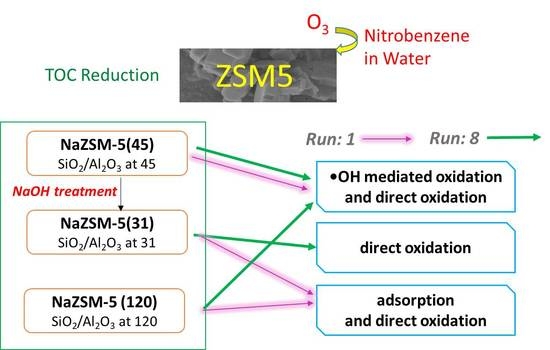
Investigation of Catalytic Ozonation of Recalcitrant Organic Chemicals in Aqueous Solution over Various ZSM-5 Zeolites
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characteristics of Catalysts
2.2. TOC Removal of Nitrobenzene Solution in SAPs, SOP, and COPs
2.3. Changes of Solution pH Values during SAP, SOP, and COP Treatments
2.4. Influences of Repeated Uses of Catalysts on COPs
2.5. Influences of NaHCO3 on COPs
3. Materials and Methods
3.1. Materials
3.2. Preparation of Catalysts
3.3. Characterization of Catalysts
3.4. Ozonation of Nitrobenzene Solution
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lin, Y.; Han, X.; Lu, H.; Zhou, J. Study of archaea community structure during the biodegradation process of nitrobenzene wastewater in an anaerobic baffled reactor. Int. Biodeter. Biodegr. 2013, 85, 499–505. [Google Scholar] [CrossRef]
- Qi, F.; Xu, B.; Chen, Z.; Feng, L.; Zhang, L.; Sun, D. Catalytic ozonation of 2-isopropyl-3-methoxypyrazine in water by γ-AlOOH and γ-Al2O3: Comparison of removal efficiency and mechanism. Chem. Eng. J. 2013, 219, 527–536. [Google Scholar] [CrossRef]
- Dai, Q.; Wang, J.; Chen, J.; Chen, J. Ozonation catalyzed by cerium supported on activated carbon for the degradation of typical pharmaceutical wastewater. Sep. Purif. Technol. 2014, 127, 112–120. [Google Scholar] [CrossRef]
- He, Z.; Zhang, A.; Song, S.; Liu, Z.; Chen, J.; Xu, X.; Liu, W. γ-Al2O3 modified with praseodymium: an application in the heterogeneous catalytic ozonation of succinic acid in aqueous solution. Ind. Eng. Chem. Res. 2010, 49, 12345–12351. [Google Scholar] [CrossRef]
- Faria, P.; Órfão, J.; Pereira, M. Catalytic ozonation of sulfonated aromatic compounds in the presence of activated carbon. Appl. Catal. B Environ. 2008, 83, 150–159. [Google Scholar] [CrossRef]
- Wu, J.; Ma, L.; Chen, Y.; Cheng, Y.; Liu, Y.; Zha, X. Catalytic ozonation of organic pollutants from bio-treated dyeing and finishing wastewater using recycled waste iron shavings as a catalyst: Removal and pathways. Water Res. 2016, 92, 140–148. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Ma, J. Catalytic ozonation of trace nitrobenzene in water with synthetic goethite. J. Mol. Catal. A Chem. 2008, 279, 82–89. [Google Scholar] [CrossRef]
- Wang, J.; Bai, Z. Fe-based catalysts for heterogeneous catalytic ozonation of emerging contaminants in water and wastewater. Chem. Eng. J. 2017, 312, 79–98. [Google Scholar] [CrossRef]
- Radhakrishnan, R.; Oyama, S.T. Ozone decomposition over manganese oxide supported on ZrO2 and TiO2: A kinetic study using in situ laser Raman spectroscopy. J. Catal. 2001, 199, 282–290. [Google Scholar] [CrossRef]
- Hu, C.; Xing, S.; Qu, J.; He, H. Catalytic ozonation of herbicide 2, 4-D over cobalt oxide supported on mesoporous zirconia. J. Phys. Chem. C 2008, 112, 5978–5983. [Google Scholar] [CrossRef]
- Ikhlaqa, A.; Browna, D.R.; Kasprzyk-Hordernc, B. Catalytic ozonation of chlorinated VOCs on ZSM-5 zeolites and alumina: Formation of chlorides. Appl. Catal. B Environ. 2017, 200, 274–282. [Google Scholar] [CrossRef]
- Ikhlaqa, A.; Browna, D.R.; Kasprzyk-Hordernc, B. Mechanisms of catalytic ozonation on alumina and zeolites in water: Formation of hydroxyl radicals. Appl. Catal. B Environ. 2012, 123–124, 94–106. [Google Scholar] [CrossRef]
- Gao, G.; Shen, J.; Chu, W.; Chen, Z.; Yuan, L. Mechanism of enhanced diclofenac mineralization by catalytic ozonation over iron silicate-loaded pumice. Sep. Purif. Technol. 2017, 173, 55–62. [Google Scholar] [CrossRef]
- Amin, N.A.S.; Akhtar, J.; Rai, H.K. Screening of combined zeolite-ozone system for phenol and COD removal. Chem. Eng. J. 2010, 158, 520–527. [Google Scholar] [CrossRef]
- Fujita, H.; Izumi, J.; Sagehashi, M.; Fujii, T.; Sakoda, A. Adsorption and decomposition of water-dissolved ozone on high silica zeolites. Water Res. 2004, 38, 159–165. [Google Scholar] [CrossRef]
- Rajasekhar Pullabhotla, V.S.R.; Jonnalagadda, S.B. Scope of metal loaded microporous ZSM-5 zeolites in the “catazone” process of n-hexadecane at moderate conditions. Ind. Eng. Chem. Res. 2009, 48, 9097–9105. [Google Scholar] [CrossRef]
- Fujita, H.; Izumi, J.; Sagehashi, M.; Fujii, T.; Sakoda, A. Decomposition of trichloroethene on ozone-adsorbed high silica zeolites. Water Res. 2004, 38, 166–172. [Google Scholar] [CrossRef]
- Fujita, H.; Shiraishi, K.; Fujii, T.; Sakoda, A.; Izumi, J. Adsorbed phase ozonation of water-dissolved organic pollutants using high-silica zeolites. Adsorption 2005, 11, 835–839. [Google Scholar] [CrossRef]
- Ikhlaqa, A.; Browna, D.R.; Kasprzyk-Hordernc, B. Catalytic ozonation for the removal of organic contaminants in water on ZSM-5 zeolites. Appl. Catal. B Environ. 2014, 154–155, 110–122. [Google Scholar] [CrossRef]
- Ikhlaq, A.; Brown, D.R.; Kasprzyk-Hordern, B. Mechanisms of catalytic ozonation: An investigation into superoxide ion radical and hydrogen peroxide formation during catalytic ozonation on alumina and zeolites in water. Appl. Catal. B Environ. 2013, 129, 437–449. [Google Scholar] [CrossRef]
- Xu, R.; Pang, W. Chemistry-Zeolites and Porous Materials, 2nd ed.; Science Press: Beijing, China, 2004; pp. 5–15. [Google Scholar]
- Armaroli, T.; Simon, L.J.; Digne, M.; Montanari, T.; Bevilacqua, M.; Valtchev, V.; Patarin, J.; Busca, G. Effects of crystal size and Si/Al ratio on the surface properties of H-ZSM-5 zeolites. Appl. Catal. A Gen. 2006, 36, 78–84. [Google Scholar] [CrossRef]
- Chen, C.; Wei, L.; Guo, X.; Guo, S.; Yan, G. Investigation of heavy oil refinery wastewater treatment by integrated ozone and activated carbon-supported manganese oxides. Fuel Process. Technol. 2014, 124, 165–173. [Google Scholar] [CrossRef]
- Faria, P.C.C.; Órfão, J.J.M.; Pereir, M.F.R. A novel ceria–activated carbon composite for the catalytic ozonation of carboxylic acids. Catal. Commun. 2008, 9, 2121–2126. [Google Scholar] [CrossRef]
- Chen, C.; Chen, Y.; Yoza, B.A.; Du, Y.; Wang, Y.; Li, Q.X.; Yi, L.; Guo, S.; Wang, Q. Comparison of Efficiencies and Mechanisms of Catalytic Ozonation of Recalcitrant Petroleum Refinery Wastewater by Ce, Mg, and Ce-Mg Oxides Loaded Al2O3. Catalysts 2017, 7, 72. [Google Scholar] [CrossRef]
- Huang, R.; Lan, B.; Chen, Z.; Yan, H.; Zhang, Q.; Li, L. Catalytic ozonation of p-chlorobenzoic acid over MCM-41 and Fe loaded MCM-41. Chem. Eng. J. 2012, 180, 19–24. [Google Scholar] [CrossRef]
- Groen, J.C.; Peffer, L.A.A.; Moulijin, J.A.; Pérez-Ramírez, J. Mesoporosity development in ZSM-5 zeolite upon optimized desilication conditions in alkaline medium. Colloids Surf. A 2004, 241, 53–58. [Google Scholar] [CrossRef]
- Ogura, M.; Shinomiya, S.; Tateno, J.; Nara, Y. Alkali-treatment technique-new method for modification of structural and acid-catalytic properties of ZSM-5 zeolites. Appl. Catal. A Gen. 2001, 219, 33–43. [Google Scholar] [CrossRef]
- Huang, X.; Yuan, J.; Shi, J.; Shangguan, W. Ozone-assisted photocatalytic oxidation of gaseous acetaldehyde on TiO2/H-ZSM-5 catalysts. J. Hazard. Mater. 2009, 171, 827–832. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Wei, L.; Cheng, S.; Cao, Y.; Julson, J.; Gu, Z. Catalytic cracking of carinata oil for hydrocarbon biofuel over fresh and regenerated Zn/Na-ZSM-5. Appl. Catal. A Gen. 2015, 507, 44–55. [Google Scholar] [CrossRef]
- Huang, H.B.; Huang, W.; Xu, Y.; Ye, X.; Wu, M.; Shao, Q.; Ou, G.; Peng, Z.; Shi, J.; Chen, J.; et al. Catalytic oxidation of gaseous benzene with ozone overzeolite-supported metal oxide nanoparticles at room temperature. Catal. Today 2015, 258, 627–633. [Google Scholar] [CrossRef]
- Suib, S.L.; Winiecki, A.M.; Kostapapas, A. Surface states of aluminophosphate and zeolite molecular sieves. Stud. Surf. Sci. Catal. 1986, 28, 409–414. [Google Scholar]
- Ismail, M.N.; Goodrich, T.L.; Ji, Z.; Ziemer, K.S.; Warzywoda, J.; Sacco, A., Jr. Assembly of titanosilicate ETS-10 crystals on organosilane-functionalized gallium nitride surfaces. Micropore Mesopor. Mater. 2009, 118, 245–250. [Google Scholar] [CrossRef]
- Moussavi, G.; Aghapour, A.A.; Yaghmaeian, K. The degradation and mineralization of catechol using ozonation catalyzed with MgO/GAC composite in a fluidized bed reactor. Chem. Eng. J. 2014, 249, 302–310. [Google Scholar] [CrossRef]
- Gallegos, M.V.; Peluso, M.A.; Thomas, H.; Damonte, L.C.; Sambeth, J.E. Structural and optical properties of ZnO and manganese-doped ZnO. J. Alloys Compd. 2016, 689, 416–424. [Google Scholar] [CrossRef]
- Reungoat, J.; Pic, J.S.; Manéro, M.H.; Debellefontaine, H. Adsorption of nitrobenzene from water onto high silica zeolites and regeneration by ozone. Sep. Sci. Technol. 2007, 42, 1447–1463. [Google Scholar] [CrossRef] [Green Version]
- Staehelin, J.; Hoigne, J. Decomposition of ozone in water: Rate of initiation by hydroxide ions and hydrogen peroxide. Environ. Sci. Technol. 1982, 16, 676–681. [Google Scholar] [CrossRef]
- Qi, F.; Xu, B.; Chen, Z.; Ma, J.; Sun, D.; Zhang, L. Influence of aluminum oxides surface properties on catalyzed ozonation of 2, 4, 6-trichloroanisole. Sep. Purif. Technol. 2009, 66, 405–410. [Google Scholar] [CrossRef]
- Altenor, S.; Carene, B.; Emmanuel, E.; Lambert, J.; Ehrhardt, J.; Gaspard, S. Adsorption studies of methylene blue and phenol onto vetiver roots activated carbon prepared by chemical activation. J. Hazard. Mater. 2009, 165, 1029–1039. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Ma, J.; Qin, Q.; Zhai, X. Degradation of nitrobenzene by nano-TiO2 catalyzed ozonation. J. Mol. Catal. A Chem. 2007, 267, 41–48. [Google Scholar] [CrossRef]
- Zhao, L.; Sun, Z.; Ma, J.; Liu, H. Enhancement mechanism of heterogeneous catalytic ozonation by cordierite-supported copper for the degradation of nitrobenzene in aqueous solution. Environ. Sci. Technol. 2009, 43, 2047–2053. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Ma, J.; Sun, Z. Oxidation products and pathway of ceramic honeycomb-catalyzed ozonation for the degradation of nitrobenzene in aqueous solution. Appl. Catal. B Environ. 2008, 79, 244–253. [Google Scholar] [CrossRef]
- Zhang, T.; Li, C.; Ma, J.; Tian, H.; Qiang, Z. Surface hydroxyl groups of synthetic a-FeOOH in promoting OH generation from aqueous ozone: Property and activity relationship. Appl. Catal. B Environ. 2008, 82, 131–137. [Google Scholar] [CrossRef]











| Zeolites | Surface Areas m2 g−1 | Micropore Surface Areas m2 g−1 | Total Pore Volumes cm3 g−1 | Micropore Volumes cm3 g−1 | Supported Metals Contents by XRF (wt%) |
|---|---|---|---|---|---|
| pNaZSM-5(120) | 330 | 177 | 0.19 | 0.09 | - |
| Mg/NaZSM-5(120) | 324 | 223 | 0.19 | 0.11 | 0.94 |
| Zn/NaZSM-5(120) | 331 | 217 | 0.19 | 0.10 | 0.89 |
| pNaZSM-5(45) | 258 | 223 | 0.14 | 0.10 | - |
| Mg/NaZSM-5(45) | 223 | 200 | 0.11 | 0.10 | 0.90 |
| Zn/NaZSM-5(45) | 244 | 213 | 0.13 | 0.10 | 0.93 |
| pNaZSM-5(31) | 334 | 233 | 0.27 | 0.11 | - |
| Mg/NaZSM-5(31) | 326 | 237 | 0.27 | 0.11 | 0.87 |
| Zn/NaZSM-5(31) | 331 | 230 | 0.27 | 0.11 | 0.91 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Ma, W.; Yoza, B.A.; Xu, Y.; Li, Q.X.; Chen, C.; Wang, Q.; Gao, Y.; Guo, S.; Zhan, Y. Investigation of Catalytic Ozonation of Recalcitrant Organic Chemicals in Aqueous Solution over Various ZSM-5 Zeolites. Catalysts 2018, 8, 128. https://doi.org/10.3390/catal8040128
Wang Y, Ma W, Yoza BA, Xu Y, Li QX, Chen C, Wang Q, Gao Y, Guo S, Zhan Y. Investigation of Catalytic Ozonation of Recalcitrant Organic Chemicals in Aqueous Solution over Various ZSM-5 Zeolites. Catalysts. 2018; 8(4):128. https://doi.org/10.3390/catal8040128
Chicago/Turabian StyleWang, Yandan, Wenfeng Ma, Brandon A. Yoza, Yingying Xu, Qing X. Li, Chunmao Chen, Qinghong Wang, Yu Gao, Shaohui Guo, and Yali Zhan. 2018. "Investigation of Catalytic Ozonation of Recalcitrant Organic Chemicals in Aqueous Solution over Various ZSM-5 Zeolites" Catalysts 8, no. 4: 128. https://doi.org/10.3390/catal8040128