W. Photocatalysis: Basic principles, diverse forms of implementations and emerging scientifc opportunities. Adv. Energy Mater. 2017, 7, 1700841. [Google Scholar] [CrossRef]
- Li, S.; Hu, S.; Jiang, W.; Liu, Y.; Liu, Y.; Zhou, Y.; Mo, L.; Liu, J. Ag3VO4 nanoparticles decorated Bi2O2CO3 micro-flowers: An efficient visible-light-driven photocatalyst for the removal of toxic contaminants. Front. Chem. 2018, 6, 255. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Hu, S.; Jiang, W.; Liu, Y.; Liu, J.; Wang, Z. Facile synthesis of flower-like Ag3VO4/Bi2WO6 heterojunction with enhanced visible-light photocatalytic activity. J. Colloid Interface Sci. 2017, 501, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, H.; Xu, A.; Song, Z. Facile synthesis of Ag3PO4 modified with GQDs composites with enhanced visible-light photocatalytic activity. J. Mater. Sci. Mater. Electron. 2018, 29, 16691–16701. [Google Scholar] [CrossRef]
- Zheng, J.; Chang, F.; Jiao, M.; Xu, Q.; Deng, B.; Hu, X. A visible-light-driven heterojuncted composite WO3/Bi12O17Cl2: Synthesis, characterization, and improved photocatalytic performance. J. Colloid Interface Sci. 2018, 510, 20–31. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.F.; Huang, B.B.; Dai, Y. Engineering BiOX (X = Cl, Br, I) nanostructures for highly efficient photocatalytic applications. Nanoscale 2014, 6, 2009–2026. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhang, C.; Lai, C.; Zeng, G.; Huang, D.; Cheng, M.; Wang, J.; Chen, F.; Zhou, C.; Xiong, W. BiOX (X = Cl, Br, I) photocatalytic nanomaterials: Applications for fuels and environmental management. Adv. Colloid Interface Sci. 2018, 254, 76–93. [Google Scholar] [CrossRef] [PubMed]
- Li, S.J.; Hu, S.W.; Xu, K.B.; Jiang, W.; Liu, J.S.; Wang, Z.H. A novel heterostructure of BiOI nanosheets anchored onto MWCNTs with excellent visible-light photocatalytic activity. Nanomaterials 2017, 7, 22–34. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Wu, Z.; Liu, R.; Dionysiou, D.D.; Yang, K.; Wang, C.; Liu, H. Novel fluorinated Bi2MoO6 nanocrystals for efficient photocatalytic removal of water organic pollutants under different light source illumination. Appl. Catal. B 2017, 209, 1–11. [Google Scholar] [CrossRef]
- Jia, Y.; Ma, Y.; Tang, J.; Shi, W. Hierarchical nanosheet-based Bi2MoO6 microboxes for efficient photocatalytic performance. Dalton Trans. 2018, 47, 5542–5547. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yang, W.; Gao, S.; Zhu, L.; Sun, C.; Li, Q. Internal polarization modulation in Bi2MoO6 for photocatalytic performance enhancement under visible light illumination. ChemSusChem 2018, 11, 1521–1532. [Google Scholar] [CrossRef] [PubMed]
- Long, J.L.; Wang, S.C.; Chang, H.J.; Zhao, B.Z.; Liu, B.T.; Zhou, Y.G.; Wei, W.; Wang, X.X.; Huang, L.; Huang, W. Bi2MoO6 nanobelts for crystal facet-enhanced photocatalysis. Small 2014, 10, 2791–2795. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Tang, L.; Zeng, G.; Liu, Y.; Zhou, Y.; Deng, Y.; Wang, J.; Peng, B. Plasmonic Bi metal deposition and g-C3N4 coating on Bi2WO6 microspheres for efficient visible-light photocatalysis. ACS Sustain. Chem. Eng. 2017, 5, 1062–1072. [Google Scholar] [CrossRef]
- Guo, C.; Xu, J.; Wang, S.; Li, L.; Zhang, Y.; Li, X. Facile synthesis and photocatalytic application of hierarchical mesoporous Bi2MoO6 nanosheet-based microspheres. CrystEngComm 2012, 14, 3602–3608. [Google Scholar] [CrossRef]
- Ye, R.; Zhao, J.; Wickemeyer, B.B.; Toste, F.D.; Somorjai, G.A. Foundations and strategies of the construction of hybrid catalysts for optimized performances. Nat. Catal. 2018, 1, 318–325. [Google Scholar] [CrossRef]
- Ke, J.; Duan, X.; Luo, S.; Zhang, H.; Sun, H.; Liu, J.; Tade, M.; Wang, S. UV-assisted construction of 3D hierarchical rGO/Bi2MoO6 composites for enhanced photocatalytic water oxidation. Chem. Eng. J. 2017, 313, 1447–1453. [Google Scholar] [CrossRef]
- Zhao, Z.W.; Zhang, W.; Sun, Y.J.; Yu, J.Y.; Zhang, Y.X.; Wang, H.; Dong, F.; Wu, Z.B. Bi cocatalyst/Bi2MoO6 microspheres nanohybrid with SPR-promoted visible-light photocatalysis. J. Phys. Chem. C 2016, 120, 11889–11898. [Google Scholar] [CrossRef]
- Opoku, F.; Govender, K.; Sittert, C.G.C.E.V.; Govender, P. Insights into the photocatalytic mechanism of mediator-free direct Z-scheme g-C3N4/Bi2MoO6 (010) and g-C3N4/Bi2WO6 (010) heterostructures: A hybrid density functional theory study. Appl. Surf. Sci. 2018, 427, 487–498. [Google Scholar] [CrossRef]
- Ma, T.J.; Wu, J.; Mi, Y.D.; Chen, Q.H.; Ma, D.; Chai, C. Novel Z-Scheme g-C3N4/C@Bi2MoO6 composite with enhanced visible-light photocatalytic activity for naphthol degradation. Sep. Purif. Technol. 2017, 183, 54–65. [Google Scholar] [CrossRef]
- Zhang, M.Y.; Shao, C.L.; Mu, J.B.; Zhang, Z.Y.; Guo, Z.C.; Zhang, P.; Liu, Y.C. One-dimensional Bi2MoO6/TiO2 hierarchical heterostructures with enhanced photocatalytic activity. CrystEngComm 2012, 14, 605–612. [Google Scholar] [CrossRef]
- Lv, J.; Zhang, J.; Liu, J.; Li, Z.; Dai, K.; Liang, C. Bi SPR-promoted Z-scheme Bi2MoO6/CdS-diethylenetriamine composite with effectively enhanced visible light photocatalytic hydrogen evolution activity and stability. ACS Sustain. Chem. Eng. 2018, 6, 696–706. [Google Scholar] [CrossRef]
- Meng, Q.Q.; Zhou, Y.S.; Chen, G.; Hu, Y.D.; Lv, C.; Qiang, L.S.; Xing, W.N. Integrating both homojunction and heterojunction in QDs self-decorated Bi2MoO6/BCN composites to achieve an efficient photocatalyst for Cr(VI) reduction. Chem. Eng. J. 2018, 334, 334–343. [Google Scholar] [CrossRef]
- Li, X.; Su, M.; Zhu, G.; Zhang, K.; Zhang, X.; Fan, J. Fabrication of a novel few-layer WS2/Bi2MoO6 plate-on-plate heterojunction structure with enhanced visible-light photocatalytic activity. Dalton Trans. 2018, 47, 10046–10056. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.L.; Ma, Z. Flower-like Ag2MoO4/Bi2MoO6 heterojunctions with enhanced photocatalytic activity under visible light irradiation. J. Taiwan Inst. Chem. Eng. 2017, 71, 156–164. [Google Scholar] [CrossRef]
- Zhang, J.L.; Zhang, L.S.; Yu, N.; Xu, K.B.; Li, S.J.; Wang, H.L.; Liu, J.S. Flower-like Bi2S3/Bi2MoO6 heterojunction superstructures with enhanced visible-light-driven photocatalytic activity. RSC Adv. 2015, 5, 75081–75088. [Google Scholar] [CrossRef]
- Feng, Y.; Yan, X.; Liu, C.; Hong, Y.; Zhu, L.; Zhou, M.; Shi, W. Hydrothermal synthesis of CdS/Bi2MoO6 heterojunction photocatalysts with excellent visible-light-driven photocatalytic performance. Appl. Surf. Sci. 2015, 353, 87–94. [Google Scholar] [CrossRef]
- Li, S.; Jiang, W.; Hu, S.; Liu, Y.; Liu, Y.; Xu, K.; Liu, J. Hierarchical heterostructure of Bi2MoO6 micro-flowers decorated with Ag2CO3 nanoparticles for efficient visible-light-driven photocatalytic removal of toxic pollutants. Beilstein J. Nanotechnol. 2018, 9, 2297–2305. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Hu, S.; Jiang, W.; Zhou, Y.; Liu, J.; Wang, Z. Facile synthesis of cerium oxide nanoparticles decorated flower-like bismuth molybdate for enhanced photocatalytic activity toward organic pollutant degradation. J. Colloid Interface Sci. 2018, 530, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Hu, S.; Jiang, W.; Liu, Y.; Zhou, Y.; Liu, Y.; Mo, L. Hierarchical architectures of bismuth molybdate nanosheets onto nickel titanate nanofibers: Facile synthesis and efficient photocatalytic removal of tetracycline hydrochloride. J. Colloid Interface Sci. 2018, 521, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Hu, S.; Zhang, J.; Jiang, W.; Liu, J. Facile synthesis of Fe2O3 nanoparticles anchored on Bi2MoO6 microflowers with improved visible light photocatalytic activity. J. Colloid Interface Sci. 2017, 497, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Shen, X.; Liu, J.; Zhang, L. Synthesis of Ta3N5/Bi2MoO6 core-shell fiber-shaped heterojunctions as efficient and easily recyclable photocatalysts. Environ. Sci. Nano 2017, 4, 1155–1167. [Google Scholar] [CrossRef]
- Korala, L.; Germain, J.R.; Chen, E.; Pala, I.R.; Li, D.; Brock, S.L. CdS aerogels as efficient photocatalysts for degradation of organic dyes under visible light irradiation. Inorg. Chem. Front. 2017, 4, 1451–1457. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Han, L.; Wang, C.; Wang, W.; Ling, T.; Yang, J.; Dong, C.; Lin, F.; Du, X.-W. Zinc-Blende CdS Nanocubes with Coordinated Facets for Photocatalytic Water Splitting. ACS Catal. 2017, 7, 1470–1477. [Google Scholar] [CrossRef]
- Zhao, N.; Peng, J.; Liu, G.; Zhai, M. PVP-capped CdS nanopopcorns with type-II homojunctions for highly efficient visible-light-driven organic pollutant degradation and hydrogen evolution. J. Mater. Chem. A 2018. [Google Scholar] [CrossRef]
- Kandi, D.; Martha, S.; Thirumurugan, A.; Parida, K.M. Modification of BiOI microplates with CdS QDs for enhancing stability, optical property, electronic behavior toward rhodamine B decolorization, and photocatalytic hydrogen evolution. J. Phys. Chem. C 2017, 121, 4834–4849. [Google Scholar] [CrossRef]
- Kaur, A.; Kansal, S.K. Bi2WO6 nanocuboids: An efficient visible light active photocatalyst for the degradation of levofloxacin drug in aqueous phase. Chem. Eng. J. 2016, 302, 194–203. [Google Scholar] [CrossRef]
- Yang, X.; Xiang, Y.; Wang, X.; Li, S.; Chen, H.; Xing, D. Pyrene-based conjugated polymer/Bi2MoO6 Z-scheme hybrids: Facile construction and sustainable enhanced photocatalytic performance in ciprofloxacin and Cr(VI) removal under visible light irradiation. Catalysts 2018, 8, 185. [Google Scholar] [CrossRef]
- Li, S.; Hu, S.; Jiang, W.; Liu, Y.; Liu, J.; Wang, Z. Synthesis of n-type TaON microspheres decorated by p-type Ag2O with enhanced visible light photocatalytic activity. Mol. Catal. 2017, 435, 135–143. [Google Scholar] [CrossRef]
- Chang, F.; Wu, F.; Zheng, J.; Cheng, W.; Yan, W.; Deng, B.; Hu, X. In-situ establishment of binary composites a-Fe2O3/Bi12O17Cl2 with both photocatalytic and photo-Fenton features. Chemosphere 2018, 210, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Jiang, W.; Xu, K.; Hu, S.; Liu, Y.; Zhou, Y.; Liu, J. Synthesis of flower-like AgI/BiOCOOH pn heterojunctions with enhanced visible-light photocatalytic performance for the removal of toxic pollutants. Front. Chem. 2018, 6, 518. [Google Scholar]
- Chen, Y.J.; Tian, G.H.; Shi, Y.H.; Xiao, Y.T.; Fu, H.G. Hierarchical MoS2/Bi2MoO6 composites with synergistic effect for enhanced visible photocatalytic activity. Appl. Catal. B 2015, 164, 40–47. [Google Scholar] [CrossRef]








© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, S.; Liu, Y.; Long, Y.; Mo, L.; Zhang, H.; Liu, J. Facile Synthesis of Bi2MoO6 Microspheres Decorated by CdS Nanoparticles with Efficient Photocatalytic Removal of Levfloxacin Antibiotic. Catalysts 2018, 8, 477. https://doi.org/10.3390/catal8100477
Li S, Liu Y, Long Y, Mo L, Zhang H, Liu J. Facile Synthesis of Bi2MoO6 Microspheres Decorated by CdS Nanoparticles with Efficient Photocatalytic Removal of Levfloxacin Antibiotic. Catalysts. 2018; 8(10):477. https://doi.org/10.3390/catal8100477
Chicago/Turabian StyleLi, Shijie, Yanping Liu, Yunqian Long, Liuye Mo, Huiqiu Zhang, and Jianshe Liu. 2018. "Facile Synthesis of Bi2MoO6 Microspheres Decorated by CdS Nanoparticles with Efficient Photocatalytic Removal of Levfloxacin Antibiotic" Catalysts 8, no. 10: 477. https://doi.org/10.3390/catal8100477