Promotional Effect of Ce on Iron-Based Catalysts for Selective Catalytic Reduction of NO with NH3
Abstract
:1. Introduction
2. Results and Discussion
2.1. Catalytic Properties of Catalysts
2.1.1. Effect of Iron Loading on Catalytic Activity
2.1.2. Promotional Effect of Ce on Catalytic Activity
2.1.3. NO Oxidation Characteristics
2.2. Catalyst Characterization
2.2.1. BET Results
2.2.2. XRD Results
2.2.3. H2-TPR Results
2.2.4. NH3-TPD Results
2.2.5. XPS Results
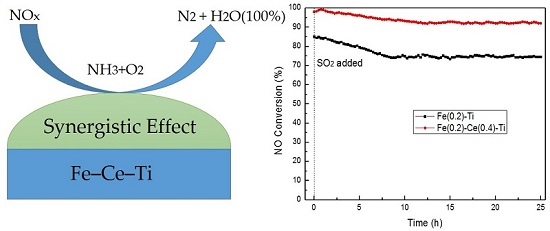
2.3. Influence of SO2 and H2O on SCR Activity of Catalysts
3. Materials and Methods
3.1. Catalysts Preparation
3.2. Catalysts Characterizations
3.3. Catalytic Activity Measurements
4. Conclusions
- (1)
- The Fe(0.2)–Ce(0.4)–Ti catalyst prepared via co-precipitation had better texture compared to than Fe(0.2)–Ti catalyst, including high surface area and high dispersion of active components on the catalyst surface.
- (2)
- Doping Ce into Fe(0.2)–Ti catalyst improved its redox ability and greatly increased the amount of acid sites on the surface.
- (3)
- As Ce load increased, the proportion of Fe3+ and Ce3+ decreased while the content of surface-adsorbed oxygen increased, all due to the interaction between Fe and Ce species.
- (4)
- The decrease of catalyst activity caused by SO2 was likely due to the deposition of ammonium sulfate and the sulfation of active components during the SCR reaction.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Yang, R.; Huang, H.F.; Chen, Y.J.; Zhang, X.X.; Lu, H.F. Performance of Cr-doped vanadia/titania catalysts for low-temperature selective catalytic reduction of NOx with NH3. Chin. J. Catal. 2015, 36, 1256–1262. [Google Scholar] [CrossRef]
- Yi, T.; Zhang, Y.B.; Li, J.W.; Yang, X.G. Promotional effect of H3PO4 on ceria catalyst for selective catalytic reduction of NO by NH3. Chin. J. Catal. 2016, 37, 300–307. [Google Scholar] [CrossRef]
- Thirupathi, B.; Smirniotis, P.G. Nickel-doped Mn/TiO2 as an efficient catalyst for the low-temperature SCR of NO with NH3: Catalytic evaluation and characterizations. J. Catal. 2012, 288, 74–83. [Google Scholar] [CrossRef]
- Jiang, S.Y.; Zhou, R.X. Ce doping effect on performance of the Fe/β catalyst for NOx reduction by NH3. Fuel Process. Technol. 2015, 133, 220–226. [Google Scholar] [CrossRef]
- Feng, B.J.; Wang, Z.; Sun, Y.Y.; Zhan, C.H.; Tang, S.F.; Li, X.B.; Huang, X. Size controlled ZSM-5 on the structure and performance of Fe catalyst in the selective catalytic reduction of NOx with NH3. Catal. Commun. 2016, 80, 20–23. [Google Scholar] [CrossRef]
- Zhu, L.; Qu, H.X.; Zhang, L.; Zhou, Q.W. Direct synthesis, characterization and catalytic performance of Al-Fe-SBA-15 materials in selective catalytic reduction of NO with NH3. Catal. Commun. 2016, 73, 118–122. [Google Scholar] [CrossRef]
- Putluru, S.S.R.; Schill, L.; Jensen, A.D.; Siret, B.; Tabaries, F.; Fehrmann, R. Mn/TiO2 and Mn-Fe/TiO2 catalysts synthesized by deposition precipitation-promising for selective catalytic reduction of NO with NH3 at low temperatures. Appl. Catal. B 2015, 165, 628–635. [Google Scholar] [CrossRef]
- Qi, G.S.; Yang, R.T. Low-temperature selective catalytic reduction of NO with NH3 over iron and manganese oxides supported on titania. Appl. Catal. B 2003, 44, 217–225. [Google Scholar] [CrossRef]
- Zhou, G.Y.; Zhong, B.C.; Wang, W.H.; Guan, X.J.; Huang, B.C.; Ye, D.Q.; Wu, H.J. In situ DRIFTS study of NO reduction by NH3 over Fe-Ce-Mn/ZSM-5 catalysts. Catal. Today 2011, 175, 157–163. [Google Scholar] [CrossRef]
- Zhang, J.; Qu, H.X. Low-temperature selective catalytic reduction of NOx with NH3 over Fe-Cu mixed oxide/ZSM-5 catalysts containing Fe2CuO4 phase. Res. Chem. Intermed. 2015, 41, 4961–4975. [Google Scholar] [CrossRef]
- Wang, X.B.; Gui, K.T. Fe2O3 particles as superior catalysts for low temperature selective catalytic reduction of NO with NH3. J. Environ. Sci. 2013, 25, 2469–2475. [Google Scholar] [CrossRef]
- Shan, W.B.; Liu, F.D.; Yu, Y.B.; He, H. The use of ceria for the selective catalytic reduction of NOx with NH3. Chin. J. Catal. 2014, 35, 1251–1259. [Google Scholar] [CrossRef]
- Shen, B.X.; Wang, F.M.; Liu, T. Homogeneous MnOx-CeO2 pellets prepared by a one-step hydrolysis process for low-temperature NH3-SCR. Powder Technol. 2014, 253, 152–157. [Google Scholar] [CrossRef]
- Shen, B.X.; Yao, Y.; Ma, H.Q.; Liu, T. Ceria modified MnOx/TiO2-pillared clays catalysts for the selective catalytic reduction of NO with NH3 at low temperature. Chin. J. Catal. 2011, 32, 1803–1811. [Google Scholar] [CrossRef]
- Shen, B.X.; Liu, T.; Zhao, N.; Yang, X.Y.; Deng, L.D. Iron-doped Mn-Ce/TiO2 catalyst for low temperature selective catalytic reduction of NO with NH3. J. Environ. Sci. 2010, 22, 1447–1454. [Google Scholar] [CrossRef]
- Zhu, L.; Zhang, L.; Qu, H.X.; Zhong, Q. A study on chemisorbed oxygen and reaction process of Fe-CuOx/ZSM-5 via ultrasonic impregnation method for low-temperature NH3-SCR. J. Mol. Catal. A 2015, 409, 207–215. [Google Scholar] [CrossRef]
- Peng, Y.; Yu, W.W.; Su, W.K.; Huang, X.; Li, J.H. An experimental and DFT study of the adsorption and oxidation of NH3 on a CeO2 catalyst modified by Fe, Mn, La and Y. Catal. Today 2015, 242, 300–307. [Google Scholar] [CrossRef]
- Li, J.; Jia, L.W.; Jin, W.Y.; Xia, F.; Wang, J.M. Effects of Ce-doping on the structure and NH3-SCR activity of Fe/Beta catalyst. Rare Metal Mater. Eng. 2015, 44, 1612–1616. [Google Scholar]
- Shu, Y.; Sun, H.; Quan, X.; Chen, S. Enhancement of catalytic activity over the iron-modified Ce/TiO2 catalyst for selective catalytic reduction of NOx with ammonia. J. Phys. Chem. C 2012, 116, 25319–25327. [Google Scholar] [CrossRef]
- Long, R.Q.; Yang, R.T. Temperature-programmed desorption/surface Reaction (TPD/TPSR) study of Fe-exchanged ZSM-5 for selective catalytic reduction of nitric oxide by ammonia. J. Catal. 2001, 198, 20–28. [Google Scholar] [CrossRef]
- Koebel, M.; Elsener, M.; Madia, G. Reaction pathways in the selective catalytic reduction process with NO and NO2 at low temperatures. Ind. Eng. Chem. Res. 2001, 40, 52–59. [Google Scholar] [CrossRef]
- Romero-Sáez, M.; Divakar, D.; Aranzabal, A.; González-Velasco, J.R.; González-Marcos, J.A. Catalytic oxidation of trichloroethylene over Fe-ZSM-5: Influence of the preparation method on the iron species and the catalytic behavior. Appl. Catal. B 2016, 180, 210–218. [Google Scholar] [CrossRef]
- Cao, F.; Su, S.; Xiang, J.; Wang, P.Y.; Hu, S.; Sun, L.S.; Zhang, A.C. The activity and mechanism study of Fe-Mn-Ce/γ-Al2O3 catalyst for low temperature selective catalytic reduction of NO with NH3. Fuel 2015, 139, 232–239. [Google Scholar] [CrossRef]
- Wan, Q.; Duan, L.; He, K.B.; Li, J.H. Removal of gaseous elemental mercury over a CeO2-WO3/TiO2 nanocomposite in simulated coal-fired flue gas. Chem. Eng. J. 2011, 170, 512–517. [Google Scholar] [CrossRef]
- Yao, X.J.; Xiong, Y.; Zou, W.; Zhang, L.; Wu, S.G.; Dong, X.; Gao, F.; Deng, Y.; Tang, C.J.; Chen, Z.; et al. Correlation between the physicochemical properties and catalytic performances of CexSn1−xO2 mixed oxides for NO reduction by CO. Appl. Catal. B 2014, 144, 152–165. [Google Scholar] [CrossRef]
- Zhang, L.; Zou, W.X.; Ma, K.L.; Cao, Y.; Xiong, Y.; Wu, S.G.; Tang, C.J.; Gao, F.; Dong, L. Sulfated temperature effects on the catalytic activity of CeO2 in NH3-selective catalytic reduction conditions. J. Phys. Chem. C 2015, 119, 1155–1163. [Google Scholar] [CrossRef]
- Zhang, X.P.; Shen, B.X.; Wang, K.; Chen, J.H. A contrastive study of the introduction of cobalt as a modifier for active components and supports of catalysts for NH3-SCR. J. Ind. Eng. Chem. 2013, 19, 1272–1279. [Google Scholar] [CrossRef]
- Wu, S.G.; Zhang, L.; Wang, X.B.; Zou, W.X.; Cao, Y.; Sun, J.F.; Tang, C.J.; Gao, F.; Deng, Y.; Dong, L. Synthesis, characterization and catalytic performance of FeMnTiOx mixed oxides catalyst prepared by a CTAB-assisted process for mid-low temperature NH3-SCR. Appl. Catal. A 2015, 505, 235–242. [Google Scholar] [CrossRef]
- Zhang, L.; Li, L.L.; Cao, Y.; Xiong, Y.; Wu, S.G.; Sun, J.F.; Tang, C.J.; Gao, F.; Dong, L. Promotional effect of doping SnO2 into TiO2 over a CeO2/TiO2 catalyst for selective catalytic reduction of NO by NH3. Catal. Sci. Technol. 2015, 5, 2188–2196. [Google Scholar] [CrossRef]
- Shen, B.X.; Yao, Y.; Chen, J.H.; Zhang, X.P. Alkali metal deactivation of Mn-CeOx/Zr-delaminated-clay for the low-temperature selective catalytic reduction of NOx with NH3. Microporous Mesoporous Mater. 2013, 180, 262–269. [Google Scholar]
- Cheng, K.; Liu, J.; Zhang, T.; Li, J.M.; Zhao, Z.; Wei, Y.C.; Jiang, G.Y.; Duan, A.J. Effect of Ce doping of TiO2 support on NH3-SCR activity over V2O5-WO3/CeO2-TiO2 catalyst. J. Environ. Sci. 2014, 26, 2106–2113. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.B.; Jin, R.B.; Wang, H.Q.; Liu, Y. Effect of ceria doping on SO2 resistance of Mn/TiO2 for selective catalytic reduction of NO with NH3 at low temperature. Catal. Commun. 2009, 10, 935–939. [Google Scholar] [CrossRef]
- Gu, T.T.; Liu, Y.; Weng, X.L.; Wang, H.Q.; Wu, Z.B. The enhanced performance of ceria with surface sulfation for selective catalytic reduction of NO by NH3. Catal. Commun. 2010, 12, 310–313. [Google Scholar] [CrossRef]
- Zhang, Q.L.; Zhang, J.H.; Song, Z.X.; Ning, P.; Li, H.; Liu, X. A novel and environmentally friendly SO42−/CeO2 catalyst for the selective catalytic reduction of NO with NH3. J. Ind. Eng. Chem. 2016, 34, 165–171. [Google Scholar] [CrossRef]
- Kumar, P.A.; Reddy, M.P.; Ju, L.K.; Hyun-Sook, B.; Phil, H.H. Low temperature propylene SCR of NO by copper alumina catalyst. J. Mol. Catal. A 2008, 291, 66–74. [Google Scholar] [CrossRef]
- Shu, Y.; Aikebaier, T.; Quan, X.; Chen, S.; Yu, H.T. Selective catalytic reaction of NOx with NH3 over Ce-Fe/TiO2-loaded wire-mesh honeycomb: Resistance to SO2 poisoning. Appl. Catal. B 2014, 150–151, 630–635. [Google Scholar] [CrossRef]
- Huang, J.H.; Tong, Z.Q.; Huang, Y.; Zhang, J.F. Selective catalytic reduction of NO with NH3 at low temperatures over iron and manganese oxides supported on mesoporous silica. Appl. Catal. B 2008, 78, 309–314. [Google Scholar] [CrossRef]
- Shen, B.X.; Zhang, X.P.; Ma, H.Q.; Yao, Y.; Liu, T. A comparative study of Mn/CeO2, Mn/ZrO2 and Mn/Ce-ZrO2 for low temperature selective catalytic reduction of NO with NH3 in the presence of SO2 and H2O. J. Environ. Sci. 2013, 25, 791–800. [Google Scholar] [CrossRef]
- Chang, H.Z.; Ma, L.; Yang, S.J.; Li, J.H.; Chen, L.; Wang, W.; Hao, J.M. Comparison of preparation methods for ceria catalyst and the effect of surface and bulk sulfates on its activity toward NH3-SCR. J. Hazard. Mater. 2013, 262, 782–788. [Google Scholar] [CrossRef] [PubMed]
- Waqif, M.; Bazin, P.; Saur, O.; Lavalley, J.C.; Blanchard, G.; Touret, O. Study of ceria sulfation. Appl. Catal. B 1997, 11, 193–205. [Google Scholar] [CrossRef]
- Yu, J.; Guo, F.; Wang, Y.L.; Zhu, J.H.; Liu, Y.Y.; Su, F.B.; Gao, S.Q.; Xu, G.W. Sulfur poisoning resistant mesoporous Mn-base catalyst for low-temperature SCR of NO with NH3. Appl. Catal. B 2010, 95, 160–168. [Google Scholar] [CrossRef]












| Sample | BET (cm2·g−1) | Pore Volume (cm3·g−1) |
|---|---|---|
| Fe(0.2)–Ti | 76.04 | 0.2783 |
| Fe(0.2)–Ce(0.02)–Ti | 117.42 | 0.2611 |
| Fe(0.2)–Ce(0.05)–Ti | 141.75 | 0.3219 |
| Fe(0.2)–Ce(0.2)–Ti | 210.85 | 0.6094 |
| Fe(0.2)–Ce(0.4)–Ti | 146.82 | 0.6456 |
| Fe(0.2)–Ce(0.8)–Ti | 105.18 | 0.3964 |
| Catalyst | Fe | Ce | Ti | O | Fe/Ti | Ce/Ti | Fe3+/(Fe2+ + Fe3+) (%) | Ce4+/Ce3+ | Oβ/(Oα + Oβ) (%) |
|---|---|---|---|---|---|---|---|---|---|
| Fe(0.2)–Ti | 4.60 | - | 17.42 | 53.66 | 0.264 | - | 39.49 | - | 5.9 |
| Ce(0.4)–Ti | - | 1.94 | 17.75 | 51.21 | - | 0.109 | - | 2.23 | 8.5 |
| Fe(0.2)–Ce(0.4)–Ti | 3.47 | 4.50 | 11.44 | 53.07 | 0.303 | 0.393 | 29.22 | 3.43 | 20.1 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Zhang, L.; Wu, S.; Zou, W.; Yu, S.; Shao, Y.; Dong, L. Promotional Effect of Ce on Iron-Based Catalysts for Selective Catalytic Reduction of NO with NH3. Catalysts 2016, 6, 112. https://doi.org/10.3390/catal6080112
Wang X, Zhang L, Wu S, Zou W, Yu S, Shao Y, Dong L. Promotional Effect of Ce on Iron-Based Catalysts for Selective Catalytic Reduction of NO with NH3. Catalysts. 2016; 6(8):112. https://doi.org/10.3390/catal6080112
Chicago/Turabian StyleWang, Xiaobo, Lei Zhang, Shiguo Wu, Weixin Zou, Shuohan Yu, Ye Shao, and Lin Dong. 2016. "Promotional Effect of Ce on Iron-Based Catalysts for Selective Catalytic Reduction of NO with NH3" Catalysts 6, no. 8: 112. https://doi.org/10.3390/catal6080112