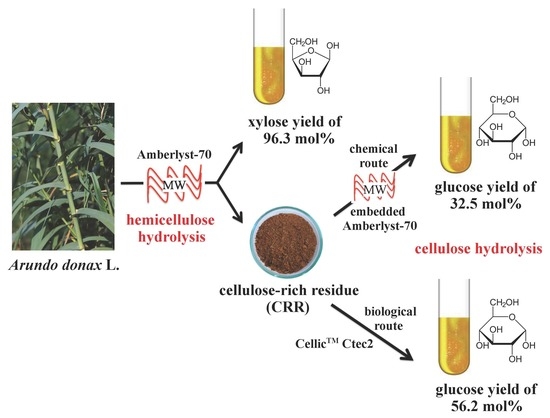
Multi-Step Exploitation of Raw Arundo donax L. for the Selective Synthesis of Second-Generation Sugars by Chemical and Biological Route
Abstract
:1. Introduction
2. Results and Discussion
2.1. Microwave-Assisted Hydrolysis of Giant Reed Hemicellulose to Xylose Catalysed by Amberlyst-70
2.2. Chemical Hydrolysis of Cellulose-Rich Residue (CRR) to Glucose
2.3. Enzymatic Hydrolysis of Cellulose-Rich Residue (CRR) to Glucose
3. Materials and Methods
3.1. Materials
3.2. Chemical Hydrolysis of Arundo donax L.
3.3. High Performance Liquid Chromatography (HPLC)
3.4. Compositional Analysis of the Feedstocks
3.5. X-ray Diffraction Method (XRD) Analysis
3.6. Enzymatic Hydrolysis of Arundo donax L.
3.7. Fourier Transformation Infrared Spectroscopy (FT-IR)
3.8. Definitions
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- De Bari, I.; Cuna, D.; Di Fidio, N. Biorefineries: Biofuels, biochemical, and bioproducts. In Biofuels Production and Processing Technology; Riazi, M.R., Chiaramonti, D., Eds.; CRC Press Taylor & Francis Group: Boca Raton, FL, USA, 2017; pp. 533–561. [Google Scholar]
- Loow, Y.L.; Wu, T.Y.; Jahim, J.M.; Mohammad, A.W.; Teoh, W.H. Typical conversion of lignocellulosic biomass into reducing sugars using dilute acid hydrolysis and alkaline pretreatment. Cellulose 2016, 23, 1491–1520. [Google Scholar] [CrossRef]
- Ro, K.S.; Libra, J.A.; Bae, S.; Berge, N.D.; Flora, J.R.; Pecenka, R. Combustion behavior of animal-manure-based hydrochar and pyrochar. ACS Sustain. Chem. Eng. 2018, 7, 470–478. [Google Scholar] [CrossRef]
- Harder, R.; Wielemaker, R.; Larsen, T.A.; Zeeman, G.; Öberg, G. Recycling nutrients contained in human excreta to agriculture: Pathways, processes, and products. Critical Rev. Environ. Sci. Technol. 2019, 49, 695–743. [Google Scholar] [CrossRef] [Green Version]
- Okoro, O.V.; Sun, Z.; Birch, J. Meat processing waste as a potential feedstock for biochemicals and biofuels —A review of possible conversion technologies. J. Clean. Prod. 2017, 142, 1583–1608. [Google Scholar] [CrossRef]
- Ndiaye, M.; Arhaliass, A.; Legrand, J.; Roelens, G.; Kerihuel, A. Reuse of waste animal fat in biodiesel: Biorefining heavily-degraded contaminant-rich waste animal fat and formulation as diesel fuel additive. Renew. Energy 2020, 145, 1073–1079. [Google Scholar] [CrossRef]
- Yeh, T.M.; Dickinson, J.G.; Franck, A.; Linic, S.; Thompson, L.T., Jr.; Savage, P.E. Hydrothermal catalytic production of fuels and chemicals from aquatic biomass. J. Chem. Technol. Biotechnol. 2013, 88, 13–24. [Google Scholar] [CrossRef] [Green Version]
- Bharathiraja, B.; Chakravarthy, M.; Kumar, R.R.; Yogendran, D.; Yuvaraj, D.; Jayamuthunagai, J.; Kumar, R.P.; Palani, S. Aquatic biomass (algae) as a future feedstock for bio-refineries: A review on cultivation, processing and products. Renew. Sustain. Energy Rev. 2015, 47, 634–653. [Google Scholar] [CrossRef]
- Kohli, K.; Prajapati, R.; Sharma, B.K. Bio-based chemicals from renewable biomass for integrated biorefineries. Energies 2019, 12, 233. [Google Scholar] [CrossRef] [Green Version]
- Esteban, J.; Yustos, P.; Ladero, M. Catalytic processes from biomass-derived hexoses and pentoses: A recent literature overview. Catalysts 2018, 8, 637. [Google Scholar] [CrossRef] [Green Version]
- Kucharska, K.; Rybarczyk, P.; Hołowacz, I.; Łukajtis, R.; Glinka, M.; Kamiński, M. Pretreatment of lignocellulosic materials as substrates for fermentation processes. Molecules 2018, 23, 2937. [Google Scholar] [CrossRef] [Green Version]
- Kumar, A.K.; Sharma, S. Recent updates on different methods of pretreatment of lignocellulosic feedstocks: A review. Bioresour. Bioprocess. 2017, 4, 7–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hassan, S.S.; Williams, G.A.; Jaiswal, A.K. Emerging technologies for the pretreatment of lignocellulosic biomass. Biores. Technol. 2018, 262, 310–318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galia, G.; Schiavo, B.; Antonetti, C.; Raspolli Galletti, A.M.; Interrante, L.; Lessi, M.; Scialdone, O.; Valenti, M.G. Autohydrolysis pretreatment of Arundo donax: A comparison between microwave-assisted batch and fast heating rate flow-through reaction systems. Biotechnol. Biofuels 2015, 8, 218–235. [Google Scholar] [CrossRef] [PubMed]
- Muthuvelu, K.S.; Rajarathinam, R.; Kanagaraj, L.P.; Ranganathan, R.V.; Dhanasekaran, K.; Manickam, N.K. Evaluation and characterization of novel sources of sustainable lignocellulosic residues for bioethanol production using ultrasound-assisted alkaline pre-treatment. Waste Manag. 2019, 87, 368–374. [Google Scholar] [CrossRef] [PubMed]
- Antonetti, C.; Bonari, E.; Licursi, D.; Nassi o Di Nasso, N.; Raspolli Galletti, A.M. Hydrothermal conversion of giant reed to furfural and levulinic acid: Optimization of the process under microwave irradiation and investigation of distinctive agronomic parameters. Molecules 2015, 20, 21232–21253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Antonetti, C.; Fulignati, S.; Licursi, D.; Raspolli Galletti, A.M. Turning point toward the sustainable production of 5-hydroxymethyl-2-furaldehyde in water: Metal salts for its synthesis from fructose and inulin. ACS Sustain. Chem. Eng. 2019, 7, 6830–6838. [Google Scholar] [CrossRef]
- Ozsel, B.K.; Ozturk, D.; Nis, B. One-pot hydrothermal conversion of different residues to value-added chemicals using new acidic carbonaceous catalyst. Biores. Technol. 2019, 289, 121627–121632. [Google Scholar] [CrossRef] [PubMed]
- Licursi, D.; Antonetti, C.; Fulignati, S.; Corsini, A.; Boschi, N.; Raspolli Galletti, A.M. Smart valorization of waste biomass: Exhausted lemon peels, coffee silverskins and paper wastes for the productionof levulinic acid. Chem. Eng. Trans. 2018, 65, 637–642. [Google Scholar] [CrossRef]
- Licursi, D.; Antonetti, C.; Fulignati, S.; Vitolo, S.; Puccini, M.; Ribechini, E.; Bernazzani, L.; Raspolli Galletti, A.M. In-depth characterization of valuable char obtained from hydrothermal conversion of hazelnut shells to levulinic acid. Biores. Technol. 2017, 244, 880–888. [Google Scholar] [CrossRef]
- Giang, C.H.; Osatiashtiani, A.; dos Santos, V.C.; Lee, A.F.; Wilson, D.R.; Waldron, K.W.; Wilson, K. Valorisation of Vietnamese rice straw waste: Catalytic aqueous phase reforming of hydrolyzate from steam explosion to platform chemicals. Catalysts 2014, 4, 414–426. [Google Scholar] [CrossRef]
- Solarte-Toro, J.C.; Romer-García, J.M.; Martínez-Patiño, J.C.; Ruiz-Ramos, E.; Castro-Galiano, E.; Cardona-Alzate, C.A. Acid pretreatment of lignocellulosic biomass for energy vectors production: A review focused on operational conditions and techno-economic assessment for bioethanol production. Renew. Sustain. Energy Rev. 2019, 107, 587–601. [Google Scholar] [CrossRef]
- Zhu, Y.; Huang, J.; Sun, S.; Wu, A.; Li, H. Effect of dilute acid and alkali pretreatments on the catalytic performance of bamboo-derived carbonaceous magnetic solid acid. Catalysts 2019, 9, 245. [Google Scholar] [CrossRef] [Green Version]
- Scordia, D.; Cosentino, S.L.; Lee, J.W.; Jeffries, T.W. Bioconversion of giant reed (Arundo donax L.) hemicellulose hydrolyzate to ethanol by Scheffersomyces stipites CBS6054. Biomass Bioenergy 2012, 39, 296–305. [Google Scholar] [CrossRef]
- Di Fidio, N.; Antonetti, C.; Raspolli Galletti, A.M. Microwave-assisted cascade exploitation of giant reed (Arundo donax L.) to xylose and levulinic acid catalysed by ferric chloride. Biores. Technol. 2019, 293, 122050–122058. [Google Scholar] [CrossRef]
- Loow, Y.L.; Wu, T.Y.; Tan, K.A.; Lim, Y.S.; Siow, L.F.; Jahim, J.M.; Mohammad, A.W.; Teoh, W.H. Recent advances in the application of ionorganic salt pretreatment for transforming lignocellulosic biomass into reducing sugars. J. Agric. Food Chem. 2015, 63, 8349–8363. [Google Scholar] [CrossRef]
- Komolwanich, T.; Tatijarern, P.; Prasertwasu, S.; Khumsupan, D.; Chaisuwan, T.; Luengnaruemitchai, A.; Wongkasemjit, S. Comparative potentiality of Kans grass (Saccharum spontaneum) and Giant reed (Arundo donax) as lignocellulosic feedstocks for the release of monomeric sugars by microwave/chemical pretreatment. Cellulose 2014, 21, 1327–1340. [Google Scholar] [CrossRef]
- Zhang, Q.; Hu, J.; Lee, D.J. Pretreatment of biomass using ionic liquids: Research updates. Renew. Energy 2017, 11, 77–84. [Google Scholar] [CrossRef]
- Wang, F.L.; Li, S.; Sun, Y.X.; Han, H.Y.; Zhang, B.X.; Hu, B.Z.; Gao, Y.F.; Hu, X.M. Ionic liquids as efficient pretreatment solvents for lignocellulosic biomass. RSC Adv. 2017, 7, 47990–47998. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Hu, H.; Gong, Z.; Yang, G.; Li, R.; Chen, L.; Huang, L.; Luo, X. Near-complete removal of non-cellulosic components from bamboo by 1-pentanol induced organosolv pretreatment under mild conditions for robust cellulose enzymatic hydrolysis. Cellulose 2019, 26, 3801–3814. [Google Scholar] [CrossRef]
- Romaní, A.; Larramendi, A.; Yáñez, R.; Cancela, A.; Sánchez, A.; Teixeira, J.A.; Domingues, L. Valorizatin of Eucalyptus nitens bark by organosolv pretreatment for the production of advanced biofuels. Ind. Crops Prod. 2019, 132, 327–335. [Google Scholar] [CrossRef] [Green Version]
- De Bari, I.; Liuzzi, F.; Villone, A.; Braccio, G. Hydrolysis of concentrated suspensions of steam pretreated Arundo donax. Appl. Energy 2013, 102, 179–189. [Google Scholar] [CrossRef]
- Nitsos, C.; Roya, U.; Christakopoulus, P. Organosolv fractionation of softwood biomass for biofuel and biorefinery applications. Energies 2018, 11, 50. [Google Scholar] [CrossRef] [Green Version]
- Diwan, B.; Parkhey, P.; Gupta, P. Platform study on the development of a nondetoxified rice straw hydrolyzate to its application in lipid production from Mortierella alpine. ACS Sustain. Chem. Eng. 2018, 6, 1225–1234. [Google Scholar] [CrossRef]
- Shatalov, A.A.; Pereira, H. Xylose production from giant reed (Arundo donax L.): Modeling and optimization of dilute acid hydrolysis. Carbohydr. Polym. 2012, 87, 210–217. [Google Scholar] [CrossRef]
- Bhagia, S.; Li, H.; Gao, X.; Kumar, R.; Wyman, C.E. Flowthrough pretreatment with very dilute acid provides insights into high lignin contribution to biomass recalcitrance. Biotechnol. Biofuels 2016, 9, 245–259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Humbird, D.; Davis, R.; Tao, L.; Kinchin, C.; Hsu, D.; Aden, A.; Schoen, P.; Lukas, J.; Olthof, B.; Worley, M.; et al. Process Design and Economics for Biochemical Conversion of Lignocellulosic Biomass to Ethanol: Dilute-Acid Pretreatment and Enzymatic Hydrolysis of Corn Stover; NREL Technical Report NREL/TP-5100-47764; National Renewable Energy Laboratory: Golden, CO, USA, 2011.
- Chung, N.H.; Dien, L.Q.; Cuong, T.D.; Lieu, N.V.; Hoang, P.H. Influence of the acidity of solid catalyst HSO3-ZSM-5 on the hydrolysis of pretreated corncob. RSC Adv. 2018, 8, 41776–41781. [Google Scholar] [CrossRef] [Green Version]
- Qi, W.; He, C.; Wang, Q.; Liu, S.; Yu, Q.; Wang, W.; Leksawasdi, N.; Wang, C.; Yuan, Z. Carbon-based solid acid pretreatment in corncob saccharification: Specific xylose production and efficient enzymatic hydrolysis. ACS Sustain. Chem. Eng. 2018, 6, 3640–3648. [Google Scholar] [CrossRef]
- Meena, S.; Navatha, S.; Devi, B.P.; Prasad, R.B.N.; Pandey, A.; Sukumaran, R.K. Evaluation of Amberlyst15 for hydrolysis of alkali pretreated rice straw and fermentation to ethanol. Biochem. Eng. J. 2015, 102, 49–53. [Google Scholar] [CrossRef]
- You, T.; Shao, L.; Wang, R.; Zhang, L.; Xu, F. Facile isothermal solid acid catalyzed ionic liquid pretreatments to enhance the combined sugars production from Arundo donax Linn. Biotechnol. Biofuels 2016, 9, 177–189. [Google Scholar] [CrossRef] [Green Version]
- Qi, X.; Yan, L.; Shen, F.; Qiu, M. Mechanochemical-assisted hydrolysis of pretreated rice straw into glucose and xylose in water by weakly acidic solid catalyst. Biores. Technol. 2019, 273, 687–691. [Google Scholar] [CrossRef]
- Vu, A.; Wickramasinghe, S.R.; Qian, X. Polymeric solid acid catalysts for lignocellulosic biomass. Ind. Eng. Chem. Res. 2018, 57, 4514–4525. [Google Scholar] [CrossRef]
- Kobayashi, H.; Kaiki, H.; Shrotri, A.; Techikawara, K.; Fukuoka, A. Hydrolysis of woody biomass by a biomass-derived reusable heterogeneous catalyst. Chem. Sci. 2016, 7, 692–696. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dragoni, F.; Ragaglini, G.; Corneli, E.; Nassi o Di Nasso, N.; Tozzini, C.; Cattani, S.; Bonari, E. Giant reed (Arundo donax L.) for biogas production: Land use saving and nitrogen utilization efficiency compared with arable crops. Ital. J. Agron. 2015, 10, 192–201. [Google Scholar] [CrossRef] [Green Version]
- Pirozzi, D.; Fiorentino, N.; Impagliazzo, A.; Sannino, F.; Yousuf, A.; Zuccaro, G.; Fagnano, M. Lipid production from Arundo donax grown under different agronomical conditions. Renew. Energy 2015, 77, 456–462. [Google Scholar] [CrossRef] [Green Version]
- Shatalov, A.A.; Morais, A.R.C.; Duarte, L.C.; Carvalheiro, F. Selective single-stage xylan-to-xylose hydrolysis and its effect on enzymatic digestibility of energy crops giant reed and cardoon for bioethanol production. Ind. Crop. Prod. 2017, 95, 104–112. [Google Scholar] [CrossRef]
- Licursi, D.; Antonetti, C.; Mattonai, M.; Pérez-Armada, L.; Rivas, S.; Ribechini, E.; Raspolli Galletti, A.M. Multi-valorisation of giant reed (Arundo donax L.) to give levulinic acid and valuable phenolic antioxidants. Ind. Crop. Prod. 2018, 112, 6–17. [Google Scholar] [CrossRef]
- Tasselli, G.; Filippucci, S.; Borsella, E.; D’Antonio, S.; Gelosia, M.; Cavalaglio, G.; Turchetti, B.; Sannino, C.; Onofri, A.; Mastrolitti, S.; et al. Yeast lipids from cardoon stalks, stranded driftwood and olive tree pruning residues as possible extra sources of oils for producing biofuels and biochemical. Biotechnol. Biofuels 2018, 11, 147–162. [Google Scholar] [CrossRef]
- Kucharska, K.; Cieśliński, H.; Rybarczyk, P.; Słupek, E.; Łukajtis, R.; Wychodnik, K.; Kamiński, M. Fermentative conversion of two-step pre-treated lignocellulosic biomass to hydrogen. Catalysts 2019, 9, 858. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.; Chi, P.; Bilal, M.; Cheng, H. Biosynthetic strategies to produce xylitol: An economical venture. Appl. Microbiol. Biotechnol. 2019, 103, 5143–5160. [Google Scholar] [CrossRef]
- Silvester, L.; Ramos, F.; Thuriot-Roukos, J.; Heyte, S.; Araque, M.; Paul, S.; Wojcieszak, R. Fully integrated high-throughput methodology for the study of Ni- and Cu- supported catalysts for glucose hydrogenation. Catal. Today 2019, 338, 72–80. [Google Scholar] [CrossRef]
- Takagaki, A. Rational design of metal oxide solid acids for sugar conversion. Catalysts 2019, 9, 907. [Google Scholar] [CrossRef] [Green Version]
- Liao, Y.T.; Matsagar, B.M.; Wu, K.C.W. Metal-organic framework (MOF)-derived effective solid catalysts for valorization of lignocellulosic biomass. ACS Sustain. Chem. Eng. 2018, 6, 13628–13643. [Google Scholar] [CrossRef]
- Di Fidio, N.; Liuzzi, F.; Mastrolitti, S.; Albergo, R.; De Bari, I. Single cell oil production from undetoxified Arundo donax L. hydrolysate by Cutaneotrichosporon curvatus. J. Microbiol. Biotechnol. 2019, 29, 256–267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siril, P.F.; Cross, H.E.; Brown, D.R. New polystyrene sulfonic acid resin catalysts with enhanced acidic and catalytic properties. J. Mol. Catal. A Chem. 2008, 279, 63–68. [Google Scholar] [CrossRef]
- Antonetti, C.; Raspolli Galletti, A.M.; Fulignati, S.; Licursi, D. Amberlyst A-70; a surprisingly active catalyst for the MW-assisted dehydration of fructose and inulin to HMF in water. Catal. Commun. 2017, 97, 146–150. [Google Scholar] [CrossRef]
- Biancalana, L.; Fulignati, S.; Antonetti, C.; Zacchini, S.; Provinciali, G.; Pampaloni, G.; Raspolli Galletti, A.M.; Marchetti, F. Ruthenium p-cymene complexes with α-diimine ligands as catalytic precursor for the transfer hydrogenation of ethyl levulinate to γ-valerolactone. New J. Chem. 2018, 42, 17574–17586. [Google Scholar] [CrossRef]
- Priecel, P.; Lopez-Sanchez, J.A. Advantages and limitations of microwave reactors: From chemical synthesis to the catalytic valorization of biobased chemicals. ACS Sustain. Chem. Eng. 2019, 7, 3–21. [Google Scholar] [CrossRef] [Green Version]
- Antonetti, C.; Licursi, D.; Raspolli Galletti, A.M.; Martinelli, M.; Tellini, F.; Valentini, G.; Gambineri, F. Application of microwave irradiation for the removal of polychlorinated biphenyls from siloxane transformer and hydrocarbon engine oils. Chemosphere 2016, 159, 72–79. [Google Scholar] [CrossRef]
- Horikoshi, S.; Minagawa, T.; Tsubaki, S.; Onda, A.; Serpone, N. Is selective heating of the sulfonic acid catalyst AC-SO3H by microwave radiation crucial in the acid hydrolysis of cellulose to glucose in aqueous media? Catalysts 2017, 7, 231. [Google Scholar] [CrossRef] [Green Version]
- Antonetti, C.; Toniolo, L.; Cavinato, G.; Forte, C.; Ghignoli, C.; Ishak, R.; Cavani, F.; Raspolli Galletti, A.M. A hybrid polyketone-SiO2 support for palladium catalysts and their applications in cinnamaldehyde hydrogenation and in 1-phenylethanol oxidation. Appl. Catal. A Gen. 2015, 496, 40–50. [Google Scholar] [CrossRef]
- Li, H.; Qu, Y.; Yang, Y.; Chang, S.; Xu, J. Microwave irradiation—A green and efficient way to pretreat biomass. Biores. Technol. 2016, 199, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Liu, J.; Zheng, P.; Li, M.; Zhou, Y.; Huang, L.; Chen, L.; Shuai, L. Promoting enzymatic hydrolysis of lignocellulosic biomass by inexpensive soy protein. Biotechnol. Biofuels 2019, 12, 51–63. [Google Scholar] [CrossRef] [Green Version]
- Ju, X.; Bowden, M.; Engelhard, M.; Zhang, X. Investigating commercial cellulose performances toward specific biomass recalcitrance factors using reference substrates. Appl. Microbiol. Biotechnol. 2014, 98, 4409–4420. [Google Scholar] [CrossRef] [PubMed]
- Jung, C.D.; Yu, J.H.; Eom, I.Y.; Hong, K.S. Sugar yields from sunflower stalks treated by hydrothermolysis and subsequent enzymatic hydrolysis. Biores. Technol. 2013, 138, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Vlachos, D.G.; Quiroz, N.R.; Norton, A.M.; Nguyen, H.; Vasiliadou, E.S. A review of homogeneous metal salt solutions for biomass upgrading and other select organic reactions. ACS Catal. 2019, 9, 9923–9952. [Google Scholar] [CrossRef]
- Xiros, C.; Janssen, M.; Byström, R.; Børresen, B.T.; Cannella, D.; Jørgensen, H.; Koppram, R.; Larsson, C.; Olsson, L.; Tillman, A.M.; et al. Toward a sustainable biorefinery using high-gravity technology. Biofuels Bioprod. Biorefining 2017, 11, 15–27. [Google Scholar] [CrossRef] [Green Version]
- Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, A.; Sluiter, J.; Templeton, D. Preparation of Samples for Compositional Analysis; NREL/TP-510-42620; National Renewable Energy Laboratory: Golden, CO, USA, 2008.
- Sluiter, A.; Hames, B.; Hyman, D.; Payne, C.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Wolfe, J. Determination of Total Solids in Biomass and Total Dissolved Solids in Liquid Process. Samples; NREL/TP-510–42621; National Renewable Energy Laboratory: Golden, CO, USA, 2008.
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Crocker, D. Determination of Structural Carbohydrates and Lignin in Biomass; NREL/TP-510–42618; National Renewable Energy Laboratory: Golden, CO, USA, 2008.
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Liuter, J.; Templeton, D. Determination of Ash in Biomass; NREL/TP-510–42622; National Renewable Energy Laboratory: Golden, CO, USA, 2008.
- Sluiter, A.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D. Determination of Extractives in Biomass; NREL/TP-510–42619; National Renewable Energy Laboratory: Golden, CO, USA, 2008.
- Zamani, A. Introduction to lignocellulose-based products. In Biofuel and Biorefinery Technologies; Karimi, K., Ed.; Springer International Publishing: Cham, Switzerland, 2015; Volume 1, pp. 1–36. [Google Scholar]
- Li, Y.C.; Gou, Z.X.; Zhang, Y.; Xia, Z.Y.; Tang, Y.Q.; Kida, K. Inhibitor tolerance of a recombinant flocculating industrial Saccharomyces cerevisiae strain during glucose and xylose co-fermentation. Braz. J. Microbiol. 2017, 48, 791–800. [Google Scholar] [CrossRef]
- Fiore, V.; Scalici, T.; Valenza, A. Characterization of a new natural fiber from Arundo donax L. as potential reinforcement of polymer composites. Carbohydr. Polym. 2014, 106, 77–83. [Google Scholar] [CrossRef]
- Mattonai, M.; Pawcenis, D.; Del Seppia, S.; Łojewska, J.; Ribechini, E. Effect of ball-milling on crystallinity index, degree of polymerization and thermal stability of cellulose. Biores. Technol. 2018, 270, 270–277. [Google Scholar] [CrossRef]
- Chen, B.; Wang, X.; Leng, W.; Kan, Y.; Mei, C.; Zhai, S. Spectroscopic/microscopic elucidation for chemical changes during acid pretreatment. J. Biores. Bioprod. 2019, 4, 192–199. [Google Scholar] [CrossRef]
- Brienzo, M.; Abud, Y.; Ferreira, S.; Corrales, R.C.N.R.; Ferreira-Leitão, V.S.; de Souza, W.; Sant’Anna, C. Characterization of anatomy, lignin distribution, and response to pretreatment of sugarcane culm and internode. Ind. Crop. Prod. 2016, 84, 305–313. [Google Scholar] [CrossRef] [Green Version]
- Ji, Z.; Zhang, X.; Ling, Z.; Sun, R.C.; Xu, F. Tissue specific response of Miscanthus x giganteus to dilute acid pretreatment for enhancing cellulose digestibility. Carbohydr. Polym. 2016, 154, 247–256. [Google Scholar] [CrossRef] [PubMed]
- Fulignati, S.; Antonetti, C.; Licursi, D.; Pieraccioni, M.; Wilbers, E.; Heeres, H.J.; Raspolli Galletti, A.M. Insight into the hydrogenation of pure and crude HMF to furan diols using Ru/C as catalyst. Appl. Catal. A Gen. 2019, 578, 122–133. [Google Scholar] [CrossRef]
- Van Dyk, J.S.; Pletschke, B.I. A review of lignocellulose bioconversion using enzymatic hydrolysis and synergistic cooperation between enzymes—Factors affecting enzymes, conversion and synergy. Biotechnol. Adv. 2012, 30, 1458–1480. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, Y.; Zhang, L.; Zhang, R.; Liu, G.; Cheng, G. Understanding changes in cellulose crystalline structure of lignocellulosic biomass during ionic liquid pretreatment by XRD. Biores. Technol. 2014, 151, 402–405. [Google Scholar] [CrossRef]
- Berlin, A.; Balakshin, M.; Gilkes, N.; Kadla, J.; Maximenko, V.; Kubo, S.; Saddler, J. Inhibition of cellulose, xylanase and β-glucosidase activities by softwood lignin preparations. J. Biotechnol. 2006, 125, 198–209. [Google Scholar] [CrossRef]
- Aliberti, A.; Ventorino, V.; Robertiello, A.; Galasso, M.; Blaiotta, G.; Comite, E.; Faraco, V.; Pepe, O. Effect of cellulase, substrate concentrations, and configuration processes on cellulosic ethanol production from pretreated Arundo donax. BioResources 2017, 12, 5321–5342. [Google Scholar] [CrossRef] [Green Version]
- Ventorino, V.; Robertiello, A.; Viscardi, S.; Ambrosiano, A.; Faraco, V.; Pepe, O. Bio-based chemical production from Arundo donax feedstock fermentation using Cosenzaea myxofaciens BPM1. BioResources 2016, 11, 6566–6581. [Google Scholar] [CrossRef] [Green Version]
- Lemons e Silva, C.F.; Artigas Schirmer, M.; Nobuyuki Maeda, R.; Araújo Barcelos, C.; Pereira, N. Potential of giant reed (Arundo donax L.) for second generation ethanol production. Electron. J. Biotechn. 2015, 18, 10–15. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Templer, R.; Murphy, R.J. High-solids loading enzymatic hydrolysis of waste papers for biofuel production. Appl. Energy 2012, 99, 23–31. [Google Scholar] [CrossRef]
- Kristensen, J.B.; Felby, C.; Jørgensen, H. Yield-determining factors in high-solids enzymatic hydrolysis of lignocellulose. Biotechnol. Biofuels 2009, 2, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Z.; Zhu, Z.; Shen, B.; Liu, L. Insights into biochar and hydrochar production and applications: A review. Energy 2019, 171, 581–598. [Google Scholar] [CrossRef]
- Antonetti, C.; Gori, S.; Licursi, D.; Frigo, S.; Antonelli, M.; López, M.; Parajó, J.C.; Raspolli Galletti, A.M. Complete exploitation of eucalyptus nitens: Optimization of hydrothermal conversion of its cellulose fraction to levulinic acid and butyl levulinate. In Proceedings of the European Biomass Conference and Exhibition (EUBCE), Lisbon, Portugal, 27–30 May 2019; pp. 1329–1336. [Google Scholar]
- Liu, Z.-H.-; Shinde, S.; Xie, S.; Hao, N.; Lin, F.; Li, M.; Yoo, C.G.; Ragauskas, A.J.; Yuan, J.S. Cooperative valorization of lignin and residual sugar to polyhydroxyalkanoate (PHA) for enhanced yield and carbon utilization in biorefineries. Sustain. Energy Fuels 2019, 3, 2024–2037. [Google Scholar] [CrossRef]
- Adney, B.; Baker, J. Measurement of Cellulase Activities: Laboratory Analytical Procedure (LAP); NREL/TP-510-42628; National Renewable Energy Laboratory: Golden, CO, USA, 2008.
- Zhang, T.; Kumar, R.; Tsai, Y.D.; Elander, R.T.; Wyman, C.E. Xylose yields and relationship to combined severity for dilute acid post-hydrolysis of xylooligomers from hydrothermal pretreatment of corn stover. Green Chem. 2015, 17, 394–403. [Google Scholar] [CrossRef]








| Raw Material | Catalyst | Final Product | Yield | Process Conditions | Reference |
|---|---|---|---|---|---|
| Corn cob | HSO3-ZSM-5 | Xylose | 28.7 wt% | 120 °C, 6 h, S/L 1/20, Cat/sub 1:1 wt/wt | [38] |
| Corn cob | Carbon-based solid acid (C-SO3H) | Xylose | 78.1 mol% | 140 °C, 6 h, S/L 1/100, Cat/sub 1:1 wt/wt | [39] |
| Giant reed | Amberlyst 35DRY | Total reducing sugars | 43.0 wt% | IL pretreatment: 120 °C, 3 h, S/L 1/20 [C4mim]Cl; acid hydrolysis: 120 °C, 1.5 h, S/L 1/20, Cat/sub 1:5 wt/wt | [41] |
| Rice straw | Carbon-based solid acid (–COOH) | Xylose | 52.1 mol% | Alkali pretreatment: 120 °C, 4 h, KOH 6 wt%, S/L 1/10; ball-milling treatment: 4 h, 500 r·min−1, Cat/sub 1:2 wt/wt; acid hydrolysis: 200 °C, 1 h, S/L 1/100, Cat/sub 1:2 wt/wt | [42] |
| Eucalyptus | Carbon-based solid acid (–COOH) | Xylose | 83.0 mol% | 215 °C, 0.5 h, S/L 1/130, Cat/sub 1:6.5 wt/wt | [44] |
| Raw Material | Catalyst | Final Product | Yield | Process Conditions | Reference |
|---|---|---|---|---|---|
| Corn cob | HSO3-ZSM-5 | Glucose | 23.0 wt% | 120 °C, 6 h, S/L 1/20, Cat/sub 1:1 wt/wt | [38] |
| Corn cob | Cellulase enzyme | Glucose | 91.6 wt% | 50 °C, 48 h, 40 FPU/g substrate | [39] |
| Rice straw | Amberlyst 15DRY | Total reducing sugars | 26.8 wt% | Alkali pretreatment: 120 °C, 1 h, NaOH 2 wt%, S/L 1/7; acid hydrolysis: 140 °C, 4 h, S/L 1/20, Cat/sub 1:1.4 wt/wt | [40] |
| Rice straw | Carbon-based solid acid (–COOH) | Glucose | 66.5 mol% | Alkali pretreatment: 120 °C, 4 h, KOH 6 wt%, S/L 1/10; ball-milling treatment: 4 h, 500 r·min−1, Cat/sub 1:2 wt/wt; acid hydrolysis: 200 °C, 1 h, S/L 1/100, Cat/sub 1:2 wt/wt | [42] |
| Corn stover | PSSA/PIL | Glucose | 20.0 wt% | 105 °C, 7 h, S/L 1/20, Cat/sub 3:1 wt/wt, [EMIM]Cl/H2O 4:1 wt/wt | [43] |
| Eucalyptus | Carbon-based solid acid (–COOH) | Glucose | 31.0 mol% | 215 °C, 0.5 h, S/L 1/130, Cat/sub 1:6.5 wt/wt | [44] |
| Run | Biomass Loading (wt%) | Cat/Sub 1 (wt/wt) | Temperature (°C) | Biomass Solubilisation (wt%) | Xylose Yield 2 (mol%) | Glucose Yield 3 (mol%) |
|---|---|---|---|---|---|---|
| 1 | 5 | 0.1 | 160 | 31.5 | 68.4 | 8.5 |
| 2 | 5 | 0.2 | 160 | 44.5 | 97.5 | 12.5 |
| 3 | 5 | 0.3 | 160 | 47.7 | 94.8 | 13.0 |
| 4 | 9 | 0.1 | 160 | 30.3 | 66.7 | 8.2 |
| 5 | 9 | 0.2 | 160 | 43.8 | 99.2 | 11.8 |
| 6 | 9 | 0.3 | 160 | 46.3 | 94.0 | 12.0 |
| 7 | 13 | 0.1 | 160 | 29.7 | 65.3 | 8.1 |
| 8 | 13 | 0.2 | 160 | 44.3 | 93.4 | 11.9 |
| 9 | 13 | 0.3 | 160 | 48.4 | 95.8 | 12.2 |
| 10 | 13 | 0.1 | 150 | 29.5 | 53.3 | 7.0 |
| 11 | 13 | 0.2 | 150 | 40.9 | 85.3 | 10.8 |
| 12 | 13 | 0.3 | 150 | 43.7 | 83.9 | 10.9 |
| 13 | 17 | 0.2 | 160 | 42.9 | 96.3 | 10.2 |
| Run | Biomass Loading (wt%) | Cat/Sub 1 (wt/wt) | Temperature (°C) | AA 2 (g/L) | FA 3 (g/L) | LA 4 (g/L) | HMF 5 (g/L) | Furfural (g/L) |
|---|---|---|---|---|---|---|---|---|
| 1 | 5 | 0.1 | 160 | 0.8 | 0.2 | 0.0 | 0.1 | 0.0 |
| 2 | 5 | 0.2 | 160 | 0.9 | 0.2 | 0.0 | 0.1 | 0.0 |
| 3 | 5 | 0.3 | 160 | 1.1 | 0.3 | 0.0 | 0.2 | 0.0 |
| 4 | 9 | 0.1 | 160 | 1.6 | 0.1 | 0.1 | 0.4 | 0.0 |
| 5 | 9 | 0.2 | 160 | 2.1 | 0.7 | 0.3 | 0.7 | 0.0 |
| 6 | 9 | 0.3 | 160 | 2.8 | 0.7 | 0.5 | 0.7 | 0.1 |
| 7 | 13 | 0.1 | 160 | 2.9 | 0.2 | 0.2 | 0.8 | 0.4 |
| 8 | 13 | 0.2 | 160 | 4.1 | 1.1 | 0.5 | 1.1 | 0.7 |
| 9 | 13 | 0.3 | 160 | 4.2 | 1.1 | 0.6 | 1.0 | 0.7 |
| 10 | 13 | 0.1 | 150 | 1.0 | 0.0 | 0.0 | 0.3 | 0.1 |
| 11 | 13 | 0.2 | 150 | 2.9 | 0.7 | 0.3 | 0.9 | 0.5 |
| 12 | 13 | 0.3 | 150 | 3.1 | 0.7 | 0.4 | 0.8 | 0.5 |
| 13 | 17 | 0.2 | 160 | 4.7 | 1.0 | 0.5 | 1.3 | 1.0 |
| Run | Time (min) | Temperature (°C) | AA 1 (g/L) | FA 2 (g/L) | LA 3 (g/L) | HMF 4 (g/L) | Furfural (g/L) | Biomass Solubilisation (wt%) | Glucose Yield 5 (mol%) |
|---|---|---|---|---|---|---|---|---|---|
| 14 | 60 | 180 | 2.5 | 1.5 | 1.9 | 0.8 | 0.6 | 17.2 | 3.9 |
| 15 | 60 | 190 | 2.5 | 1.1 | 3.2 | 1.9 | 0.8 | 35.9 | 26.4 |
| 16 | 20 | 190 | 2.2 | 1.5 | 1.6 | 0.6 | 0.5 | 27.3 | 32.5 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Fidio, N.; Raspolli Galletti, A.M.; Fulignati, S.; Licursi, D.; Liuzzi, F.; De Bari, I.; Antonetti, C. Multi-Step Exploitation of Raw Arundo donax L. for the Selective Synthesis of Second-Generation Sugars by Chemical and Biological Route. Catalysts 2020, 10, 79. https://doi.org/10.3390/catal10010079
Di Fidio N, Raspolli Galletti AM, Fulignati S, Licursi D, Liuzzi F, De Bari I, Antonetti C. Multi-Step Exploitation of Raw Arundo donax L. for the Selective Synthesis of Second-Generation Sugars by Chemical and Biological Route. Catalysts. 2020; 10(1):79. https://doi.org/10.3390/catal10010079
Chicago/Turabian StyleDi Fidio, Nicola, Anna Maria Raspolli Galletti, Sara Fulignati, Domenico Licursi, Federico Liuzzi, Isabella De Bari, and Claudia Antonetti. 2020. "Multi-Step Exploitation of Raw Arundo donax L. for the Selective Synthesis of Second-Generation Sugars by Chemical and Biological Route" Catalysts 10, no. 1: 79. https://doi.org/10.3390/catal10010079