Immobilization of Fusarium solani Cutinase onto Magnetic Genipin-Crosslinked Chitosan Beads
Abstract
:1. Introduction
2. Results and Discussion
2.1. Effects of Immobilization Conditions
2.1.1. Effect of Genipin Concentration
2.1.2. Effect of Crosslinking Time
2.1.3. Effect of Enzyme Concentration
2.1.4. Effect of Immobilization Time
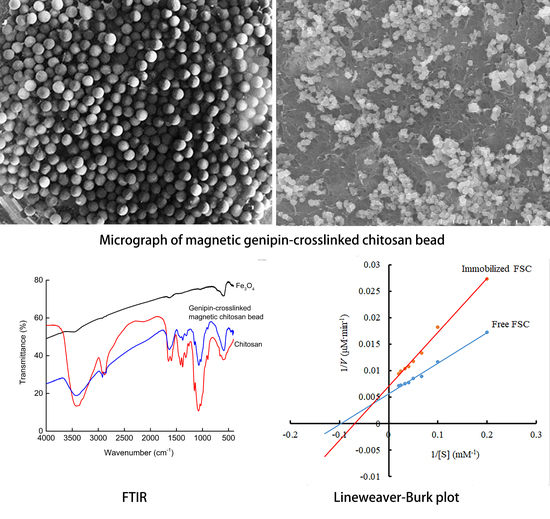
2.2. Characterization of Magnetic Genipin-Crosslinked Chitosan Beads
2.3. Effects of Reaction pH and Temperature on FSC Activity
2.4. Reusability of Immobilized FSC
2.5. Storage Stability of FSC
2.6. Kinetic Parameters
3. Materials and Methods
3.1. Materials
3.2. Immobilization of FSC onto Magnetic Chitosan Beads
3.3. Characterization of Magnetic Genipin-Crosslinked Chitosan Beads
3.3.1. Scanning Electron Microscopy (SEM)
3.3.2. Fourier Transform Infrared (FTIR) Spectroscopy
3.4. Analysis of Enzyme Activity and Protein Concentration
3.5. Effects of pH and Temperature on Enzyme Stability
3.6. Storage Stability and Reusability
3.7. Determination of Kinetic Parameters
3.8. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Su, A.; Shirke, A.; Baik, J.; Zou, Y.; Gross, R. Immobilized cutinases: Preparation, solvent tolerance and thermal stability. Enzym. Microb. Technol. 2018, 116, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Purdy, R.E.; Kolattukudy, P.E. Hydrolysis of plant cuticle by plant pathogens. Properties of cutinase I, cutinase II, and a nonspecific esterase isolated from Fusarium solani pisi. Biochemistry 1975, 14, 2832–2840. [Google Scholar] [CrossRef] [PubMed]
- Ping, L.-F.; Chen, X.-Y.; Yuan, X.-L.; Zhang, M.; Chai, Y.-J.; Shan, S.-D. Application and comparison in biosynthesis and biodegradation by Fusarium solani and Aspergillus fumigatus cutinases. Int. J. Biol. Macromol. 2017, 104, 1238–1245. [Google Scholar] [CrossRef] [PubMed]
- Sulaiman, S.; Yamato, S.; Kanaya, E.; Kim, J.-J.; Koga, Y.; Takano, K.; Kanaya, S. Isolation of a novel cutinase homolog with polyethylene terephthalate-degrading activity from leaf-branch compost by using a metagenomic approach. Appl. Environ. Microbiol. 2012, 78, 1556–1562. [Google Scholar] [CrossRef] [Green Version]
- Shi, K.; Su, T.; Wang, Z. Comparison of poly(butylene succinate) biodegradation by Fusarium solani cutinase and Candida antarctica lipase. Polym. Degrad. Stab. 2019, 164, 55–60. [Google Scholar] [CrossRef]
- Su, L.; Hong, R.; Guo, X.; Wu, J.; Xia, Y. Short-chain aliphatic ester synthesis using Thermobifida fusca cutinase. Food Chem. 2016, 206, 131–136. [Google Scholar] [CrossRef]
- Mateo, C.; Palomo, J.M.; Fernandez-Lorente, G.; Guisan, J.M.; Fernandez-Lafuente, R. Improvement of enzyme activity, stability and selectivity via immobilization techniques. Enzym. Microb. Technol. 2007, 40, 1451–1463. [Google Scholar] [CrossRef]
- Hegde, K.; Veeranki, V.D. Studies on Immobilization of Cutinases from Thermobifida fusca on Glutaraldehyde Activated Chitosan Beads. Br. Biotechnol. J. 2014, 4, 1049–1063. [Google Scholar] [CrossRef]
- Nikolaivits, E.; Makris, G.; Topakas, E. Immobilization of a Cutinase from Fusarium oxysporum and Application in Pineapple Flavor Synthesis. J. Agric. Food Chem. 2017, 65, 3505–3511. [Google Scholar] [CrossRef]
- Sousa, I.T.; Lourenço, N.M.T.; Afonso, C.A.M.; Taipa, M.A. Protein stabilization with a dipeptide-mimic triazine-scaffolded synthetic affinity ligand. J. Mol. Recognit. 2013, 26, 104–112. [Google Scholar] [CrossRef]
- Kumari, V.; Kumar, S.; Kaur, I.; Bhalla, T.C. Graft copolymerization of acrylamide on chitosan-co-chitin and its application for immobilization of Aspergillus sp. RL2Ct cutinase. Bioorg. Chem. 2017, 70, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.S.; Singh, R.P.; Kennedy, J.F. Immobilization of yeast inulinase on chitosan beads for the hydrolysis of inulin in a batch system. Int. J. Biol. Macromol. 2017, 95, 87–93. [Google Scholar] [CrossRef]
- Dinçer, A.; Becerik, S.; Aydemir, T. Immobilization of tyrosinase on chitosan–clay composite beads. Int. J. Biol. Macromol. 2012, 50, 815–820. [Google Scholar] [CrossRef] [PubMed]
- Durán, N.; Rosa, M.A.; D’Annibale, A.; Gianfreda, L. Applications of laccases and tyrosinases (phenoloxidases) immobilized on different supports: A review. Enzym. Microb. Technol. 2002, 31, 907–931. [Google Scholar] [CrossRef]
- Delmar, K.; Bianco-Peled, H. The dramatic effect of small pH changes on the properties of chitosan hydrogels crosslinked with genipin. Carbohydr. Polym. 2015, 127, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Chiono, V.; Pulieri, E.; Vozzi, G.; Ciardelli, G.; Ahluwalia, A.; Giusti, P. Genipin-crosslinked chitosan/gelatin blends for biomedical applications. J. Mater. Sci. Mater. Med. 2008, 19, 889–898. [Google Scholar] [CrossRef]
- Butler, M.F.; Ng, Y.-F.; Pudney, P.D.A. Mechanism and kinetics of the crosslinking reaction between biopolymers containing primary amine groups and genipin. J. Polym. Sci. Part A Polym. Chem. 2003, 41, 3941–3953. [Google Scholar] [CrossRef]
- Nickerson, M.T.; Patel, J.; Heyd, D.V.; Rousseau, D.; Paulson, A.T. Kinetic and mechanistic considerations in the gelation of genipin-crosslinked gelatin. Int. J. Biol. Macromol. 2006, 39, 298–302. [Google Scholar] [CrossRef]
- Tsai, T.-R.; Tseng, T.-Y.; Chen, C.-F.; Tsai, T.-H. Identification and determination of geniposide contained in Gardenia jasminoides and in two preparations of mixed traditional Chinese medicines. J. Chromatogr. A 2002, 961, 83–88. [Google Scholar] [CrossRef]
- Ma, H.F.; Meng, G.; Cui, B.K.; Si, J.; Dai, Y.C. Chitosan crosslinked with genipin as supporting matrix for biodegradation of synthetic dyes: Laccase immobilization and characterization. Chem. Eng. Res. Des. 2018, 132, 664–676. [Google Scholar] [CrossRef]
- Jiang, D.-S.; Long, S.-Y.; Huang, J.; Xiao, H.-Y.; Zhou, J.-Y. Immobilization of Pycnoporus sanguineus laccase on magnetic chitosan microspheres. Biochem. Eng. J. 2005, 25, 15–23. [Google Scholar] [CrossRef]
- Sathishkumar, P.; Kamala-Kannan, S.; Cho, M.; Kim, J.S.; Hadibarata, T.; Salim, M.R.; Oh, B.-T. Laccase immobilization on cellulose nanofiber: The catalytic efficiency and recyclic application for simulated dye effluent treatment. J. Mol. Catal. B Enzym. 2014, 100, 111–120. [Google Scholar] [CrossRef]
- Kašpar, O.; Tokárová, V.; Nyanhongo, G.S.; Gübitz, G.; Štěpánek, F. Effect of cross-linking method on the activity of spray-dried chitosan microparticles with immobilized laccase. Food Bioprod. Process. 2013, 91, 525–533. [Google Scholar] [CrossRef]
- Zheng, F.; Cui, B.-K.; Wu, X.-J.; Meng, G.; Liu, H.-X.; Si, J. Immobilization of laccase onto chitosan beads to enhance its capability to degrade synthetic dyes. Int. Biodeterior. Biodegrad. 2016, 110, 69–78. [Google Scholar] [CrossRef]
- Halder, S.K.; Maity, C.; Jana, A.; Ghosh, K.; Das, A.; Paul, T.; Mohapatra, P.K.D.; Pati, B.R.; Mondal, K.C. Chitinases biosynthesis by immobilized Aeromonas hydrophila SBK1 by prawn shells valorization and application of enzyme cocktail for fungal protoplast preparation. J. Biosci. Bioeng. 2014, 117, 170–177. [Google Scholar] [CrossRef]
- Gomes, F.M.; Pereira, E.B.; de Castro, H.F. Immobilization of Lipase on Chitin and Its Use in Nonconventional Biocatalysis. Biomacromolecules 2004, 5, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Krajewska, B.; Piwowarska, Z. Free vs. chitosan-immobilized urease: Microenvironmental effects on enzyme inhibitions. Biocatal. Biotransform. 2005, 23, 225–232. [Google Scholar] [CrossRef]
- González-Sáiz, J.M.; Pizarro, C. Polyacrylamide gels as support for enzyme immobilization by entrapment. Effect of polyelectrolyte carrier, pH and temperature on enzyme action and kinetics parameters. Eur. Polym. J. 2001, 37, 435–444. [Google Scholar] [CrossRef]
- Bhandari, S.; Gupta, V.K.; Singh, H. Enhanced stabilization of mungbean thiol protease immobilized on glutaraldehyde-activated chitosan beads. Biocatal. Biotransform. 2009, 27, 71–77. [Google Scholar] [CrossRef]
- Gopinath, S.; Sugunan, S. Enzymes immobilized on montmorillonite K 10: Effect of adsorption and grafting on the surface properties and the enzyme activity. Appl. Clay Sci. 2007, 35, 67–75. [Google Scholar] [CrossRef]
- Arıca, M.Y.; Bayramoǧlu, G.; Bıçak, N. Characterisation of tyrosinase immobilised onto spacer-arm attached glycidyl methacrylate-based reactive microbeads. Process Biochem. 2004, 39, 2007–2017. [Google Scholar] [CrossRef]
- Vu, T.K.H.; Le, V.V.M. Biochemical Studies on the Immobilization of the Enzyme Invertase (EC.3.2.1.26) in Alginate Gel and its Kinetics. Asean Food J. 2008, 15, 73–78. [Google Scholar]
- Hu, X.; Gao, Z.; Wang, Z.; Su, T.; Yang, L.; Li, P. Enzymatic degradation of poly(butylene succinate) by cutinase cloned from Fusarium solani. Polym. Degrad. Stab. 2016, 134, 211–219. [Google Scholar] [CrossRef]
- Davies, K.A.; De Lorono, I.; Foster, S.J.; Li, D.; Johnstone, K.; Ashby, A.M. Evidence for a role of cutinase in pathogenicity of Pyrenopeziza brassicae on brassicas. Physiol. Mol. Plant Pathol. 2000, 57, 63–75. [Google Scholar] [CrossRef]





| Kinetic Parameters | Free FSC | Immobilized FSC |
|---|---|---|
| Km (mM) | 10.3 | 14.4 |
| Vmax (μM·min−1) | 178.6 | 140.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Su, T.; Zhao, J. Immobilization of Fusarium solani Cutinase onto Magnetic Genipin-Crosslinked Chitosan Beads. Catalysts 2021, 11, 1158. https://doi.org/10.3390/catal11101158
Wang Z, Su T, Zhao J. Immobilization of Fusarium solani Cutinase onto Magnetic Genipin-Crosslinked Chitosan Beads. Catalysts. 2021; 11(10):1158. https://doi.org/10.3390/catal11101158
Chicago/Turabian StyleWang, Zhanyong, Tingting Su, and Jingjing Zhao. 2021. "Immobilization of Fusarium solani Cutinase onto Magnetic Genipin-Crosslinked Chitosan Beads" Catalysts 11, no. 10: 1158. https://doi.org/10.3390/catal11101158