8-Oxoguanine DNA Glycosylase (OGG1) Cys326 Variant: Increased Risk for Worse Outcome of Patients with Locally Advanced Rectal Cancer after Multimodal Therapy
Abstract
:Simple Summary
Abstract
1. Introduction
2. Results
2.1. Eligible Patients
2.2. Patient Baseline, Tumor, and Treatment Characteristics in Relation to Clinical Outcome
2.3. Genetic Polymorphisms and Initial Tumor Staging Parameters
2.4. Genetic Polymorphisms and Tumor Response
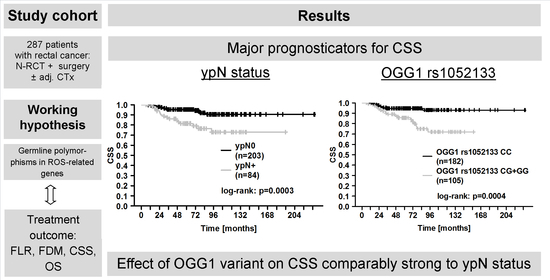
2.5. Genetic Polymorphisms and Clinical Outcome
2.6. Multiparametric Assessment for Clinical Outcome
2.7. Subgroup Analyses for Parameters Affecting Clinical Outcome
2.8. Functional Assessment of OGG1 rs1052133
3. Discussion
4. Patients and Methods
4.1. Eligibility Criteria
4.2. Disease Staging
4.3. Multimodal Treatment Sequence
4.4. Assessment of Treatment Outcome
4.5. Genotyping
4.6. Micronuclei Assessment
4.7. Cell viability Assessment
4.8. Ethical Approval
4.9. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fietkau, R.; Zettl, H.; Klocking, S.; Kundt, G. Incidence, therapy and prognosis of colorectal cancer in different age groups. A population-based cohort study of the Rostock Cancer Registry. Strahlenther. Onkol. 2004, 180, 478–487. [Google Scholar] [CrossRef]
- Belluco, C.; De Paoli, A.; Canzonieri, V.; Sigon, R.; Fornasarig, M.; Buonadonna, A.; Boz, G.; Innocente, R.; Perin, T.; Cossaro, M.; et al. Long-term outcome of patients with complete pathologic response after neoadjuvant chemoradiation for cT3 rectal cancer: Implications for local excision surgical strategies. Ann. Surg. Oncol. 2011, 18, 3686–3693. [Google Scholar] [CrossRef] [Green Version]
- Bosset, J.-F.; Calais, G.; Mineur, L.; Maingon, P.; Stojanovic-Rundic, S.; Bensadoun, R.-J.; Bardet, E.; Beny, A.; Ollier, J.-C.; Bolla, M.; et al. Fluorouracil-based adjuvant chemotherapy after preoperative chemoradiotherapy in rectal cancer: Long-term results of the EORTC 22921 randomised study. Lancet Oncol. 2014, 15, 184–190. [Google Scholar] [CrossRef]
- Gérard, J.-P.; Conroy, T.; Bonnetain, F.; Bouché, O.; Chapet, O.; Closon-Dejardin, M.-T.; Untereiner, M.; LeDuc, B.; Francois, É.; Maurel, J.; et al. Preoperative radiotherapy with or without concurrent fluorouracil and leucovorin in T3-4 rectal cancers: Results of FFCD 9203. J. Clin. Oncol. 2006, 24, 4620–4625. [Google Scholar] [CrossRef]
- Kapiteijn, E.; Marijnen, C.A.; Nagtegaal, I.; Putter, H.; Steup, W.H.; Wiggers, T.; Rutten, H.J.; Pahlman, L.; Glimelius, B.; Van Krieken, J.H.J.; et al. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer. New Engl. J. Med. 2001, 345, 638–646. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sauer, R.; Becker, H.; Hohenberger, W.; Rödel, C.; Wittekind, C.; Fietkau, R.; Martus, P.; Tschmelitsch, J.; Hager, E.; Hess, C.F.; et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. New Engl. J. Med. 2004, 351, 1731–1740. [Google Scholar] [CrossRef] [Green Version]
- Sauer, R.; Liersch, T.; Merkel, S.; Fietkau, R.; Hohenberger, W.; Hess, C.; Becker, H.; Raab, H.-R.; Villanueva, M.-T.; Witzigmann, H.; et al. Preoperative versus postoperative chemoradiotherapy for locally advanced rectal cancer: Results of the German CAO/ARO/AIO-94 randomized phase III trial after a median follow-up of 11 years. J. Clin. Oncol. 2012, 30, 1926–1933. [Google Scholar] [CrossRef] [PubMed]
- Bahadoer, R.R.; A Dijkstra, E.; Van Etten, B.; Marijnen, C.A.M.; Putter, H.; Kranenbarg, E.M.-K.; Roodvoets, A.G.H.; Nagtegaal, I.D.; Beets-Tan, R.G.H.; Blomqvist, L.K.; et al. Short-course radiotherapy followed by chemotherapy before total mesorectal excision (TME) versus preoperative chemoradiotherapy, TME, and optional adjuvant chemotherapy in locally advanced rectal cancer (RAPIDO): A randomised, open-label, phase 3 trial. Lancet Oncol. 2021, 22, 29–42. [Google Scholar] [CrossRef]
- Fokas, E.; Liersch, T.; Fietkau, R.; Hohenberger, W.; Beissbarth, T.; Hess, C.; Becker, H.; Ghadimi, M.; Mrak, K.; Merkel, S.; et al. Tumor regression grading after preoperative chemoradiotherapy for locally advanced rectal carcinoma revisited: Updated results of the CAO/ARO/AIO-94 trial. J. Clin. Oncol. 2014, 32, 1554–1562. [Google Scholar] [CrossRef]
- Ahn, J.; Ambrosone, C.B.; Kanetsky, P.A.; Tian, C.; Lehman, T.A.; Kropp, S.; Helmbold, I.; Von Fournier, D.; Haase, W.; Sautter-Bihl, M.L.; et al. Polymorphisms in genes related to oxidative stress (MPO, MnSOD, CAT) and survival after treatment for breast cancer. Cancer Res. 2005, 65, 1105–1111. [Google Scholar]
- Hubackova, M.; Vaclavikova, R.; Ehrlichova, M.; Mrhalova, M.; Kodet, R.; Kubackova, K.; Vrána, D.; Gut, I.; Soucek, P. Association of superoxide dismutases and NAD(P)H quinone oxidoreductases with prognosis of patients with breast carcinomas. Int. J. Cancer 2012, 130, 338–348. [Google Scholar] [CrossRef]
- Yao, S.; Barlow, W.E.; Albain, K.S.; Choi, J.-Y.; Zhao, H.; Livingston, R.B.; Davis, W.; Rae, J.M.; Yeh, I.-T.; Hutchins, L.F.; et al. Manganese superoxide dismutase polymorphism, treatment-related toxicity and disease-free survival in SWOG 8897 clinical trial for breast cancer. Breast Cancer Res. Treat 2010, 124, 433–439. [Google Scholar] [CrossRef] [Green Version]
- Forsberg, L.; Lyrenäs, L.; Morgenstern, R.; De Faire, U. A common functional C-T substitution polymorphism in the promoter region of the human catalase gene influences transcription factor binding, reporter gene transcription and is correlated to blood catalase levels. Free Radic. Biol. Med. 2001, 30, 500–505. [Google Scholar] [CrossRef]
- Rajaraman, P.; Hutchinson, A.; Rothman, N.; Black, P.M.; Fine, H.A.; Loeffler, J.S.; Selker, R.G.; Shapiro, W.R.; Linet, M.S.; Inskip, P.D. Oxidative response gene polymorphisms and risk of adult brain tumors. Neuro Oncol. 2008, 10, 709–715. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, H.-I.; Lin, Y.-T.; Jung, C.-R.; Hwang, B.-F. Interaction Between Catalase Gene Promoter Polymorphisms and Indoor Environmental Exposure in Childhood Allergic Rhinitis. Epidemiology 2017, 28 (Suppl. S1), S126–S132. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Gong, W.; Shen, H.; Li, X.; Ding, L.; Han, L.; Zhang, H.; Zhu, B.; Liu, X. Correlation between CAT polymorphism and susceptibility to DMAc-induced abnormal liver function: A case-control study of Chinese population. Biomarkers 2018, 23, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, M.; Schirmer, M.A.; Tzvetkov, M.V.; Kreuz, M.; Ziepert, M.; Wojnowski, L.; Kube, D.; Pfreundschuh, M.; Trümper, L.; Loeffler, M.; et al. A functional polymorphism in the NAD(P)H oxidase subunit CYBA is related to gene expression, enzyme activity, and outcome in non-Hodgkin lymphoma. Cancer Res. 2010, 70, 2328–2338. [Google Scholar] [CrossRef] [Green Version]
- Bhatti, P.; Stewart, P.A.; Hutchinson, A.; Rothman, N.; Linet, M.S.; Inskip, P.D.; Rajaraman, P. Lead exposure, polymorphisms in genes related to oxidative stress, and risk of adult brain tumors. Cancer Epidemiol. Biomark. Prev. 2009, 18, 1841–1848. [Google Scholar] [CrossRef] [Green Version]
- Soerensen, M.; Christensen, K.; Stevnsner, T.; Christiansen, L. The Mn-superoxide dismutase single nucleotide polymorphism rs4880 and the glutathione peroxidase 1 single nucleotide polymorphism rs1050450 are associated with aging and longevity in the oldest old. Mech. Ageing Dev. 2009, 130, 308–314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, T.Y.; Barnett, M.J.; Kristal, A.R.; Ambrosone, C.B.; King, I.B.; Thornquist, M.D.; Goodman, G.E.; Neuhouser, M.L. Genetic variation in myeloperoxidase modifies the association of serum alpha-tocopherol with aggressive prostate cancer among current smokers. J. Nutr. 2011, 141, 1731–1737. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kershaw, R.M.; Hodges, N.J. Repair of oxidative DNA damage is delayed in the Ser326Cys polymorphic variant of the base excision repair protein OGG1. Mutagenesis 2012, 27, 501–510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hansen, R.; Saebø, M.; Skjelbred, C.F.; Nexø, B.A.; Hagen, P.C.; Bock, G.; Lothe, I.M.B.; Johnson, E.; Aase, S.; Hansteen, I.-L.; et al. GPX Pro198Leu and OGG1 Ser326Cys polymorphisms and risk of development of colorectal adenomas and colorectal cancer. Cancer Lett. 2005, 229, 85–91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leibold, T.; Shia, J.; Ruo, L.; Minsky, B.D.; Akhurst, T.; Ginsberg, M.J.G.S.; Larson, S.; Riedel, E.; Wong, W.D.; Guillem, J.G. Prognostic implications of the distribution of lymph node metastases in rectal cancer after neoadjuvant chemoradiotherapy. J. Clin. Oncol. 2008, 26, 2106–2111. [Google Scholar] [CrossRef] [PubMed]
- Gerard, J.P.; Azria, D.; Gourgou-Bourgade, S.; Martel-Laffay, I.; Hennequin, C.; Vendrely, P.-L.E.; François, E.; De la Roche, G.; Bouché, O. Comparison of two neoadjuvant chemoradiotherapy regimens for locally advanced rectal cancer: Results of the phase III trial ACCORD 12/0405-Prodige 2. J. Clin. Oncol. 2010, 28, 1638–1644. [Google Scholar] [CrossRef]
- Rödel, C.; Graeven, U.; Fietkau, R.; Hohenberger, W.; Hothorn, T.; Arnold, D.; Hofheinz, R.-D.; Ghadimi, M.; Wolff, H.A.; Lang-Welzenbach, M. Oxaliplatin added to fluorouracil-based preoperative chemoradiotherapy and postoperative chemotherapy of locally advanced rectal cancer (the German CAO/ARO/AIO-04 study): Final results of the multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2015, 16, 979–989. [Google Scholar] [CrossRef]
- Breugom, A.J.; Swets, M.; Bosset, J.-F.; Collette, L.; Sainato, A.; Cionini, L.; Glynne-Jones, R.; Counsell, N.; Bastiaannet, E.; Broek, C.B.M.V.D.; et al. Adjuvant chemotherapy after preoperative (chemo)radiotherapy and surgery for patients with rectal cancer: A systematic review and meta-analysis of individual patient data. Lancet Oncol. 2015, 16, 200–207. [Google Scholar] [CrossRef]
- Kasai, H.; Crain, P.; Kuchino, Y.; Nishimura, S.; Ootsuyama, A.; Tanooka, H. Formation of 8-hydroxyguanine moiety in cellular DNA by agents producing oxygen radicals and evidence for its repair. Carcinogenesis 1986, 7, 1849–1851. [Google Scholar] [CrossRef]
- Shibutani, S.; Takeshita, M.; Grollman, A.P. Insertion of specific bases during DNA synthesis past the oxidation-damaged base 8-oxodG. Nature 1991, 349, 431–434. [Google Scholar] [CrossRef]
- Arai, K.; Morishita, K.; Shinmura, K.; Kohno, T.; Kim, S.-R.; Nohmi, T.; Taniwaki, M.; Ohwada, S.; Yokota, J. Cloning of a human homolog of the yeast OGG1 gene that is involved in the repair of oxidative DNA damage. Oncogene 1997, 14, 2857–2861. [Google Scholar] [CrossRef] [Green Version]
- Nishioka, K.; Ohtsubo, T.; Oda, H.; Fujiwara, T.; Kang, D.; Sugimachi, K.; Nakabeppu, Y. Expression and differential intracellular localization of two major forms of human 8-oxoguanine DNA glycosylase encoded by alternatively spliced OGG1 mRNAs. Mol. Biol. Cell 1999, 10, 1637–1652. [Google Scholar] [CrossRef] [Green Version]
- Baptiste, B.A.; Katchur, S.R.; Fivenson, E.M.; Croteau, D.L.; Rumsey, W.L.; Bohr, V.A. Enhanced mitochondrial DNA repair of the common disease-associated variant, Ser326Cys, of hOGG1 through small molecule intervention. Free Radic. Biol. Med. 2018, 124, 149–162. [Google Scholar] [CrossRef]
- Yamane, A.; Kohno, T.; Ito, K.; Sunaga, N.; Aoki, K.; Yoshimura, K.; Murakami, H.; Nojima, Y.; Yokota, J. Differential ability of polymorphic OGG1 proteins to suppress mutagenesis induced by 8-hydroxyguanine in human cell in vivo. Carcinogenesis 2004, 25, 1689–1694. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morreall, J.; Limpose, K.; Sheppard, C.; Kow, Y.W.; Werner, E.; Doetsch, P.W. Inactivation of a common OGG1 variant by TNF-alpha in mammalian cells. DNA Repair 2015, 26, 15–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luna, L.; Rolseth, V.; Hildrestrand, G.A.; Otterlei, M.; Dantzer, F.; Bjørås, M.; Seeberg, E. Dynamic relocalization of hOGG1 during the cell cycle is disrupted in cells harbouring the hOGG1-Cys326 polymorphic variant. Nucleic Acids Res. 2005, 33, 1813–1824. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Svejstrup, J.Q. Mechanisms of transcription-coupled DNA repair. Nat. Rev. Mol. Cell Biol. 2002, 3, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Smart, D.J.; Chipman, J.K.; Hodges, N.J. Activity of OGG1 variants in the repair of pro-oxidant-induced 8-oxo-2-deoxyguanosine. DNA Repair 2006, 5, 1337–1345. [Google Scholar] [CrossRef] [PubMed]
- Sinitsky, M.Y.; Minina, V.I.; Asanov, M.A.; Yuzhalin, A.E.; Ponasenko, A.V.; Druzhinin, V.G. Association of DNA repair gene polymorphisms with genotoxic stress in underground coal miners. Mutagenesis 2017, 32, 501–509. [Google Scholar] [CrossRef] [Green Version]
- Preston, T.J.; Henderson, J.T.; McCallum, G.P.; Wells, P.G. Base excision repair of reactive oxygen species-initiated 7,8-dihydro-8-oxo-2’-deoxyguanosine inhibits the cytotoxicity of platinum anticancer drugs. Mol. Cancer Ther. 2009, 8, 2015–2026. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arizono, K.; Osada, Y.; Kuroda, Y. DNA repair gene hOGG1 codon 326 and XRCC1 codon 399 polymorphisms and bladder cancer risk in a Japanese population. Jpn. J. Clin. Oncol. 2008, 38, 186–191. [Google Scholar] [CrossRef]
- Jiao, X.; Huang, J.; Wu, S.; Hu, Y.; Su, X.; Luo, C.; Broelsch, C. hOGG1 Ser326Cys polymorphism and susceptibility to gallbladder cancer in a Chinese population. Int. J. Cancer 2007, 121, 501–505. [Google Scholar] [CrossRef]
- Srivastava, A.; Srivastava, K.; Pandey, S.N.; Choudhuri, G.; Mittal, B. Single-nucleotide polymorphisms of DNA repair genes OGG1 and XRCC1: Association with gallbladder cancer in North Indian population. Ann. Surg. Oncol. 2009, 16, 1695–1703. [Google Scholar] [CrossRef]
- Duan, W.-X.; Hua, R.-X.; Yi, W.; Shen, L.-J.; Jin, Z.-X.; Zhao, Y.-H.; Yi, D.-H.; Chen, W.-S.; Yu, S.-Q. The association between OGG1 Ser326Cys polymorphism and lung cancer susceptibility: A meta-analysis of 27 studies. PLoS ONE 2012, 7, e35970. [Google Scholar] [CrossRef]
- Sorensen, M.; Raaschou-Nielsen, O.; Hansen, R.D.; Tjonneland, A.; Overvad, K.; Vogel, U. Interactions between the OGG1 Ser326Cys polymorphism and intake of fruit and vegetables in relation to lung cancer. Free Radic. Res. 2006, 40, 885–891. [Google Scholar] [CrossRef]
- Xu, Z.; Yu, L.; Zhang, X. Association between the hOGG1 Ser326Cys polymorphism and lung cancer susceptibility: A meta-analysis based on 22,475 subjects. Diagn. Pathol. 2013, 8, 144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitra, A.K.; Singh, S.V.; Garg, V.K.; Sharma, M.; Chaturvedi, R.; Rath, S.K. Protective association exhibited by the single nucleotide polymorphism (SNP) rs1052133 in the gene human 8-oxoguanine DNA glycosylase (hOGG1) with the risk of squamous cell carcinomas of the head & neck (SCCHN) among north Indians. Indian J. Med. Res. 2011, 133, 605–612. [Google Scholar]
- Sliwinski, T.; Przybylowska, K.; Markiewicz, L.; Rusin, P.; Pietruszewska, W.; Zelinska-Blizniewska, H.; Olszewski, J.; Morawiec-Sztandera, A.; Mlynarski, W.; Majsterek, I. MUTYH Tyr165Cys, OGG1 Ser326Cys and XPD Lys751Gln polymorphisms and head neck cancer susceptibility: A case control study. Mol. Biol. Rep. 2011, 38, 1251–1261. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; He, B.-S.; Pan, Y.-Q.; Xu, Y.-Q.; Wang, S.-K. Association of OGG1 Ser326Cys polymorphism with colorectal cancer risk: A meta-analysis. Int. J. Colorectal. Dis. 2011, 26, 1525–1530. [Google Scholar] [CrossRef]
- Ghelmani, Y.; Asadian, F.; Antikchi, M.H.; Dastgheib, S.A.; Shaker, S.H.; Jafari-Nedooshan, J.; Neamatzadeh, H. Association Between the hOGG1 1245C>G (rs1052133) Polymorphism and Susceptibility to Colorectal Cancer: A Meta-analysis Based on 7010 Cases and 10,674 Controls. J. Gastrointest Cancer 2021, 52, 389–398. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Xu, A.; Zhu, J. The effect of oxoguanine glycosylase 1 rs1052133 polymorphism on colorectal cancer risk in Caucasian population. Tumour Biol. 2014, 35, 513–517. [Google Scholar] [CrossRef]
- Aggarwal, N.; Donald, N.D.; Malik, S.; Selvendran, S.S.; McPhail, M.J.; Monahan, K.J. The Association of Low-Penetrance Variants in DNA Repair Genes with Colorectal Cancer: A Systematic Review and Meta-Analysis. Clin. Transl. Gastroenterol. 2017, 8, e109. [Google Scholar] [CrossRef]
- Reeves, S.G.; Meldrum, C.; Groombridge, C.; Spigelman, A.; Suchy, J.; Kurzawski, G.; Lubinski, J.; Scott, R.J. DNA repair gene polymorphisms and risk of early onset colorectal cancer in Lynch syndrome. Cancer Epidemiol. 2012, 36, 183–189. [Google Scholar] [CrossRef]
- Rödel, C.; Liersch, T.; Becker, H.; Fietkau, R.; Hohenberger, W.; Hothorn, T.; Graeven, U.; Arnold, D.; Lang-Welzenbach, M.; Raab, H.-R. Preoperative chemoradiotherapy and postoperative chemotherapy with fluorouracil and oxaliplatin versus fluorouracil alone in locally advanced rectal cancer: Initial results of the German CAO/ARO/AIO-04 randomised phase 3 trial. Lancet Oncol. 2012, 13, 679–687. [Google Scholar] [CrossRef]
- Dröge, L.H.; Hennies, S.; Lorenzen, S.; Conradi, L.-C.; Quack, H.; Liersch, T.; Helms, C.; Frank, M.A.; Schirmer, M.A.; Rave-Fränk, M.; et al. Prognostic value of the micronucleus assay for clinical endpoints in neoadjuvant radiochemotherapy for rectal cancer. BMC Cancer 2021, 21, 219. [Google Scholar] [CrossRef]
- Fenech, M. Cytokinesis-block micronucleus cytome assay. Nat. Protoc. 2007, 2, 1084–1104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gross, A.; Ziepert, M.; Scholz, M. KMWin—A convenient tool for graphical presentation of results from Kaplan-Meier survival time analysis. PLoS ONE 2012, 7, e38960. [Google Scholar] [CrossRef] [PubMed]





| Gene | SNP | Genomic Localization 1 | Affected Element 2 | Alleles 3 | MAF 4 | References |
|---|---|---|---|---|---|---|
| CAT | rs1001179 | 11:34438684 | upstream, −250 bp | C > T | 0.216 | [13,14] |
| rs769214 | 11:34438170 | upstream, −764 bp | A > G | 0.346 | [15,16] | |
| CYBA | rs1049255 | 16:88643329 | 3′-UTR | A > G | 0.495 | [17] |
| GPX1 | rs1050450 | 3:49357401 | coding, missense | C > T | 0.239 | [18,19] |
| MPO | rs2333227 | 17:58281401 | upstream, −466 bp | G > A | 0.192 | [10,20] |
| OGG1 | rs1052133 | 3:9757089 | coding, missense | C > G | 0.203 | [21,22] |
| SOD2 | rs4880 | 6:159692840 | coding, missense | T > C | 0.486 | [10,12,14,19] |
| SOD3 | rs699473 | 4:24795181 | upstream, −297 bp | T > C | 0.356 | [14] |
| Item | Numbers (%) |
|---|---|
| Age (years): median (min–max) | 64.4 (20.8–85.4) |
| Female | 95 (33.1) |
| T category 1 | |
| T1 | 0 (0) |
| T2 | 6 (2.1) |
| T3 | 256 (89.2) |
| T4 | 25 (8.7) |
| Nodal status 1 | |
| N0 | 60 (20.9) |
| N+ | 227 (79.1) |
| AJCC stage 2 | |
| II | 60 (20.9) |
| III | 227 (79.1) |
| Radiotherapy 3 | |
| Completed as planned | 282 (98.3) |
| 80% ≤ dose < 100% | 5 (1.7) |
| Type of chemotherapy within N-RCT | |
| 5-FU mono | 184 (64.1) |
| 5-FU + oxaliplatin 4 | 103 (35.9) |
| Chemotherapy within N-RCT completed 5 | 273 (95.1) |
| Pathological complete response | 48 (16.7) |
| T category upon N-RCT | |
| ypT0 | 52 (18.1) |
| ypT1 | 32 (11.2) |
| ypT2 | 71 (24.7) |
| ypT3 | 123 (42.9) |
| ypT4 | 9 (3.1) |
| N category upon N-RCT | |
| ypN0 | 203 (70.7) |
| ypN1 | 61 (21.3) |
| ypN2 | 23 (8.0) |
| Postoperative chemotherapy | 214 (74.6) |
| Variable | FLR | FDM | CSS | OS | ||||
|---|---|---|---|---|---|---|---|---|
| Hazard Ratio (95% CI) | p Value | Hazard Ratio (95% CI) | p Value | Hazard Ratio (95% CI) | p Value | Hazard Ratio (95% CI) | p Value | |
| Age (per year) | 0.98 (0.93–1.03) | 0.36 | 1.00 (0.98–1.03) | 0.96 | 1.01 (0.97–1.04) | 0.78 | 1.03 (1.00–1.06) | 0.02 |
| Sex Female (95) vs. male (192) | 1.12 (0.38–3.28) | 0.84 | 0.76 (0.42–1.38) | 0.37 | 1.32 (0.63–2.78) | 0.46 | 1.07 (0.60–1.91) | 0.81 |
| T category T4 (25) vs. T2-T3 (262) | 0.70 (0.09–5.34) | 0.73 | 0.35 (0.09–1.44) | 0.15 | 0.67 (0.16–2.83) | 0.59 | 0.96 (0.38–2.42) | 0.94 |
| N category N+ (227) vs. N0 (60) | 1.23 (0.49–3.05) | 0.66 | 0.74 (0.46–1.22) | 0.24 | 0.70 (0.35–1.39) | 0.31 | 0.74 (0.44–1.23) | 0.24 |
| Radiation technique 1 VMAT (119) vs. 3D-RT (157) | 0.24 (0.05–1.07) | 0.06 | 0.89 (0.52–1.53) | 0.67 | 0.82 (0.38–1.77) | 0.61 | 0.62 (0.33–1.15) | 0.13 |
| N-RCT with oxaliplatin Yes (103) vs. no (184) | 0.66 (0.21–2.08) | 0.48 | 0.78 (0.44–1.37) | 0.38 | 1.06 (0.50–2.22) | 0.88 | 1.39 (0.81–2.38) | 0.23 |
| pCR Yes (48) vs. no (239) | 0.69 (0.16–3.04) | 0.62 | 0.26 (0.81–0.83) | 0.02 | 0.16 (0.02–1.18) | 0.07 | 0.09 (0.01–0.64) | 0.02 |
| ypN+ Yes (84) vs. no (203) | 1.85 (0.66–5.19) | 0.25 | 3.09 (1.83–5.23) | <0.001 | 3.47 (1.69–7.15) | <0.001 | 2.48 (1.46–4.24) | <0.001 |
| Adjuvant chemotherapy Yes (214) vs. no (73) | 1.17 (0.33–4.17 | 0.81 | 0.99 (0.53–1.85) | 0.98 | 0.72 (0.31–1.63) | 0.42 | 0.66 (0.36–1.21) | 0.18 |
| OGG1 rs1052133 GG + GC (105) vs. CC (182) | 2.77 (0.99–7.79) | 0.05 | 1.72 (1.02–2.91) | 0.04 | 3.64 (1.70–7.78) | <0.001 | 2.12 (1.24–3.63) | 0.006 |
| Variable | FLR | FDM | CSS | OS | ||||
|---|---|---|---|---|---|---|---|---|
| Hazard Ratio (95% CI) | p Value | Hazard Ratio (95% CI) | p Value | Hazard Ratio (95% CI) | p Value | Hazard Ratio (95% CI) | p Value | |
| Age (per year) | 0.98 (0.94–1.03) | 0.43 | 1.00 (0.98–1.03) | 0.88 | 1.01 (0.98–1.04) | 0.67 | 1.03 (1.01–1.06) | 0.02 |
| pCR 2 Yes (48) vs. no (239) | 0.76 (0.16–3.69) | 0.73 | 0.37 (0.11–1.24) | 0.11 | 0.22 (0.03–1.73) | 0.15 | 0.11 (0.01–0.77) | 0.03 |
| ypN+ 3 Yes (84) vs. no (203) | 1.58 (0.53–4.76) | 0.41 | 2.51 (1.46–4.32) | <0.001 | 2.41 (1.14–5.07) | 0.02 | 1.71 (0.99–2.94) | 0.06 |
| OGG1 rs1052133 GG + GC (105) vs. CC (182) | 2.63 (0.92–7.48) | 0.07 | 1.64 (0.97–2.78) | 0.07 | 3.41 (1.58–7.34) | 0.002 | 2.11 (1.23–3.62) | 0.007 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leu, M.; Riebeling, T.; Dröge, L.H.; Hubert, L.; Guhlich, M.; Wolff, H.A.; Brockmöller, J.; Gaedcke, J.; Rieken, S.; Schirmer, M.A. 8-Oxoguanine DNA Glycosylase (OGG1) Cys326 Variant: Increased Risk for Worse Outcome of Patients with Locally Advanced Rectal Cancer after Multimodal Therapy. Cancers 2021, 13, 2805. https://doi.org/10.3390/cancers13112805
Leu M, Riebeling T, Dröge LH, Hubert L, Guhlich M, Wolff HA, Brockmöller J, Gaedcke J, Rieken S, Schirmer MA. 8-Oxoguanine DNA Glycosylase (OGG1) Cys326 Variant: Increased Risk for Worse Outcome of Patients with Locally Advanced Rectal Cancer after Multimodal Therapy. Cancers. 2021; 13(11):2805. https://doi.org/10.3390/cancers13112805
Chicago/Turabian StyleLeu, Martin, Theresa Riebeling, Leif Hendrik Dröge, Laura Hubert, Manuel Guhlich, Hendrik Andreas Wolff, Jürgen Brockmöller, Jochen Gaedcke, Stefan Rieken, and Markus Anton Schirmer. 2021. "8-Oxoguanine DNA Glycosylase (OGG1) Cys326 Variant: Increased Risk for Worse Outcome of Patients with Locally Advanced Rectal Cancer after Multimodal Therapy" Cancers 13, no. 11: 2805. https://doi.org/10.3390/cancers13112805