Evaluation of the Role of ITGBL1 in Ovarian Cancer
Abstract
:Simple Summary
Abstract
1. Introduction
2. Results
2.1. ITGBL1 mRNA Expression in Ovarian Cancer Tissues and Cell Lines
2.2. Production of ITGBL1-Overexpressing Cell Lines
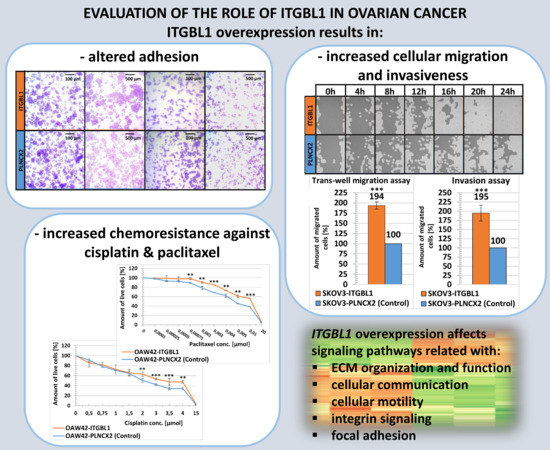
2.3. ITGBL1 Overexpression Results in Altered Adhesiveness of Ovarian Cancer Cells
2.4. ITGBL1 Overexpression Promotes Ovarian Cancer Cell Migration and Invasion Rate
2.5. ITGBL1 Has No Effect on the Proliferation Rate of Ovarian Cancer Cells
2.6. ITGBL1 Overexpression Results in Increased Chemoresistance of Ovarian Cancer Cells
2.7. Gene Expression Profiling and Signaling Pathways Related to ITGBL1
3. Discussion
3.1. Cellular Adhesion, Migration, and Invasion
3.2. Signaling Pathways and ITGBL1 Co-Expressed Genes
3.3. Technical Constraints
3.4. ITGBL1 Function
4. Materials and Methods
4.1. Cell Culture and Experimental Conditions
4.2. Total RNA Extraction and Reverse Transcription
4.3. ITGBL1 CDS Cloning and Sequencing
4.4. Generation of Stably ITGBL1-Overexpressing Cell Lines
4.5. Protein Extraction and Western Blot Analysis
4.6. The primer Design and Semi-Quantitative PCR
4.7. Scratch Assay
4.8. Transwell Migration Assay
4.9. Matrigel Transwell Invasion Assay
4.10. In Vitro Cytotoxicity Assay
4.11. Cell Proliferation Assay
4.12. Cell Attachment Assay
4.13. Cell Spreading Assay
4.14. Analysis of the Cell-Cycle Distribution
4.15. RNA Preparation for Microarrays, Hybridization and Analysis
4.16. Microarray Data Analysis
4.16.1. Microarray Preprocessing
4.16.2. Differential Expression Analysis
4.16.3. Principal Component Analysis (PCA)
4.16.4. Gene Set Enrichment Analysis
4.17. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lisio, M.-A.; Fu, L.; Goyeneche, A.; Gao, Z.-H.; Telleria, C.M. High-Grade Serous Ovarian Cancer: Basic Sciences, Clinical and Therapeutic Standpoints. Int. J. Mol. Sci. 2019, 20, 952. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kujawa, K.A.; Lisowska, K.M. Ovarian cancer—from biology to clinic. Postep. Hig. Med. Dosw. 2015, 69, 1275–1290. [Google Scholar] [CrossRef] [PubMed]
- Lisowska, K.M.; Olbryt, M.; Dudaladava, V.; Pamuła-Piłat, J.; Kujawa, K.A.; Grzybowska, E.; Jarzab, M.; Student, S.; Rzepecka, I.K.; Jarzab, B.; et al. Gene Expression Analysis in Ovarian Cancer – Faults and Hints from DNA Microarray Study. Front. Oncol. 2014, 4, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lisowska, K.M.; Olbryt, M.; Student, S.; Kujawa, K.A.; Cortez, A.J.; Simek, K.; Dansonka-Mieszkowska, A.; Rzepecka, I.K.; Tudrej, P.; Kupryjańczyk, J. Unsupervised analysis reveals two molecular subgroups of serous ovarian cancer with distinct gene expression profiles and survival. J. Cancer Res. Clin. Oncol. 2016, 142, 1239–1252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Győrffy, B.; Lánczky, A.; Szállási, Z. Implementing an online tool for genome-wide validation of survival-associated biomarkers in ovarian-cancer using microarray data from 1287 patients. Endocr.-Relat. Cancer 2012, 19, 197–208. [Google Scholar] [CrossRef] [Green Version]
- Tan, T.Z.; Yang, H.; Ye, J.; Low, J.; Choolani, M.; Tan, D.S.P.; Thiery, J.-P.; Huang, R.Y.-J. CSIOVDB: A microarray gene expression database of epithelial ovarian cancer subtype. Oncotarget 2015, 6, 43843–43852. [Google Scholar] [CrossRef] [Green Version]
- Berg, R.W.; Leung, E.; Gough, S.; Morris, C.; Yao, W.-P.; Wang, S.-X.; Ni, J.; Krissansen, G.W. Cloning and Characterization of a Novel β Integrin-Related cDNA Coding for the Protein TIED (“Ten β Integrin EGF-like Repeat Domains”) That Maps to Chromosome Band 13q33: A Divergent Stand-Alone Integrin Stalk Structure. Genomics 1999, 56, 169–178. [Google Scholar] [CrossRef]
- Takagi, J.; Beglova, N.; Yalamanchili, P.; Blacklow, S.C.; Springer, T.A. Definition of EGF-like, closely interacting modules that bear activation epitopes in integrin subunits. Proc. Natl. Acad. Sci. USA 2001, 98, 11175–11180. [Google Scholar] [CrossRef] [Green Version]
- Lin, H.-H.; Stacey, M.; Saxby, C.; Knott, V.; Chaudhry, Y.; Evans, D.J.; Gordon, S.; McKnight, A.J.; Handford, P.; Lea, S.M. Molecular Analysis of the Epidermal Growth Factor-like Short Consensus Repeat Domain-mediated Protein-Protein Interactions. J. Biol. Chem. 2001, 276, 24160–24169. [Google Scholar] [CrossRef] [Green Version]
- Appella, E.; Weber, I.T.; Blasi, F. Structure and function of epidermal growth factor-like regions in proteins. FEBS Lett. 1988, 231, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Davis, C.G. The many faces of epidermal growth factor repeats. New Biol. 1990, 2, 410–419. [Google Scholar] [PubMed]
- Kujawa, K.A.; Cortez, A.J.; Maciola, A.; Olbryt, M.; Kujawa, T.; Lisowska, K. Itgbl1 over expression stimulates ovarian cancer cell migration rate. Eur. J. Cancer 2013, 49, S740. [Google Scholar]
- Li, X.-Q.; Du, X.; Li, D.-M.; Kong, P.; Sun, Y.; Liu, P.-F.; Wang, Q.-S.; Feng, Y.-M. ITGBL1 Is a Runx2 Transcriptional Target and Promotes Breast Cancer Bone Metastasis by Activating the TGF Signaling Pathway. Cancer Res. 2015, 75, 3302–3313. [Google Scholar] [CrossRef] [Green Version]
- Gan, X.; Liu, Z.; Tong, B.; Zhou, J. Epigenetic downregulated ITGBL1 promotes non-small cell lung cancer cell invasion through Wnt/PCP signaling. Tumor Biol. 2015, 37, 1663–1669. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Xu, P.; Meng, C.; Song, C.; Blackwell, T.S.; Li, R.; Li, H.; Zhang, J.; Lv, C. lncITPF Promotes Pulmonary Fibrosis by Targeting hnRNP-L Depending on Its Host Gene ITGBL1. Mol. Ther. 2019, 27, 380–393. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.; Gong, Q.; Zhang, J.; Chen, L.; Zhang, Z.; Lu, L.; Yu, D.; Han, Y.; Zhang, D.; Chen, P.; et al. Characterization of gene expression profiles in HBV-related liver fibrosis patients and identification of ITGBL1 as a key regulator of fibrogenesis. Sci. Rep. 2017, 7, 43446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, L.-G. Fbsbioscience.Org A 6 gene signature identifies the risk of developing cirrhosis in patients with chronic hepatitis B. Front. Biosci. 2016, 21, 479–486. [Google Scholar] [CrossRef] [Green Version]
- Song, E.K.; Jeon, J.; Gil Jang, D.; Kim, H.E.; Sim, H.J.; Kwon, K.Y.; Medina-Ruiz, S.; Jang, H.-J.; Lee, A.R.; Rho, J.G.; et al. ITGBL1 modulates integrin activity to promote cartilage formation and protect against arthritis. Sci. Transl. Med. 2018, 10, eaam7486. [Google Scholar] [CrossRef] [Green Version]
- Qi, L.; Song, F.; Ding, Y. Regulatory Mechanism of ITGBL1 in the Metastasis of Colorectal Cancer. Front. Oncol. 2020, 10, 10. [Google Scholar] [CrossRef]
- Ji, Q.; Zhou, L.; Sui, H.; Yang, L.; Wu, X.; Song, Q.; Jia, R.; Li, R.; Sun, J.; Wang, Z.; et al. Primary tumors release ITGBL1-rich extracellular vesicles to promote distal metastatic tumor growth through fibroblast-niche formation. Nat. Commun. 2020, 11, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Matsuyama, T.; Ishikawa, T.; Takahashi, N.; Yamada, Y.; Yasuno, M.; Kawano, T.; Uetake, H.; Goel, A. Transcriptomic expression profiling identifies ITGBL1, an epithelial to mesenchymal transition (EMT)-associated gene, is a promising recurrence prediction biomarker in colorectal cancer. Mol. Cancer 2019, 18, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, R.; Zhuang, C.; Jiang, S.; Du, N.; Zhao, W.; Tu, L.; Cao, H.; Zhang, Z.; Chen, X. ITGBL1 Predicts a Poor Prognosis and Correlates EMT Phenotype in Gastric Cancer. J. Cancer 2017, 8, 3764–3773. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Li, S.; Yang, J.; Cui, C.; Yu, M.; Zhang, Y. ITGBL1 promotes EMT, invasion and migration by activating NF-κB signaling pathway in prostate cancer. OncoTargets Ther. 2019, 12, 3753–3763. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, L.; Wang, D.; Li, X.; Zhang, L.; Zhang, H.; Zhang, Y. Extracellular matrix protein ITGBL1 promotes ovarian cancer cell migration and adhesion through Wnt/PCP signaling and FAK/SRC pathway. Biomed. Pharmacother. 2016, 81, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Yang, P.; Lu, J. Upregulation of ITGBL1 predicts poor prognosis and promotes chemoresistance in ovarian cancer. Cancer Biomarkers 2020, 27, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Lian, X.-Y.; Ma, J.-C.; Zhou, J.-D.; Zhang, T.-J.; Wu, D.-H.; Deng, Z.-Q.; Zhang, Z.-H.; Li, X.-X.; He, P.-F.; Yan, Y.; et al. Hypermethylation of ITGBL1 is associated with poor prognosis in acute myeloid leukemia. J. Cell. Physiol. 2018, 234, 9438–9446. [Google Scholar] [CrossRef]
- Tudrej, P.; Kujawa, K.A.; Cortez, A.J.; Lisowska, K.M. Characteristics of in vitro model systems for ovarian cancer studies. Oncol. Clin. Pr. 2019, 15, 246–259. [Google Scholar] [CrossRef]
- Khalili, A.A.; Ahmad, M.R. A Review of Cell Adhesion Studies for Biomedical and Biological Applications. Int. J. Mol. Sci. 2015, 16, 18149–18184. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Yan, C.; Zheng, Z. Functional polymer surfaces for controlling cell behaviors. Mater. Today 2018, 21, 38–59. [Google Scholar] [CrossRef]
- Humphries, M.J. Cell Adhesion Assays. Adv. Struct. Safety Stud. 2009, 522, 203–210. [Google Scholar] [CrossRef]
- Cortez, A.J.; Tudrej, P.; Kujawa, K.A.; Lisowska, K.M. Advances in ovarian cancer therapy. Cancer Chemother. Pharmacol. 2017, 81, 17–38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hong, S.; Ergezen, E.; Lec, R.; Barbee, K.A. Real-time analysis of cell–surface adhesive interactions using thickness shear mode resonator. Biomaterials 2006, 27, 5813–5820. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Yu, D.; Wang, M.; Han, Y.; Lin, J.; Wei, D.; Cai, J.; Li, B.; Chen, P.; Zhang, X.X. ITGBL1 promotes cell migration and invasion through stimulating the TGF-β signalling pathway in hepatocellular carcinoma. Cell Prolif. 2020, 53. [Google Scholar] [CrossRef] [PubMed]
- Qiu, X.; Feng, J.-R.; Qiu, J.; Liu, L.; Xie, Y.; Zhang, Y.-P.; Liu, J.; Zhao, Q.-Y. ITGBL1 promotes migration, invasion and predicts a poor prognosis in colorectal cancer. Biomed. Pharmacother. 2018, 104, 172–180. [Google Scholar] [CrossRef] [PubMed]
- Balduit, A.; Agostinis, C.; Mangogna, A.; Maggi, V.; Zito, G.; Romano, F.; Romano, A.; Ceccherini, R.; Grassi, G.; Bonin, S.; et al. The Extracellular Matrix Influences Ovarian Carcinoma Cells’ Sensitivity to Cisplatinum: A First Step towards Personalized Medicine. Cancers 2020, 12, 1175. [Google Scholar] [CrossRef] [PubMed]
- Cho, A.; Howell, V.M.; Colvin, E.K. The Extracellular Matrix in Epithelial Ovarian Cancer—A Piece of a Puzzle. Front. Oncol. 2015, 5. [Google Scholar] [CrossRef] [Green Version]
- Tudrej, P.; Olbryt, M.; Zembala-Nozynska, E.; Kujawa, K.A.; Cortez, A.J.; Fiszer-Kierzkowska, A.; Piglowski, W.; Nikiel, B.; Głowala-Kosińska, M.; Bartkowska-Chrobok, A.; et al. Establishment and Characterization of the Novel High-Grade Serous Ovarian Cancer Cell Line OVPA8. Int. J. Mol. Sci. 2018, 19, 2080. [Google Scholar] [CrossRef] [Green Version]
- Ritchie, M.E.; Phipson, B.; Wu, D.; Hu, Y.; Law, C.W.; Shi, W.; Smyth, G.K. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015, 43, e47. [Google Scholar] [CrossRef]
- Smyth, G.K. Linear Models and Empirical Bayes Methods for Assessing Differential Expression in Microarray Experiments. Stat. Appl. Genet. Mol. Biol. 2004, 3, 1–25. [Google Scholar] [CrossRef]
- Simek, K.; Fujarewicz, K.; Świerniak, A.; Kimmel, M.; Jarzab, B.; Wiench, M.; Rzeszowska-Wolny, J. Using SVD and SVM methods for selection, classification, clustering and modeling of DNA microarray data. Eng. Appl. Artif. Intell. 2004, 17, 417–427. [Google Scholar] [CrossRef]
- Wall, M.E.; Dyck, P.A.; Brettin, T.S. SVDMAN—singular value decomposition analysis of microarray data. Bioinformatics 2001, 17, 566–568. [Google Scholar] [CrossRef] [PubMed]
- Liberzon, A.; Subramanian, A.; Pinchback, R.; Thorvaldsdóttir, H.; Tamayo, P.; Mesirov, J.P. Molecular signatures database (MSigDB) 3.0. Bioinformatics 2011, 27, 1739–1740. [Google Scholar] [CrossRef] [PubMed]








| Cisplatin | Paclitaxel | ||
|---|---|---|---|
| Cell lines | IC50 [µM] | Cell lines | IC50 [µM] |
| OAW42-ITGBL1 | 4.256 ± 0.491 | OAW42-ITGBL1 | 0.025 ± 0.011 |
| OAW42-PLNCX2 | 2.950 ± 0.207 | OAW42-PLNCX2 | 0.008 ± 0.002 |
| SKOV3-ITGBL1 | 8.066 ± 0.171 | SKOV3-ITGBL1 | 0.201 ± 0.041 |
| SKOV3-PLNCX2 | 6.612 ± 0.188 | SKOV3-PLNCX2 | 0.114 ± 0.024 |
| Rank | Pathway | No of Genes in Pathway | No of Genes in Gene Set | p-Value |
|---|---|---|---|---|
| 1 | KEGG_ECM_RECEPTOR_INTERACTION | 84 | 8 | 3.59 × 106 |
| 4 | REACTOME_LAMININ_INTERACTIONS | 30 | 4 | 0.0003 |
| 5 | NABA_MATRISOME | 1026 | 24 | 0.0003 |
| 8 | PID_INTEGRIN1_PATHWAY | 66 | 5 | 0.0007 |
| 11 | NABA_ECM_GLYCOPROTEINS | 196 | 8 | 0.0014 |
| 12 | REACTOME_EXTRACELLULAR_MATRIX_ORGANIZATION | 301 | 10 | 0.0017 |
| 17 | PID_REELIN_PATHWAY | 28 | 3 | 0.0034 |
| 20 | REACTOME_MET_ACTIVATES_PTK2_SIGNALING | 30 | 3 | 0.0041 |
| 22 | NABA_ECM_REGULATORS | 238 | 8 | 0.0045 |
| 31 | KEGG_FOCAL_ADHESION | 199 | 7 | 0.0061 |
| 33 | NABA_MATRISOME_ASSOCIATED | 751 | 16 | 0.0079 |
| 35 | REACTOME_DISSOLUTION_OF_FIBRIN_CLOT | 13 | 2 | 0.0084 |
| 36 | REACTOME_MET_PROMOTES_CELL_MOTILITY | 41 | 3 | 0.0099 |
| 37 | NABA_CORE_MATRISOME | 275 | 8 | 0.0104 |
| 40 | PID_INTEGRIN3_PATHWAY | 43 | 3 | 0.0112 |
| 42 | REACTOME_INTEGRIN_CELL_SURFACE_INTERACTIONS | 85 | 4 | 0.0137 |
| 47 | REACTOME_DEGRADATION_OF_THE_EXTRACELLULAR_MATRIX | 140 | 5 | 0.0183 |
| 57 | REACTOME_NON_INTEGRIN_MEMBRANE_ECM_INTERACTIONS | 59 | 3 | 0.0261 |
| 60 | PID_INTEGRIN_A9B1_PATHWAY | 25 | 2 | 0.0297 |
| 61 | PID_INTEGRIN_CS_PATHWAY | 26 | 2 | 0.0319 |
| 75 | PID_INTEGRIN_A4B1_PATHWAY | 33 | 2 | 0.0494 |
| 76 | REACTOME_ADHERENS_JUNCTIONS_INTERACTIONS | 33 | 2 | 0.0494 |
| Rank | Pathway | No. of Genes in Pathway | No. of Genes in Gene Set | p-Value |
|---|---|---|---|---|
| 1 | NABA_MATRISOME | 1026 | 35 | 7.41 × 107 |
| 2 | REACTOME_EXTRACELLULAR_MATRIX_ORGANIZATION | 301 | 16 | 4.68 × 106 |
| 4 | REACTOME_CELL_JUNCTION_ORGANIZATION | 92 | 8 | 3.96 × 105 |
| 7 | PID_ERBB_NETWORK_PATHWAY | 15 | 4 | 4.23 × 105 |
| 9 | NABA_MATRISOME_ASSOCIATED | 751 | 25 | 4.58 × 105 |
| 16 | REACTOME_CELL_CELL_JUNCTION_ORGANIZATION | 65 | 6 | 0.0003 |
| 18 | REACTOME_PI3K_EVENTS_IN_ERBB4_SIGNALING | 10 | 3 | 0.0003 |
| 21 | REACTOME_CELL_CELL_COMMUNICATION | 130 | 8 | 0.0004 |
| 28 | REACTOME_ERBB2_ACTIVATES_PTK6_SIGNALING | 13 | 3 | 0.0007 |
| 29 | REACTOME_SHC1_EVENTS_IN_ERBB4_SIGNALING | 14 | 3 | 0.0008 |
| 31 | REACTOME_NUCLEAR_SIGNALING_BY_ERBB4 | 32 | 4 | 0.0009 |
| 32 | NABA_SECRETED_FACTORS | 343 | 13 | 0.0010 |
| 33 | REACTOME_ERBB2_REGULATES_CELL_MOTILITY | 15 | 3 | 0.0010 |
| 35 | REACTOME_ADHERENS_JUNCTIONS_INTERACTIONS | 33 | 4 | 0.0010 |
| 36 | REACTOME_GRB2_EVENTS_IN_ERBB2_SIGNALING | 16 | 3 | 0.0013 |
| 37 | REACTOME_PI3K_EVENTS_IN_ERBB2_SIGNALING | 16 | 3 | 0.0013 |
| 44 | PID_INTEGRIN1_PATHWAY | 66 | 5 | 0.0021 |
| 49 | REACTOME_SHC1_EVENTS_IN_ERBB2_SIGNALING | 22 | 3 | 0.0033 |
| 50 | REACTOME_DEGRADATION_OF_THE_EXTRACELLULAR_MATRIX | 140 | 7 | 0.0033 |
| 51 | REACTOME_CONSTITUTIVE_SIGNALING_BY_ABERRANT_PI3K_IN_CANCER | 75 | 5 | 0.0037 |
| 55 | REACTOME_SIGNALING_BY_EGFR_IN_CANCER | 25 | 3 | 0.0047 |
| 58 | NABA_CORE_MATRISOME | 275 | 10 | 0.0049 |
| 59 | REACTOME_SIGNALING_BY_ERBB2_IN_CANCER | 26 | 3 | 0.0053 |
| 60 | NABA_ECM_REGULATORS | 238 | 9 | 0.0058 |
| 61 | REACTOME_INTEGRIN_CELL_SURFACE_INTERACTIONS | 85 | 5 | 0.0063 |
| 62 | REACTOME_SIGNALING_BY_PTK6 | 54 | 4 | 0.0064 |
| 65 | REACTOME_DOWNREGULATION_OF_ERBB2_SIGNALING | 29 | 3 | 0.0072 |
| 69 | REACTOME_COLLAGEN_FORMATION | 90 | 5 | 0.0080 |
| 70 | REACTOME_SIGNALING_BY_ERBB4 | 58 | 4 | 0.0083 |
| 77 | REACTOME_TYPE_I_HEMIDESMOSOME_ASSEMBLY | 11 | 2 | 0.0095 |
| 78 | KEGG_CELL_ADHESION_MOLECULES_CAMS | 133 | 6 | 0.0103 |
| 87 | REACTOME_DISSOLUTION_OF_FIBRIN_CLOT | 13 | 2 | 0.0133 |
| 88 | REACTOME_PI3K_AKT_SIGNALING_IN_CANCER | 102 | 5 | 0.0134 |
| 90 | PID_ERBB4_PATHWAY | 38 | 3 | 0.0152 |
| 99 | REACTOME_NEGATIVE_REGULATION_OF_THE_PI3K_AKT_NETWORK | 110 | 5 | 0.0180 |
| 100 | REACTOME_INTERLEUKIN_4_AND_INTERLEUKIN_13_SIGNALING | 111 | 5 | 0.0186 |
| 101 | NABA_ECM_GLYCOPROTEINS | 196 | 7 | 0.0190 |
| 103 | REACTOME_ECM_PROTEOGLYCANS | 76 | 4 | 0.0207 |
| 104 | PID_INTEGRIN3_PATHWAY | 43 | 3 | 0.0211 |
| 109 | PID_A6B1_A6B4_INTEGRIN_PATHWAY | 46 | 3 | 0.0252 |
| 113 | KEGG_ECM_RECEPTOR_INTERACTION | 84 | 4 | 0.0286 |
| 123 | REACTOME_SIGNALING_BY_ERBB2 | 50 | 3 | 0.0313 |
| 125 | KEGG_ERBB_SIGNALING_PATHWAY | 87 | 4 | 0.0320 |
| 143 | PID_INTEGRIN_CS_PATHWAY | 26 | 2 | 0.0492 |
| Primer | Sequence (5′→3′) | Primer Annealing Temperature | |
| Primers used for cloning of the whole ITGBL1 coding sequence (CDS) | |||
| F-cloning | AAAAAAAGATCTTCCTGCCGCCTCCCTCGGTG | 65 °C | |
| R-cloning | CATGGTTTCCTTCGCATTTATGTCGACTAATGGCCCAG | ||
| Primers used for concurrent detection of all four ITGBL1 isoforms | |||
| F-PCR | GCTCTGGGAGGGGTAAATGTG | 59 °C | |
| R-PCR | TGCACTTCCCACAATGACAAGAA | ||
| Primers used for PCR internal control | |||
| 18S rRNA_L | CATGGCCGTTCTTAGTTGGTG | 59 °C | |
| 18S rRNA_R | GTGCAGCCCCGGACATCTAA | ||
| Primers used for detection of different ITGBL1 mRNA isoforms | |||
| Primer Name | Sequence (5′→3′) | Annealing Temp. | mRNA Variant |
| w1_F(1) | CCTGTGTGAGTGCCATGAGT | 61.4 °C | 1 & 2 |
| w2_R | CTTCTGTTTCATCGTCTATGCATTC | ||
| w3_F | TAGTTGCAGTGATGGGAGCA | 61.4 °C | 3 |
| w2_R | CTTCTGTTTCATCGTCTATGCATTC | ||
| w4_pozaX1_F(2) | CTCTCCACTGAGGGGTTTGG | 61.4 °C | 4 |
| w1_R(2) | GTGACATGTACCTGCATTAGAGC | ||
| Application | Host/Clonality | Localization of Immunogen | Immunogen Sequence | Source (cat. no) | Dilution |
|---|---|---|---|---|---|
| Primary | |||||
| ITGBL1 | Rabbit/Polyclonal | Exon: 6, 7, 8 | VCGECTCHDVDPTGDWGDIHGDTCECDERDCRAVYDRYSDDFCSGHGQCNCGRCDCKAGWYGKKCEHPQSCTLSAEESIRKCQGSSDLPCSGRGKCECGKCTCYPPGDRRVYGKTCECDDRRCEDLDGV | Sigma-Aldrich (HPA005676) | 1:750 |
| ITGBL1 | Rabbit/Polyclonal | Exon: 1 (fragment), 2 | MRPPGFRNFLLLASSLLFAGLSAVPQSFSPSLRSWPGAACRLSRAESERR | Thermo Fisher Scientific (PA5-42123) | 1:1000 |
| ITGBL1 | Rabbit/Polyclonal | Exon: 7 (fragment), 8 | PCSGRGKCECGKCTCYPPGDRRVYGKT | ABGENT (Ap8781c) | 1:3000 |
| ITGBL1 | Rabbit/Polyclonal | Exon: 3 (fragment), 4, 5 (fragment) | CSNAGTCHCGRCKCDNSDGSGLVYGKFCECDDRECIDDETEEICGGHGKC | ProSci (29-712) | 1:300 |
| β-actin | Mouse/Monoclonal | ------------ | ------------ | Milipore (MAB1501) | 1:5000 |
| Secondary | |||||
| Anti-Rabbit IgG (HRP) | Goat | ------------ | ------------ | Millipore (AP132P) | 1:1000 |
| Anti-Mouse IgG (HRP) | Donkey | ------------ | ------------ | R&D Systems (HAF018) | 1:5000 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cortez, A.J.; Kujawa, K.A.; Wilk, A.M.; Sojka, D.R.; Syrkis, J.P.; Olbryt, M.; Lisowska, K.M. Evaluation of the Role of ITGBL1 in Ovarian Cancer. Cancers 2020, 12, 2676. https://doi.org/10.3390/cancers12092676
Cortez AJ, Kujawa KA, Wilk AM, Sojka DR, Syrkis JP, Olbryt M, Lisowska KM. Evaluation of the Role of ITGBL1 in Ovarian Cancer. Cancers. 2020; 12(9):2676. https://doi.org/10.3390/cancers12092676
Chicago/Turabian StyleCortez, Alexander Jorge, Katarzyna Aleksandra Kujawa, Agata Małgorzata Wilk, Damian Robert Sojka, Joanna Patrycja Syrkis, Magdalena Olbryt, and Katarzyna Marta Lisowska. 2020. "Evaluation of the Role of ITGBL1 in Ovarian Cancer" Cancers 12, no. 9: 2676. https://doi.org/10.3390/cancers12092676