Mutation of the Cell Cycle Regulator p27kip1 Drives Pseudohypoxic Pheochromocytoma Development
Abstract
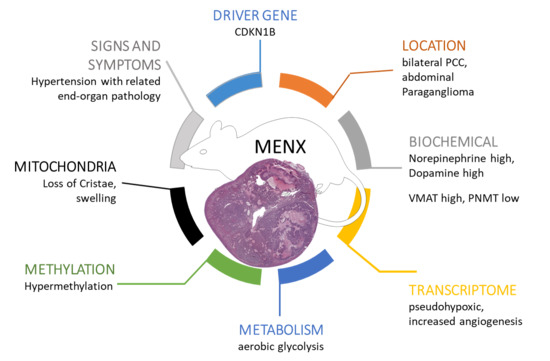
:Simple Summary
Abstract
1. Introduction
2. Results
2.1. Catecholamine Secretion Profile
2.2. Transcriptome Analysis
2.3. TCA Cycle Metabolomics
2.4. Methylation
2.5. Tumor Neoangiogenesis
2.6. Histopathology
2.7. Mitochondria Morphology
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Preparation of Tissue
4.3. Mouse Xenografts
4.4. Multispectral Optoacoustic Tomography (MSOT)
4.5. Tissue Embedding and Histology
4.6. Light-Sheed Fluorescent Microscopy
4.7. Clearing
4.8. Histology after LSFM for Co-Localization
4.9. Immunohistochemistry
4.10. Metabolomics
4.11. Catecholamines
4.12. Quantitative Real-Time PCR (qRT-PCR) Analysis
4.13. RNA-Seq Analysis
4.14. Consensus Clustering
4.15. Transmission Electron Microscopy (EM)
4.16. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 2-HG | 2-hydroxyglutarate |
| HYP | hyperplasia |
| IHC | immunohistochemistry |
| EPI | epinephrine |
| LSFM | light sheet fluorescence microscopy |
| MSOT | multispectral optoacoustic tomography |
| NE | norepinephrine |
| OXPHOS | oxidative phosphorylation |
| PCC | pheochromocytoma |
| PGL | paraganglioma |
| PPGL | pheochromocyomat and paraganglioma |
| p-PPGL | pseudohypoxic pheochromocytoma and paraganglioma |
| WT | wildtype |
References
- Eisenhofer, G.; Bornstein, S.R.; Brouwers, F.M.; Cheung, N.K.; Dahia, P.L.; de Krijger, R.R.; Giordano, T.J.; Greene, L.A.; Goldstein, D.S.; Lehnert, H.; et al. Malignant pheochromocytoma: Current status and initiatives for future progress. Endocr. Relat. Cancer 2004, 11, 423–436. [Google Scholar] [CrossRef] [PubMed]
- Daly, A.F.; Castermans, E.; Oudijk, L.; Guitelman, M.A.; Beckers, P.; Potorac, I.; Neggers, S.J.C.M.M.; Sacre, N.; Van Der Lely, A.-J.; Bours, V.; et al. Pheochromocytomas and pituitary adenomas in three patients with MAX exon deletions. Endocr. Relat. Cancer 2018, 25, L37–L42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fishbein, L.; Leshchiner, I.; Walter, V.; Danilova, L.; Robertson, A.G.; Johnson, A.R.; Lichtenberg, T.M.; Murray, B.A.; Ghayee, H.K.; Else, T.; et al. Comprehensive Molecular Characterization of Pheochromocytoma and Paraganglioma. Cancer Cell 2017, 31, 181–193. [Google Scholar] [CrossRef] [PubMed]
- Fishbein, L.; Ben-Maimon, S.; Keefe, S.; Cengel, K.; Pryma, D.A.; Loaiza-Bonilla, A.; Fraker, D.L.; Nathanson, K.L.; Cohen, D.L. SDHB mutation carriers with malignant pheochromocytoma respond better to CVD. Endocr. Relat. Cancer 2017, 24, L51–L55. [Google Scholar] [CrossRef]
- Lussey-Lepoutre, C.; Buffet, A.; Morin, A.; Goncalves, J.; Favier, J. Rodent models of pheochromocytoma, parallels in rodent and human tumorigenesis. Cell Tissue Res. 2018, 372, 379–392. [Google Scholar] [CrossRef]
- Powers, J.F.; Cochran, B.; Baleja, J.D.; Sikes, H.D.; Pattison, A.D.; Zhang, X.; Lomakin, I.; Shepard-Barry, A.; Pacak, K.; Moon, S.J.; et al. A xenograft and cell line model of SDH-deficient pheochromocytoma derived from Sdhb+/- rats. Endocr. Relat. Cancer 2020, 27, 337–354. [Google Scholar] [CrossRef]
- Fritz, A.; Walch, A.; Piotrowska, K.; Rosemann, M.; Schäffer, E.; Weber, K.; Timper, A.; Wildner, G.; Graw, J.; Höfler, H.; et al. Recessive transmission of a multiple endocrine neoplasia syndrome in the rat. Cancer Res. 2002, 62, 3048–3051. [Google Scholar]
- Wiedemann, T.; Peitzsch, M.; Qin, N.; Neff, F.; Ehrhart-Bornstein, M.; Eisenhofer, G.; Pellegata, N.S. Morphology, Biochemistry, and Pathophysiology of MENX-Related Pheochromocytoma Recapitulate the Clinical Features. Endocrinology 2016, 157, 3157–3166. [Google Scholar] [CrossRef] [Green Version]
- Eisenhofer, G.; Huynh, T.-T.; Elkahloun, A.; Morris, J.C.; Bratslavsky, G.; Linehan, W.M.; Zhuang, Z.; Balgley, B.M.; Lee, C.S.; Mannelli, M.; et al. Differential expression of the regulated catecholamine secretory pathway in different hereditary forms of pheochromocytoma. Am. J. Physiol. Metab. 2008, 295, E1223–E1233. [Google Scholar] [CrossRef] [Green Version]
- Molatore, S.; Kiermaier, E.; Jung, C.B.; Lee, M.; Pulz, E.; Höfler, H.; Atkinson, M.J.; Pellegata, N.S. Characterization of a naturally-occurring p27 mutation predisposing to multiple endocrine tumors. Mol. Cancer 2010, 9, 116. [Google Scholar] [CrossRef] [Green Version]
- Flynn, A.; Dwight, T.; Harris, J.; Benn, D.; Zhou, L.; Hogg, A.; Catchpoole, D.; James, P.; Duncan, E.L.; Trainer, A.; et al. Pheo-Type: A Diagnostic Gene-expression Assay for the Classification of Pheochromocytoma and Paraganglioma. J. Clin. Endocrinol. Metab. 2016, 101, 1034–1043. [Google Scholar] [CrossRef] [PubMed]
- Fishbein, L.; Wilkerson, M.D. Chromaffin cell biology: Inferences from The Cancer Genome Atlas. Cell Tissue Res. 2018, 372, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Burnichon, N.; Vescovo, L.; Amar, L.; Libé, R.; De Reynies, A.; Venisse, A.; Jouanno, E.; Laurendeau, I.; Parfait, B.; Bertherat, J.; et al. Integrative genomic analysis reveals somatic mutations in pheochromocytoma and paraganglioma. Hum. Mol. Genet. 2011, 20, 3974–3985. [Google Scholar] [CrossRef] [PubMed]
- Fliedner, S.M.; Shankavaram, U.; Marzouca, G.; Elkahloun, A.; Jochmanova, I.; Daerr, R.; Linehan, W.M.; Timmers, H.; Tischler, A.S.; Papaspyrou, K.; et al. Hypoxia-Inducible Factor 2α Mutation-Related Paragangliomas Classify as Discrete Pseudohypoxic Subcluster. Neoplasia 2016, 18, 567–576. [Google Scholar] [CrossRef] [Green Version]
- Morin, A.; Letouzé, E.; Gimenez-Roqueplo, A.-P.; Favier, J. Oncometabolites-driven tumorigenesis: From genetics to targeted therapy. Int. J. Cancer 2014, 135, 2237–2248. [Google Scholar] [CrossRef]
- Shenoy, N.; Bhagat, T.D.; Cheville, J.C.; Lohse, C.; Bhattacharyya, S.; Tischer, A.; Machha, V.; Gordon-Mitchell, S.; Choudhary, G.S.; Wong, L.-F.; et al. Ascorbic acid–induced TET activation mitigates adverse hydroxymethylcytosine loss in renal cell carcinoma. J. Clin. Investig. 2019, 129, 1612–1625. [Google Scholar] [CrossRef] [Green Version]
- Xiao, M.; Yang, H.; Xu, W.; Ma, S.; Lin, H.; Zhu, H.; Liu, L.; Liu, Y.; Yang, C.; Xu, Y.; et al. Inhibition of -KG-dependent histone and DNA demethylases by fumarate and succinate that are accumulated in mutations of FH and SDH tumor suppressors. Genes Dev. 2012, 26, 1326–1338. [Google Scholar] [CrossRef] [Green Version]
- Fernandez, A.F.; Bayon, G.F.; Sierra, M.I.; Urdinguio, R.G.; Toraño, E.G.; García, M.G.; Carella, A.; López, V.; Santamarina, P.; Pérez, R.F.; et al. Loss of 5hmC identifies a new type of aberrant DNA hypermethylation in glioma. Hum. Mol. Genet. 2018, 27, 3046–3059. [Google Scholar] [CrossRef]
- Letouzé, E.; Martinelli, C.; Loriot, C.; Burnichon, N.; Abermil, N.; Ottolenghi, C.; Janin, M.; Menara, M.; Nguyen, A.T.; Bénit, P.; et al. SDH Mutations Establish a Hypermethylator Phenotype in Paraganglioma. Cancer Cell 2013, 23, 739–752. [Google Scholar] [CrossRef] [Green Version]
- Favier, J.; Igaz, P.; Burnichon, N.; Amar, L.; Libé, R.; Badoual, C.; Tissier, F.; Bertherat, J.; Plouin, P.-F.; Jeunemaitre, X.; et al. Rationale for Anti-angiogenic Therapy in Pheochromocytoma and Paraganglioma. Endocr. Pathol. 2011, 23, 34–42. [Google Scholar] [CrossRef]
- Diot, G.; MetzAmbros, S.; Noske, A.; Liapis, E.; Schroeder, B.; Ovsepian, S.V.; Meier, R.; Rummeny, E.; Ntziachristos, V. Multispectral Optoacoustic Tomography (MSOT) of Human Breast Cancer. Clin. Cancer Res. 2017, 23, 6912–6922. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thompson LD: Pheochromocytoma of the Adrenal gland Scaled Score (PASS) to separate benign from malignant neoplasms: A clinicopathologic and immunophenotypic study of 100 cases. Am. J. Surg. Pathol. 2002, 26, 551–566. [CrossRef] [PubMed]
- Kimura, N.; Takayanagi, R.; Takizawa, N.; Itagaki, E.; Katabami, T.; Kakoi, N.; Rakugi, H.; Ikeda, Y.; Tanabe, A.; Nigawara, T.; et al. Pathological grading for predicting metastasis in phaeochromocytoma and paraganglioma. Endocr. Relat. Cancer 2014, 21, 405–414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Molatore, S.; Liyanarachchi, S.; Irmler, M.; Perren, A.; Mannelli, M.; Ercolino, T.; Beuschlein, F.; Jarzab, B.; Wloch, J.; Ziaja, J.; et al. Pheochromocytoma in rats with multiple endocrine neoplasia (MENX) shares gene expression patterns with human pheochromocytoma. Proc. Natl. Acad. Sci. USA 2010, 107, 18493–18498. [Google Scholar] [CrossRef] [Green Version]
- Mannelli, M.; Rapizzi, E.; Fucci, R.; Canu, L.; Ercolino, T.; Luconi, M.; Young, W.F. 15 YEARS OF PARAGANGLIOMA: Metabolism and pheochromocytoma/paraganglioma. Endocr. Relat. Cancer 2015, 22, T83–T90. [Google Scholar] [CrossRef] [Green Version]
- Coupland, R.E. Electron Microscopic Observations on the Structure of the Rat Adrenal Medulla. I. The Ultrastructure and Organization of Chromaffin Cells in the Normal Adrenal Medulla. J. Anat. 1965, 99, 231–254. [Google Scholar]
- Pellegata, N.S.; Quintanilla-Martinez, L.; Siggelkow, H.; Samson, E.; Bink, K.; Höfler, H.; Fend, F.; Graw, J.; Atkinson, M.J. Germ-line mutations in p27Kip1 cause a multiple endocrine neoplasia syndrome in rats and humans. Proc. Natl. Acad. Sci. USA 2006, 103, 15558–15563. [Google Scholar] [CrossRef] [Green Version]
- Agarwal, S.K.; Mateo, C.M.; Marx, S.J. Rare Germline Mutations in Cyclin-Dependent Kinase Inhibitor Genes in Multiple Endocrine Neoplasia Type 1 and Related States. J. Clin. Endocrinol. Metab. 2009, 94, 1826–1834. [Google Scholar] [CrossRef] [Green Version]
- Pellegata, N.S.; Quintanilla-Martinez, L.; Keller, G.; Liyanarachchi, S.; Höfler, H.; Atkinson, M.J.; Fend, F. Human pheochromocytomas show reduced p27Kip1 expression that is not associated with somatic gene mutations and rarely with deletions. Virchows Arch. 2007, 451, 37–46. [Google Scholar] [CrossRef]
- Gameiro, P.A.; Yang, J.; Metelo, A.M.; Pérez-Carro, R.; Baker, R.; Wang, Z.; Arreola, A.; Rathmell, W.K.; Olumi, A.; López-Larrubia, P.; et al. In Vivo HIF-Mediated Reductive Carboxylation Is Regulated by Citrate Levels and Sensitizes VHL-Deficient Cells to Glutamine Deprivation. Cell Metab. 2013, 17, 372–385. [Google Scholar] [CrossRef] [Green Version]
- Jochmanova, I.; Zhuang, Z.; Pacak, K. Pheochromocytoma: Gasping for Air. Horm. Cancer 2015, 6, 191–205. [Google Scholar] [CrossRef] [PubMed]
- Cogliati, S.; Enriquez, J.A.; Scorrano, L. Mitochondrial Cristae: Where Beauty Meets Functionality. Trends Biochem. Sci. 2016, 41, 261–273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lian, C.G.; Xu, Y.; Ceol, C.; Wu, F.; Larson, A.; Dresser, K.; Xu, W.; Tan, L.; Hu, Y.; Zhan, Q.; et al. Loss of 5-Hydroxymethylcytosine Is an Epigenetic Hallmark of Melanoma. Cell 2012, 150, 1135–1146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loriot, C.; Domingues, M.; Berger, A.; Menara, M.; Ruel, M.; Morin, A.; Castro-Vega, L.-J.; Letouzé, E.; Martinelli, C.; Bemelmans, A.-P.; et al. Deciphering the molecular basis of invasiveness in Sdhb-deficient cells. Oncotarget 2015, 6, 32955–32965. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morin, A.; Goncalves, J.; Moog, S.; Castro-Vega, L.-J.; Job, S.; Buffet, A.; Fontenille, M.-J.; Woszczyk, J.; Gimenez-Roqueplo, A.-P.; Letouzé, E.; et al. TET-Mediated Hypermethylation Primes SDH-Deficient Cells for HIF2α-Driven Mesenchymal Transition. Cell Rep. 2020, 30, 4551–4566.e7. [Google Scholar] [CrossRef] [PubMed]
- Tischler, A.S.; Powers, J.; Pignatello, M.; Tsokas, P.; Downing, J.C.; McClain, R.M. Vitamin D3-induced proliferative lesions in the rat adrenal medulla. Toxicol. Sci. 1999, 51, 9–18. [Google Scholar] [CrossRef] [Green Version]
- Carmeliet, P.; Jain, R.K. Principles and mechanisms of vessel normalization for cancer and other angiogenic diseases. Nat. Rev. Drug Discov. 2011, 10, 417–427. [Google Scholar] [CrossRef]
- Ertürk, A.; Becker, K.; Jährling, N.; Mauch, C.P.; Hojer, C.D.; Egen, J.G.; Hellal, F.; Bradke, F.; Sheng, M.; Dodt, H.-U. Three-dimensional imaging of solvent-cleared organs using 3DISCO. Nat. Protoc. 2012, 7, 1983–1995. [Google Scholar] [CrossRef]
- Salazar-Roa, M.; Malumbres, M. Fueling the Cell Division Cycle. Trends Cell Biol. 2017, 27, 69–81. [Google Scholar] [CrossRef]
- Pacak, K. Phaeochromocytoma: A catecholamine and oxidative stress disorder. Endocr. Regul. 2011, 45, 65–90. [Google Scholar] [CrossRef]
- Burhans, W.C.; Heintz, N.H. The cell cycle is a redox cycle: Linking phase-specific targets to cell fate. Free Radic. Biol. Med. 2009, 47, 1282–1293. [Google Scholar] [CrossRef] [PubMed]
- Owusu-Ansah, E.; Yavari, A.; Mandal, S.; Banerjee, U. Distinct mitochondrial retrograde signals control the G1-S cell cycle checkpoint. Nat. Genet. 2008, 40, 356–361. [Google Scholar] [CrossRef] [PubMed]
- Lepoutre-Lussey, C.; Thibault, C.; Buffet, A.; Morin, A.; Badoual, C.; Bénit, P.; Rustin, P.; Ottolenghi, C.; Janin, M.; Castro-Vega, L.J.; et al. From Nf1 to Sdhb knockout: Successes and failures in the quest for animal models of pheochromocytoma. Mol. Cell. Endocrinol. 2016, 421, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Hill, H.; Christie, A.; Kim, M.S.; Holloman, E.; Pavia-Jimenez, A.; Homayoun, F.; Ma, Y.; Patel, N.; Yell, P.; et al. Targeting renal cell carcinoma with a HIF-2 antagonist. Nat. Cell Biol. 2016, 539, 112–117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dima, A.; Burton, N.C.; Ntziachristos, V. Multispectral optoacoustic tomography at 64, 128, and 256 channels. J. Biomed. Opt. 2014, 19, 036021. [Google Scholar] [CrossRef] [Green Version]
- Dean-Ben, X.L.; Ntziachristos, V.; Razansky, D. Acceleration of Optoacoustic Model-Based Reconstruction Using Angular Image Discretization. IEEE Trans. Med. Imaging 2012, 31, 1154–1162. [Google Scholar] [CrossRef]
- Neuschmelting, V.; Kim, K.; Malekzadeh-Najafabadi, J.; Jebiwott, S.; Prakash, J.; Scherz, A.; Coleman, J.A.; Kircher, M.F.; Ntziachristos, V. WST11 Vascular Targeted Photodynamic Therapy Effect Monitoring by Multispectral Optoacoustic Tomography (MSOT) in Mice. Theranostics 2018, 8, 723–734. [Google Scholar] [CrossRef]
- Eisenhofer, G. Analytical differences between the determination of plasma catecholamines by liquid chromatography with electrochemical detection and by radioenzymatic assay. J. Chromatogr. B Biomed. Sci. Appl. 1986, 377, 328–333. [Google Scholar] [CrossRef]
- Eisenhofer, G.; Goldstein, D.S.; Stull, R.; Keiser, H.R.; Sunderland, T.; Murphy, D.L.; Kopin, I.J. Simultaneous liquid-chromatographic determination of 3,4-dihydroxyphenylglycol, catecholamines, and 3,4-dihydroxyphenylalanine in plasma, and their responses to inhibition of monoamine oxidase. Clin. Chem. 1986, 32, 2030–2033. [Google Scholar] [CrossRef]
- Colaprico, A.; Silva, T.C.; Olsen, C.; Garofano, L.; Cava, C.; Garolini, D.; Sabedot, T.S.; Malta, T.M.; Pagnotta, S.M.; Castiglioni, I.; et al. TCGAbiolinks: An R/Bioconductor package for integrative analysis of TCGA data. Nucleic Acids Res. 2016, 44, e71. [Google Scholar] [CrossRef]
- Abrams, Z.B.; Johnson, T.S.; Huang, K.; Payne, P.R.O.; Coombes, K. A protocol to evaluate RNA sequencing normalization methods. BMC Bioinform. 2019, 20, 679. [Google Scholar]
- Metsalu, T.; Vilo, J. ClustVis: A web tool for visualizing clustering of multivariate data using Principal Component Analysis and heatmap. Nucleic Acids Res. 2015, 43, W566–W570. [Google Scholar] [CrossRef] [PubMed]
- Glytsou, C.; Calvo, E.; Cogliati, S.; Mehrotra, A.; Anastasia, I.; Rigoni, G.; Raimondi, A.; Shintani, N.; Loureiro, M.; Vazquez, J.; et al. Optic Atrophy 1 Is Epistatic to the Core MICOS Component MIC60 in Mitochondrial Cristae Shape Control. Cell Rep. 2016, 17, 3024–3034. [Google Scholar] [CrossRef] [PubMed] [Green Version]






| MENX (Total = 31) | |||
|---|---|---|---|
| Diagnosis | 1–2 mo (n = 4) | 4–5 mo (n = 4) | >7 mo (n = 23) |
| Hyperplasia | 4/4 | 3/4 | 1/23 |
| Early PCC | 1/4 | 3/23 | |
| PCC | 19/23 | ||
| Major findings | |||
| Architecture: | |||
| regular, nests can get wider, focal loss of reticulin network | 4/4 | 3/4 | 23/23 |
| enlarged irregular nests, diffuse, small nodule formation and loss of reticulin network | 1/4 | 13/23 | |
| enlarged irregular nests with distinct large nodule formation and loss of reticulin network | 9/23 | ||
| Pleomorphism | |||
| grade 1 | 2/4 | 22/23 | |
| grade 2 | 1/23 | ||
| Increased cellularity | 4/4 | 4/4 | 23/23 |
| Pyknoses | 2/4 | 2/4 | 21/23 |
| Mitosis, atypical mitosis | 2/4 | 14/23 | |
| Small comedonecrosis and/or fatty degeneration | 12/23 | ||
| Spindle cell architecture (>10% of tumor volume) | 2/23 | ||
| Invasion through the capsule into periadrenal adipose tissue | 3/23 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohr, H.; Ballke, S.; Bechmann, N.; Gulde, S.; Malekzadeh-Najafabadi, J.; Peitzsch, M.; Ntziachristos, V.; Steiger, K.; Wiedemann, T.; Pellegata, N.S. Mutation of the Cell Cycle Regulator p27kip1 Drives Pseudohypoxic Pheochromocytoma Development. Cancers 2021, 13, 126. https://doi.org/10.3390/cancers13010126
Mohr H, Ballke S, Bechmann N, Gulde S, Malekzadeh-Najafabadi J, Peitzsch M, Ntziachristos V, Steiger K, Wiedemann T, Pellegata NS. Mutation of the Cell Cycle Regulator p27kip1 Drives Pseudohypoxic Pheochromocytoma Development. Cancers. 2021; 13(1):126. https://doi.org/10.3390/cancers13010126
Chicago/Turabian StyleMohr, Hermine, Simone Ballke, Nicole Bechmann, Sebastian Gulde, Jaber Malekzadeh-Najafabadi, Mirko Peitzsch, Vasilis Ntziachristos, Katja Steiger, Tobias Wiedemann, and Natalia S. Pellegata. 2021. "Mutation of the Cell Cycle Regulator p27kip1 Drives Pseudohypoxic Pheochromocytoma Development" Cancers 13, no. 1: 126. https://doi.org/10.3390/cancers13010126