Maltose Production Using Starch from Cassava Bagasse Catalyzed by Cross-Linked β-Amylase Aggregates
Abstract
:1. Introduction
2. Results and Discussion
2.1. β-Amylase Immobilization
2.2. Optimization of β-Amylase Immobilization Using CLEAs Technology
2.3. Effects of pH and Temperature on the Activity and the Stability of β-Amylase Preparations
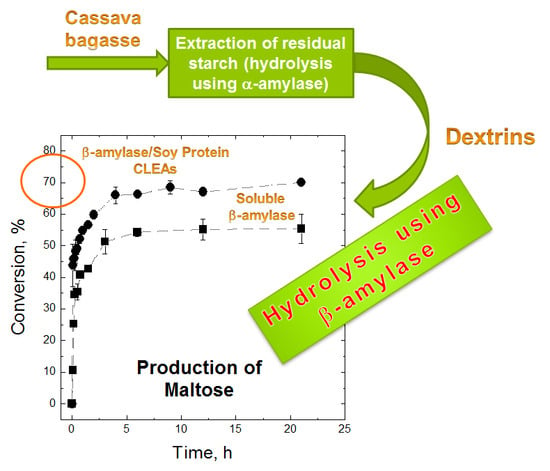
2.4. Maltose Production
3. Materials and Methods
3.1. Materials
3.2. Enzymatic Activity Assay
3.3. β-Amylase Immobilization Using CLEA Technique
3.4. Immobilizations on Chitosan Based Supports by Adsorption, Covalent Attachment and Encapsulation
3.5. Effect of pH and Temperature on Activity and Stability of β-Amylase Preparations
3.6. Cassava Bagasse Compositional Analysis
3.7. Residual Starch Extraction
3.8. Maltose Production
3.9. Reuse Assays
3.10. Protein Assay
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Food and Agriculture Organization (FAO). Food Outlook—Biannual Report on Global Food Markets; FAO: Rome, Italy, 2017; Volume November. [Google Scholar]
- Pandey, A.; Soccol, C.R.; Nigam, P.; Soccol, V.T.; Vanderberghe, L.P.S.; Mohan, R. Biotechnological potential of agro-industrial residues: II cassava bagasse. Bioresour. Technol. 2000, 74, 81–87. [Google Scholar] [CrossRef]
- Leonel, M.; Cereda, M.P. Starch extration from cassava fibrous residue. Ciência e Tecnol. Aliment. 2000, 20, 122–127. [Google Scholar] [CrossRef]
- Sriroth, K.; Chollakup, R.; Chotineeranat, S.; Piyachomkwan, K.; Oates, C.G. Processing of cassava waste for improved biomass utilization. Bioresour. Technol. 2000, 71, 63–69. [Google Scholar] [CrossRef]
- Prado, I.N.; Martins, A.D.S.; Alcalde, C.R.; Zeoula, L.M.; Marques, J.D.A. Performance of heifers fed diets containing corn or cassava hull as energy source and cottonseed meal or yeast as protein source. Rev. Bras. Zootec. 2000, 29, 278–287. [Google Scholar] [CrossRef]
- Piddocke, M.P.; Kreisz, S.; Heldt-Hansen, H.P.; Nielsen, K.F.; Olsson, L. Physiological characterization of brewer’s yeast in high-gravity beer fermentations with glucose or maltose syrups as adjuncts. Appl. Microbiol. Biotechnol. 2009, 84, 453–464. [Google Scholar] [CrossRef] [PubMed]
- BeMiller, J.N.; Huber, K.C. Carbohydrates. In Fennema’s Food Chemistry; Damodaran, S., Parkin, K.L., Fennema, O.R., Eds.; CRC Press: Boca Raton, FL, USA, 2007; pp. 83–151. [Google Scholar]
- Synowiecki, J. The use of starch processing enzymes in the food industry. In Industrial Enzymes; Polaina, J., MacCabe, A.P., Eds.; Springer: Dordrecht, The Netherlands, 2007; ISBN 9781402053764. [Google Scholar]
- Das, R.; Mishra, H.; Srivastava, A.; Kayastha, A.M. Covalent immobilization of beta-amylase onto functionalized molybdenum sulfide nanosheets, its kinetics and stability studies: A gateway to boost enzyme application. Chem. Eng. J. 2017, 328, 215–227. [Google Scholar] [CrossRef]
- Ma, Y.; Stewart, D.C.; Eglinton, J.K.; Logue, S.J.; Langridge, P.; Evans, D.E. Comparative Enzyme Kinetics of Two Allelic Forms of Barley (Hordeum vulgare L.) Beta -amylase. J. Cereal Sci. 2000, 31, 335–344. [Google Scholar] [CrossRef]
- Baker, W.L.; Smiley, K.L. Beta-amylase sulphydryl and disulphide group reactions. Additional aspects on enzyme inhibition by ascorbic acid. J. Inst. Brew. 1985, 91, 25–30. [Google Scholar] [CrossRef]
- Mikami, B.; Yoon, H.; Yoshigi, N. The Crystal Structure of the Sevenfold Mutant of Barley β-Amylase with Increased Thermostability at 2.5 Å Resolution. J. Mol. Biol. 1999, 285, 1235–1243. [Google Scholar] [CrossRef] [PubMed]
- Yoshigi, N.; Okada, Y.; Maeba, H.; Sahara, H.; Tamaki, T. Construction of a Plasmid Used for the Expression of a Sevenfold-Mutant Barley β-Amylase with Increased Thermostability in Escherichia coli and Properties of the Sevenfold-Mutant β-Amylase. J. Biochem. 1995, 118, 562–567. [Google Scholar] [CrossRef] [PubMed]
- Brady, D.; Jordaan, J. Advances in enzyme immobilisation. Biotechnol. Lett. 2009, 31, 1639–1650. [Google Scholar] [CrossRef] [PubMed]
- Cantone, S.; Ferrario, V.; Corici, L.; Ebert, C.; Fattor, D.; Spizzo, P.; Gardossi, L. Efficient immobilisation of industrial biocatalysts: Criteria and constraints for the selection of organic polymeric carriers and immobilisation methods. Chem. Soc. Rev. 2013, 42, 6262–6276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DiCosimo, R.; McAuliffe, J.; Poulose, A.J.; Bohlmann, G. Industrial use of immobilized enzymes. Chem. Soc. Rev. 2013, 42, 6437–6474. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, R.A.; van Pelt, S. Enzyme immobilisation in biocatalysis: Why, what and how. Chem. Soc. Rev. 2013, 42, 6223–6235. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Galan, C.; Berenguer-Murcia, Á.; Fernandez-Lafuente, R.; Rodrigues, R.C. Potential of different enzyme immobilization strategies to improve enzyme performance. Adv. Synth. Catal. 2011, 353, 2885–2904. [Google Scholar] [CrossRef]
- Guzik, U.; Hupert-Kocurek, K.; Wojcieszyńska, D. Immobilization as a Strategy for Improving Enzyme Properties-Application to Oxidoreductases. Molecules 2014, 19, 8995–9018. [Google Scholar] [CrossRef] [PubMed]
- Mateo, C.; Palomo, J.M.; Fernandez-Lorente, G.; Guisan, J.M.; Fernandez-Lafuente, R. Improvement of enzyme activity, stability and selectivity via immobilization techniques. Enzyme Microb. Technol. 2007, 40, 1451–1463. [Google Scholar] [CrossRef]
- Balcão, V.M.; Vila, M.M.D.C. Structural and functional stabilization of protein entities: State-of-the-art. Adv. Drug Deliv. Rev. 2015, 93, 25–41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iyer, P.V.; Ananthanarayan, L. Enzyme stability and stabilization—Aqueous and non-aqueous environment. Process Biochem. 2008, 43, 1019–1032. [Google Scholar] [CrossRef]
- Rodrigues, R.C.; Ortiz, C.; Berenguer-Murcia, A.; Torres, R.; Fernández-Lafuente, R.; Berenguer-Murcia, Á.; Torres, R.; Fernández-Lafuente, R. Modifying enzyme activity and selectivity by immobilization. Chem. Soc. Rev. 2013, 42, 6290–6307. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, O.; Ortiz, C.; Berenguer-Murcia, Á.; Torres, R.; Rodrigues, R.C.; Fernandez-Lafuente, R. Strategies for the one-step immobilization–purification of enzymes as industrial biocatalysts. Biotechnol. Adv. 2015, 33, 435–456. [Google Scholar] [CrossRef] [PubMed]
- Biró, E.; Németh, Á.S.; Sisak, C.; Feczkó, T.; Gyenis, J. Preparation of chitosan particles suitable for enzyme immobilization. J. Biochem. Biophys. Methods 2008, 70, 1240–1246. [Google Scholar] [CrossRef] [PubMed]
- Krajewska, B. Application of chitin- and chitosan-based materials for enzyme immobilizations: A review. Enzyme Microb. Technol. 2004, 35, 126–139. [Google Scholar] [CrossRef]
- Malmiri, H.J.; Jahanian, M.A.G.; Berenjian, A. Potential applications of chitosan nanoparticles as novel support in enzyme immobilization. Am. J. Biochem. Biotechnol. 2012, 8, 203–219. [Google Scholar] [CrossRef]
- Sjoholm, K.H.; Cooney, M.; Minteer, S.D. Effects of degree of deacetylation on enzyme immobilization in hydrophobically modified chitosan. Carbohydr. Polym. 2009, 77, 420–424. [Google Scholar] [CrossRef]
- Jesionowski, T.; Zdarta, J.; Krajewska, B. Enzyme immobilization by adsorption: A review. Adsorption 2014, 20, 801–821. [Google Scholar] [CrossRef]
- Santos, J.C.S.; Barbosa, O.; Ortiz, C.; Berenguer-Murcia, A.; Rodrigues, R.C.; Fernandez-Lafuente, R. Importance of the support properties for immobilization or purification of enzymes. ChemCatChem 2015, 7, 2413–2432. [Google Scholar] [CrossRef]
- Barbosa, O.; Ortiz, C.; Berenguer-Murcia, Á.; Torres, R.; Rodrigues, R.C.; Fernandez-Lafuente, R. Glutaraldehyde in bio-catalysts design: A useful crosslinker and a versatile tool in enzyme immobilization. RSC Adv. 2014, 4, 1583–1600. [Google Scholar] [CrossRef]
- Migneault, I.; Dartiguenave, C.; Bertrand, M.J.; Waldron, K.C. Glutaraldehyde: Behavior in aqueous solution, reaction with proteins, and application to enzyme crosslinking. Biotechniques 2004, 37, 790–802. [Google Scholar] [PubMed]
- Wine, Y.; Cohen-Hadar, N.; Freeman, A.; Frolow, F. Elucidation of the mechanism and end products of glutaraldehyde crosslinking reaction by X-ray structure analysis. Biotechnol. Bioeng. 2007, 98, 711–718. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, O.; Torres, R.; Ortiz, C.; Berenguer-Murcia, Á.; Rodrigues, R.C.; Fernandez-Lafuente, R. Heterofunctional supports in enzyme immobilization: From traditional immobilization protocols to opportunities in tuning enzyme properties. Biomacromolecules 2013, 14, 2433–2462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barbosa, O.; Torres, R.; Ortiz, C.; Fernandez-Lafuente, R. Versatility of glutaraldehyde to immobilize lipases: Effect of the immobilization protocol on the properties of lipase B from Candida antarctica. Process Biochem. 2012, 47, 1220–1227. [Google Scholar] [CrossRef]
- Betancor, L.; López-Gallego, F.; Hidalgo, A.; Alonso-Morales, N.; Mateo, G.D.-O.C.; Fernández-Lafuente, R.; Guisán, J.M. Different mechanisms of protein immobilization on glutaraldehyde activated supports: Effect of support activation and immobilization conditions. Enzyme Microb. Technol. 2006, 39, 877–882. [Google Scholar] [CrossRef]
- López-Gallego, F.; Betancor, L.; Mateo, C.; Hidalgo, A.; Alonso-Morales, N.; Dellamora-Ortiz, G.; Guisán, J.M.; Fernández-Lafuente, R. Enzyme stabilization by glutaraldehyde crosslinking of adsorbed proteins on aminated supports. J. Biotechnol. 2005, 119, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Zaak, H.; Peirce, S.; de Albuquerque, T.; Sassi, M.; Fernandez-Lafuente, R. Exploiting the Versatility of Aminated Supports Activated with Glutaraldehyde to Immobilize β-galactosidase from Aspergillus oryzae. Catalysts 2017, 7, 250. [Google Scholar] [CrossRef]
- Vazquez-Ortega, P.G.; Alcaraz-Fructuoso, M.T.; Rojas-Contreras, J.A.; López-Miranda, J.; Fernandez-Lafuente, R. Stabilization of dimeric β-glucosidase from Aspergillus niger via glutaraldehyde immobilization under different conditions. Enzyme Microb. Technol. 2018, 110, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Peniche, C.; Argüelles-Monal, W.; Peniche, H.; Acosta, N. Chitosan: An Attractive Biocompatible Polymer for Microencapsulation. Macromol. Biosci. 2003, 3, 511–520. [Google Scholar] [CrossRef]
- Zhang, Y.; Tao, L.; Li, S.; Wei, Y. Synthesis of Multiresponsive and Dynamic Chitosan-Based Hydrogels for Controlled Release of Bioactive Molecules. Biomacromolecules 2011, 12, 2894–2901. [Google Scholar] [CrossRef] [PubMed]
- Betancor, L.; Luckarift, H.R. Bioinspired enzyme encapsulation for biocatalysis. Trends Biotechnol. 2008, 26, 566–572. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, S. Carrageenan for encapsulation and immobilization of flavor, fragrance, probiotics, and enzymes: A review. J. Carbohydr. Chem. 2017, 36, 1–19. [Google Scholar] [CrossRef]
- Pierre, A.C. The sol-gel encapsulation of enzymes. Biocatal. Biotransformation 2004, 22, 145–170. [Google Scholar] [CrossRef]
- Cao, L.; van Rantwijk, F.; Sheldon, R.A. Cross-Linked Enzyme Aggregates: A Simple and Effective Method for the Immobilization of Penicillin Acylase. Org. Lett. 2000, 2, 1361–1364. [Google Scholar] [CrossRef] [PubMed]
- Schoevaart, R.; Wolbers, M.W.; Golubovic, M.; Ottens, M.; Kieboom, A.P.G.; van Rantwijk, F.; van der Wielen, L.A.M.; Sheldon, R.A. Preparation, optimization, and structures of cross-linked enzyme aggregates (CLEAs). Biotechnol. Bioeng. 2004, 87, 754–762. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, R.A. Cross-linked enzyme aggregates (CLEA®s): Stable and recyclable biocatalysts. Biochem. Soc. Trans. 2007, 35, 1583–1587. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, R.A. Cross-linked enzyme aggregates as industrial biocatalysts. Org. Process Res. Dev. 2011, 15, 213–223. [Google Scholar] [CrossRef]
- Talekar, S.; Joshi, A.; Joshi, G.; Kamat, P.; Haripurkar, R.; Kambale, S. Parameters in preparation and characterization of cross linked enzyme aggregates (CLEAs). RSC Adv. 2013, 3, 12485–12511. [Google Scholar] [CrossRef]
- Galvis, M.; Barbosa, O.; Ruiz, M.; Cruz, J.; Ortiz, C.; Torres, R.; Fernandez-Lafuente, R. Chemical amination of lipase B from Candida antarctica is an efficient solution for the preparation of crosslinked enzyme aggregates. Process Biochem. 2012, 47, 2373–2378. [Google Scholar] [CrossRef]
- Rodrigues, R.C.; Barbosa, O.; Ortiz, C.; Berenguer-Murcia, Á.; Torres, R.; Fernandez-Lafuente, R. Amination of enzymes to improve biocatalyst performance: Coupling genetic modification and physicochemical tools. RSC Adv. 2014, 4, 38350–38374. [Google Scholar] [CrossRef]
- López-Gallego, F.; Betancor, L.; Hidalgo, A.; Alonso, N.; Fernández-Lafuente, R.; Guisán, J.M. Co-aggregation of Enzymes and Polyethyleneimine: A Simple Method To Prepare Stable and Immobilized Derivatives of Glutaryl Acylase. Biomacromolecules 2005, 6, 1839–1842. [Google Scholar] [CrossRef] [PubMed]
- Wilson, L.; Fernández-Lorente, G.; Fernández-Lafuente, R.; Illanes, A.; Guisán, J.M.; Palomo, J.M. CLEAs of lipases and poly-ionic polymers: A simple way of preparing stable biocatalysts with improved properties. Enzyme Microb. Technol. 2006, 39, 750–755. [Google Scholar] [CrossRef]
- Ahumada, K.; Urrutia, P.; Illanes, A.; Wilson, L. Production of combi-CLEAs of glycosidases utilized for aroma enhancement in wine. Food Bioprod. Process. 2015, 94, 555–560. [Google Scholar] [CrossRef]
- Cruz, J.; Barbosa, O.; Rodrigues, R.C.; Fernandez-Lafuente, R.; Torres, R.; Ortiz, C. Optimized preparation of CALB-CLEAs by response surface methodology: The necessity to employ a feeder to have an effective crosslinking. J. Mol. Catal. B Enzym. 2012, 80, 7–14. [Google Scholar] [CrossRef]
- Cui, J.D.; Sun, L.M.; Li, L.L. A simple technique of preparing stable CLEAs of Phenylalanine ammonia lyase using co-aggregation with starch and bovine serum albumin. Appl. Biochem. Biotechnol. 2013, 170, 1827–1837. [Google Scholar] [CrossRef] [PubMed]
- Dal Magro, L.; Hertz, P.F.; Fernandez-Lafuente, R.; Klein, M.P.; Rodrigues, R.C. Preparation and characterization of a Combi-CLEAs from pectinases and cellulases: A potential biocatalyst for grape juice clarification. RSC Adv. 2016, 6, 27242–27251. [Google Scholar] [CrossRef]
- Mafra, A.C.O.; Kopp, W.; Beltrame, M.B.; Giordano, R.L.C.; Ribeiro, M.P.A.; Tardioli, P.W. Diffusion effects of bovine serum albumin on cross-linked aggregates of catalase. J. Mol. Catal. B Enzym. 2016, 133, 107–116. [Google Scholar] [CrossRef]
- Shah, S.; Sharma, A.; Gupta, M.N. Preparation of cross-linked enzyme aggregates by using bovine serum albumin as a proteic feeder. Anal. Biochem. 2006, 351, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Torabizadeh, H.; Tavakoli, M.; Safari, M. Immobilization of thermostable α-amylase from Bacillus licheniformis by cross-linked enzyme aggregates method using calcium and sodium ions as additives. J. Mol. Catal. B Enzym. 2014, 108, 13–20. [Google Scholar] [CrossRef]
- Perkins, E.G. Composition of soybean and soybean products. In Practical Handbook of Soybean Processing and Utilization; Erickson, D.R., Ed.; AOCS Press: St. Louis, France, 1995; ISBN 978-0-935315-63-9. [Google Scholar]
- Iwabuchi, S.; Yamauchi, F. Determination of glycinin and β-conglycinin in soybean proteins by immunological methods. J. Agric. Food Chem. 1987, 35, 200–205. [Google Scholar] [CrossRef]
- Thanh, V.H.; Shibasaki, K. Major proteins of soybean seeds. subunit structure of β-conglycinin. J. Agric. Food Chem. 1978, 26, 692–695. [Google Scholar] [CrossRef]
- Utsumi, S.; Inaba, H.; Mori, T. Heterogeneity of soybean glycinin. Phytochemistry 1981, 20, 585–589. [Google Scholar] [CrossRef]
- Noda, T.; Furuta, S.; Suda, I. Sweet potato beta-amylase immobilized on chitosan beads and its application in the semi-continuous production of maltose. Carbohydr. Polym. 2001, 44, 189–195. [Google Scholar] [CrossRef]
- Vretblad, P.; Axen, R. Preparation and properties of an immobilized Barley β-amylase. Biotechnol. Bioeng. 1973, 15, 783–794. [Google Scholar] [CrossRef]
- Martensson, K.A.J. Preparation of an immobilized two-enzyme system, beta-amylase-Pullulanase, to an acrylic copolymer for the conversion of starch to maltose. I. Preparation and stability of immobilized beta-amylase. Biotechnol. Bioeng. 1974, XVI, 579–591. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.J.; Khan, F.H.; Husain, Q. Application of immobilized Ipomoea batata beta amylase in the saccharification of starch. J. Appl. Biol. Sci. 2011, 5, 33–39. [Google Scholar]
- Tavano, O.L.; Fernandez-Lafuente, R.; Goulart, A.J.; Monti, R. Optimization of the immobilization of sweet potato amylase using glutaraldehyde-agarose support. Characterization of the immobilized enzyme. Process Biochem. 2013, 48, 1054–1058. [Google Scholar] [CrossRef]
- Talekar, S.; Desai, S.; Pillai, M.; Nagavekar, N.; Ambarkar, S.; Surnis, S.; Ladole, M.; Nadar, S.; Mulla, M. Carrier free co-immobilization of glucoamylase and pullulanase as combi-cross linked enzyme aggregates (combi-CLEAs). RSC Adv. 2013, 3, 2265–2271. [Google Scholar] [CrossRef]
- Talekar, S.; Ghodake, V.; Ghotage, T.; Rathod, P.; Deshmukh, P.; Nadar, S.; Mulla, M.; Ladole, M. Novel magnetic cross-linked enzyme aggregates (magnetic CLEAs) of alpha amylase. Bioresour. Technol. 2012, 123, 542–547. [Google Scholar] [CrossRef] [PubMed]
- Talekar, S.; Waingade, S.; Gaikwad, V.; Patil, S.; Nagavekar, N. Preparation and characterization of cross linked enzyme aggregates (CLEAs) of Bacillus amyloliquefaciens alpha amylase. J. Biochem. Technol. 2012, 3, 349–353. [Google Scholar]
- Yoshigi, N.; Okada, Y.; Sahara, H.; Koshino, S. Expression in Escherichia coli of cDNA encoding barley beta-amylase and properties of recombinant beta-amylase. Biosci. Biotechnol. Biochem. 1994, 58, 1080–1086. [Google Scholar] [CrossRef] [PubMed]
- Gaouar, O.; Zakhia, N.; Aymard, C.; Rios, G.M. Production of maltose syrup by bioconversion of cassava starch in an ultrafiltration reactor. Ind. Crops Prod. 1998, 7, 159–167. [Google Scholar] [CrossRef]
- Shiraishi, F.; Kawakami, K.; Yuasa, A.; Kojima, T.; Kusunoki, K. Kinetic expression for maltose production from soluble starch by simultaneous use of beta-amylase and debranching enzymes. Biotechnol. Bioeng. 1987, 30, 374–380. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, A.; Pletschke, B.I. Magnetic cross-linked enzyme aggregates (CLEAs): A novel concept towards carrier free immobilization of lignocellulolytic enzymes. Enzyme Microb. Technol. 2014, 61, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Kopp, W.; Da Costa, T.P.; Pereira, S.C.; Jafelicci, M.; Giordano, R.C.; Marques, R.F.C.; Araújo-Moreira, F.M.; Giordano, R.L.C. Easily handling penicillin G acylase magnetic cross-linked enzymes aggregates: Catalytic and morphological studies. Process Biochem. 2014, 49, 38–46. [Google Scholar] [CrossRef]
- Kumar, V.V.; Sivanesan, S.; Cabana, H. Magnetic cross-linked laccase aggregates—Bioremediation tool for decolorization of distinct classes of recalcitrant dyes. Sci. Total Environ. 2014, 487, 830–839. [Google Scholar] [CrossRef] [PubMed]
- Nadar, S.S.; Rathod, V.K. Magnetic macromolecular cross linked enzyme aggregates (CLEAs) of glucoamylase. Enzyme Microb. Technol. 2016, 83, 78–87. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-M.; Kim, M.; Park, J.-Y.; Lee, D.-H.; Lee, K.-H.; Min, J.; Kim, Y.-H. Immobilization of the cross-linked para-nitrobenzyl esterase of Bacillus subtilis aggregates onto magnetic beads. Process Biochem. 2010, 45, 259–263. [Google Scholar] [CrossRef]
- Wilson, L.; Illanes, A.; Pessela, B.C.C.; Abian, O.; Fernández-Lafuente, R.; Guisán, J.M. Encapsulation of crosslinked penicillin G acylase aggregates in lentikats: Evaluation of a novel biocatalyst in organic media. Biotechnol. Bioeng. 2004, 86, 558–562. [Google Scholar] [CrossRef] [PubMed]
- Molawa, L.; Jordaan, J.; Limson, J.; Brady, D. Modification of Alcalase SphereZymeTM by entrapment in LentiKats® to impart improved particle stability. Biocatal. Biotransformation 2013, 31, 71–78. [Google Scholar] [CrossRef]
- Cui, J.D.; Li, L.L.; Bian, H.J. Immobilization of Cross-Linked Phenylalanine Ammonia Lyase Aggregates in Microporous Silica Gel. PLoS ONE 2013, 8, e80581. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Wang, Q.; Wang, W.; Zhou, L.; Gao, J. Preparation of immobilized lipase through combination of cross-linked enzyme aggregates and biomimetic silicification. Chin. J. Catal. 2012, 33, 857–862. [Google Scholar] [CrossRef]
- Bernfeld, P. Amylases, alpha and beta. In Methods in Enzymology; Colowick, S.P., Kaplan, N.O., Eds.; Academic Press: New York, NY, USA, 1955; pp. 149–158. [Google Scholar]
- Miller, G.L. Use of DinitrosaIicyIic Acid Reagent for Determination of Reducing Sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Vieira, D.C.; Lima, L.N.; Mendes, A.A.; Adriano, W.S.; Giordano, R.C.; Giordano, R.L.C.; Tardioli, P.W. Hydrolysis of lactose in whole milk catalyzed by β-galactosidase from Kluyveromyces fragilis immobilized on chitosan-based matrix. Biochem. Eng. J. 2013, 81. [Google Scholar] [CrossRef]
- Lacerda, L.G.; Almeida, R.R.; Demiate, I.M.; Carvalho Filho, M.A.S.; Vasconcelos, E.C.; Woiciechowski, A.L.; Bannach, G.; Schnitzler, E.; Soccol, C.R. Thermoanalytical and starch content evaluation of cassava bagasse as agro-industrial residue. Brazilian Arch. Biol. Technol. 2009, 52, 143–150. [Google Scholar] [CrossRef]
- Gouveia, E.R.; Nascimento, R.T.D.; Souto-Maior, A.M.; Rocha, G.J.D.M. Validation of methodology for the chemical characterization of sugar cane bagasse. Quim. Nova 2009, 32, 1500–1503. [Google Scholar] [CrossRef]
- Giordano, R.C.; Giordano, R.L.C. Taylor-Couette vortex flow in enzymatic reactors. In Immobilization of enzymes and cells; Guisan, J.M., Ed.; Humana Press: Totowa, NJ, USA, 2006; pp. 321–332. [Google Scholar]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]





| Immobilization Method | Immobilization Yield (%) | Expressed Activity (%) | Global Yield (%) |
|---|---|---|---|
| Adsorption | 21.4 ± 3.4 | 108.9 ± 17.6 | 23.3 ± 3.8 |
| Adsorption followed by crosslinking 1 | 21.4 ± 3.4 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| Covalent attachment 2 | 100.0 ± 0.0 | 6.7 ± 0.8 | 6.7 ± 0.8 |
| Encapsulation 3 | – | – | 1.1 ± 0.5 |
| CLEA 4 | – | – | 34.2 ± 3.3 |
| CLEA 5 | – | – | 25.8 ± 1.2 |
| Assay | Feeder Protein | Global Yield (%) |
|---|---|---|
| 1 | None | 34.2 ± 3.3 |
| 2 | 25.8 ± 1.2 | |
| 3 | BSA | 54.5 ± 0.9 |
| 4 | 43.2 ± 0.4 | |
| 5 | 82.7 ± 5.8 | |
| 6 | 53.1 ± 0.1 | |
| 7 | 33.6 ± 0.5 | |
| 8 | 0 | |
| 9 | 0 | |
| 10 | 0 | |
| 11 | SPI | 45.2 ± 2.1 |
| 12 | 53.3 ± 2.4 | |
| 13 | 48.0 ± 2.3 | |
| 14 | 47.5 ± 1.5 | |
| 15 | 38.2 ± 5.2 | |
| 16 | 31.0 ± 1.8 | |
| 17 | 28.4 ± 2.9 | |
| 18 | 24.4 ± 0.5 | |
| 19 | 28.6 ± 0.0 |
| Assay 1 | Feeder Protein | Cofeeder Concentration 2 (mg/mL) | Glutaraldehyde Concentration 3 (mM) | Glutaraldehyde/Total Protein 4 Ratio (mM/mg) |
|---|---|---|---|---|
| 1 | None | 0 | 30 | 60.0 |
| 2 | 0 | 60 | 120.0 | |
| 3 | BSA | 160 | 30 | 0.74 |
| 4 | 120 | 30 | 0.98 | |
| 5 | 80 | 30 | 1.46 | |
| 6 | 60 | 30 | 1.93 | |
| 7 | 40 | 30 | 2.86 | |
| 8 | 120 | 60 | 1.97 | |
| 9 | 80 | 60 | 2.93 | |
| 10 | 40 | 60 | 5.71 | |
| 11 | SPI | 80 | 30 | 1.46 |
| 12 | 60 | 30 | 1.93 | |
| 13 | 40 | 30 | 2.86 | |
| 14 | 30 | 30 | 3.75 | |
| 15 | 20 | 30 | 5.45 | |
| 16 | 60 | 60 | 3.87 | |
| 17 | 40 | 60 | 5.71 | |
| 18 | 30 | 60 | 7.50 | |
| 19 | 20 | 60 | 10.91 |
| Immobilization Method | Adsorption | Adsorption Followed by Crosslinking | Covalent Attachment | Encapsulation |
|---|---|---|---|---|
| Carrier | Chitosan 2 wt % | Chitosan 2 wt % | Chitosan 2 wt % | Chitosan 1 wt % |
| Glutaraldehyde concentration | 0 | 0.15% | 0.80% | 0.10% |
| Temperature (°C) | 25 | 25 | 25 | 0 (Ice bath) |
| Stirring | 100 rpm | 100 rpm | 100 rpm | 50 rpm |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Araujo-Silva, R.; Mafra, A.C.O.; Rojas, M.J.; Kopp, W.; Giordano, R.D.C.; Fernandez-Lafuente, R.; Tardioli, P.W. Maltose Production Using Starch from Cassava Bagasse Catalyzed by Cross-Linked β-Amylase Aggregates. Catalysts 2018, 8, 170. https://doi.org/10.3390/catal8040170
Araujo-Silva R, Mafra ACO, Rojas MJ, Kopp W, Giordano RDC, Fernandez-Lafuente R, Tardioli PW. Maltose Production Using Starch from Cassava Bagasse Catalyzed by Cross-Linked β-Amylase Aggregates. Catalysts. 2018; 8(4):170. https://doi.org/10.3390/catal8040170
Chicago/Turabian StyleAraujo-Silva, Rafael, Agnes Cristina Oliveira Mafra, Mayerlenis Jimenez Rojas, Willian Kopp, Roberto De Campos Giordano, Roberto Fernandez-Lafuente, and Paulo Waldir Tardioli. 2018. "Maltose Production Using Starch from Cassava Bagasse Catalyzed by Cross-Linked β-Amylase Aggregates" Catalysts 8, no. 4: 170. https://doi.org/10.3390/catal8040170