Revisiting Neutrophil Gelatinase-Associated Lipocalin (NGAL) in Cancer: Saint or Sinner?
Abstract
:1. Introduction
2. Methods
3. NGAL as a Biomarker in Cancer
3.1. Solid Tumors
3.2. Leukemias
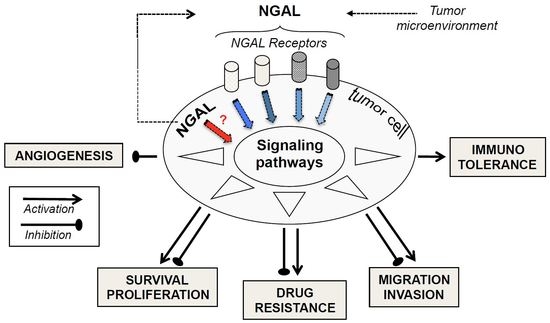
4. Detrimental and Beneficial Effects of NGAL in Cancer
5. Conclusions and Future Directions
Funding
Conflicts of Interest
Abbreviations
| AML | acute myeloid leukemia |
| ALL | acute lymphoblastic leukemia |
| CLL | chronic lymphocytic leukemia |
| CML | chronic myeloid leukemia |
| FAB | French-American-British |
| HLA-G | human leukocyte antigen G |
| Lcn-2 | ipocalin-2 |
| LRP-2 | low-density lipoprotein receptor-related protein-2 |
| Neutrophil gelatinase-associated lipocalin | NGAL |
| NGAL Ab | NGAL antibody |
| NGAL-R | NGAL-receptor |
| Nrf2/HO-1 | nuclear factor E2-related factor 2/heme oxygenase-1 |
| proMMP-9 | pro-matrix metalloproteinase-9 |
| rhNGAL | recombinant human NGAL |
| ROS | reactive oxygen species |
| Nrf2 | nuclear factor E2-related factor-2 |
| SLC22A17 | solute carrier family 22 member 17 |
| Treg | T-regulatory cell |
References
- Chakraborty, S.; Kaur, S.; Guha, S.; Batra, S.K. The multifaceted roles of neutrophil gelatinase associated lipocalin (NGAL) in inflammation and cancer. Biochim. Biophys. Acta 2012, 1826, 129–169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiao, X.; Yeoh, B.S.; Vijay-Kumar, M. Lipocalin 2: An Emerging Player in Iron Homeostasis and Inflammation. Annu. Rev. Nutr. 2017, 37, 103–130. [Google Scholar] [CrossRef] [PubMed]
- Rudd, P.M.; Mattu, T.S.; Masure, S.; Bratt, T.; Van den Steen, P.E.; Wormald, M.R.; Kuster, B.; Harvey, D.J.; Borregaard, N.; Van Damme, J.; et al. Glycosylation of natural human neutrophil gelatinase B and neutrophil gelatinase B-associated lipocalin. Biochemistry 1999, 38, 13937–13950. [Google Scholar] [CrossRef] [PubMed]
- Lippi, G.; Meschi, T.; Nouvenne, A.; Mattiuzzi, C.; Borghi, L. Neutrophil gelatinase-associated lipocalin in cancer. Adv. Clin. Chem. 2014, 64, 179–219. [Google Scholar] [PubMed]
- Kjeldsen, L.; Johnsen, A.H.; Sengelov, H.; Borregaard, N. Isolation and primary structure of NGAL, a novel protein associated with human neutrophil gelatinase. J. Biol. Chem. 1993, 268, 10425–10432. [Google Scholar] [PubMed]
- Virzi, G.M.; Clementi, A.; de Cal, M.; Cruz, D.N.; Ronco, C. Genomics and biological activity of neutrophil gelatinase-associated lipocalin in several clinical settings. Blood Purif. 2013, 35, 139–143. [Google Scholar] [CrossRef] [PubMed]
- Makris, K.; Rizos, D.; Kafkas, N.; Haliassos, A. Neutrophil gelatinase-associated lipocalin as a new biomarker in laboratory medicine. Clin. Chem. Lab. Med. 2012, 50, 1519–1532. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, N.; Choudhary, R.; Chan, J.; Wentworth, B.; Higginbotham, E.; Maisel, A.S. Neutrophil gelatinase-associated lipocalin as diagnostic and prognostic tool for cardiovascular disease and heart failure. Expert Opin. Med. Diagn. 2013, 7, 209–220. [Google Scholar] [CrossRef] [PubMed]
- Paragas, N.; Qiu, A.; Hollmen, M.; Nickolas, T.L.; Devarajan, P.; Barasch, J. NGAL-Siderocalin in kidney disease. Biochim. Biophys. Acta 2012, 1823, 1451–1458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Devarajan, P. Neutrophil gelatinase-associated lipocalin: New paths for an old shuttle. Cancer Ther. 2007, 5, 463–470. [Google Scholar] [PubMed]
- Nickolas, T.L.; Forster, C.S.; Sise, M.E.; Barasch, N.; Sola-Del Valle, D.; Viltard, M.; Buchen, C.; Kupferman, S.; Carnevali, M.L.; Bennett, M.; et al. NGAL (Lcn2) monomer is associated with tubulointerstitial damage in chronic kidney disease. Kidney Int. 2012, 82, 718–722. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martensson, J.; Xu, S.; Bell, M.; Martling, C.R.; Venge, P. Immunoassays distinguishing between HNL/NGAL released in urine from kidney epithelial cells and neutrophils. Clin. Chim. Acta 2012, 413, 1661–1667. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Moses, M.A. Lipocalin 2: A multifaceted modulator of human cancer. Cell Cycle 2009, 8, 2347–2352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bolignano, D.; Donato, V.; Lacquaniti, A.; Fazio, M.R.; Bono, C.; Coppolino, G.; Buemi, M. Neutrophil gelatinase-associated lipocalin (NGAL) in human neoplasias: A new protein enters the scene. Cancer Lett. 2010, 288, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Klausen, P.; Niemann, C.U.; Cowland, J.B.; Krabbe, K.; Borregaard, N. On mouse and man: Neutrophil gelatinase associated lipocalin is not involved in apoptosis or acute response. Eur. J. Haematol. 2005, 75, 332–340. [Google Scholar] [CrossRef] [PubMed]
- Bouchet, S.; Bauvois, B. Neutrophil Gelatinase-Associated Lipocalin (NGAL), Pro-Matrix Metalloproteinase-9 (pro-MMP-9) and Their Complex Pro-MMP-9/NGAL in Leukaemias. Cancers 2014, 6, 796–812. [Google Scholar] [CrossRef] [PubMed]
- Miharada, K.; Hiroyama, T.; Sudo, K.; Danjo, I.; Nagasawa, T.; Nakamura, Y. Lipocalin 2-mediated growth suppression is evident in human erythroid and monocyte/macrophage lineage cells. J. Cell. Physiol. 2008, 215, 526–537. [Google Scholar] [CrossRef] [PubMed]
- Borregaard, N.; Cowland, J.B. Granules of the human neutrophilic polymorphonuclear leukocyte. Blood 1997, 89, 3503–3521. [Google Scholar] [PubMed]
- Cai, L.; Rubin, J.; Han, W.; Venge, P.; Xu, S. The origin of multiple molecular forms in urine of HNL/NGAL. Clin. J. Am. Soc. Nephrol. CJASN 2010, 5, 2229–2235. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Borregaard, N.; Kjeldsen, L.; Moses, M.A. The high molecular weight urinary matrix metalloproteinase (MMP) activity is a complex of gelatinase B/MMP-9 and neutrophil gelatinase-associated lipocalin (NGAL). Modulation of MMP-9 activity by NGAL. J. Biol. Chem. 2001, 276, 37258–37265. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Bielenberg, D.R.; Rodig, S.J.; Doiron, R.; Clifton, M.C.; Kung, A.L.; Strong, R.K.; Zurakowski, D.; Moses, M.A. Lipocalin 2 promotes breast cancer progression. Proc. Natl. Acad. Sci. USA 2009, 106, 3913–3918. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Provatopoulou, X.; Gounaris, A.; Kalogera, E.; Zagouri, F.; Flessas, I.; Goussetis, E.; Nonni, A.; Papassotiriou, I.; Zografos, G. Circulating levels of matrix metalloproteinase-9 (MMP-9), neutrophil gelatinase-associated lipocalin (NGAL) and their complex MMP-9/NGAL in breast cancer disease. BMC Cancer 2009, 9, 390. [Google Scholar] [CrossRef] [PubMed]
- Song, N.; Choi, J.Y.; Sung, H.; Jeon, S.; Chung, S.; Park, S.K.; Han, W.; Lee, J.W.; Kim, M.K.; Lee, J.Y.; et al. Prediction of breast cancer survival using clinical and genetic markers by tumor subtypes. PLoS ONE 2015, 10, e0122413. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, C.A.; Yan, L.; Louis, G.; Yang, J.; Kutok, J.L.; Moses, M.A. The matrix metalloproteinase-9/neutrophil gelatinase-associated lipocalin complex plays a role in breast tumor growth and is present in the urine of breast cancer patients. Clin. Cancer Res. 2005, 11, 5390–5395. [Google Scholar] [CrossRef] [PubMed]
- Candido, S.; Maestro, R.; Polesel, J.; Catania, A.; Maira, F.; Signorelli, S.S.; McCubrey, J.A.; Libra, M. Roles of neutrophil gelatinase-associated lipocalin (NGAL) in human cancer. Oncotarget 2014, 5, 1576–1594. [Google Scholar] [CrossRef] [PubMed]
- Leng, X.; Wu, Y.; Arlinghaus, R.B. Relationships of lipocalin 2 with breast tumorigenesis and metastasis. J. Cell. Physiol. 2010, 226, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.; Kim, J.H. Lipocalin2 expressions correlate significantly with tumor differentiation in epithelial ovarian cancer. J. Histochem. Cytochem. 2009, 57, 513–521. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.J.; Huang, Y.H.; Au, H.K.; Wang, L.M.; Chu, S.T. The cancer marker neutrophil gelatinase-associated lipocalin is highly expressed in human endometrial hyperplasia. Mol. Boil. Rep. 2012, 39, 1029–1036. [Google Scholar] [CrossRef] [PubMed]
- Cymbaluk-Ploska, A.; Chudecka-Glaz, A.; Pius-Sadowska, E.; Sompolska-Rzechula, A.; Chudecka, K.; Bulsa, M.; Machalinski, B.; Menkiszak, J. Clinical Relevance of NGAL/MMP-9 Pathway in Patients with Endometrial Cancer. Dis. Markers 2017, 2017, 6589262. [Google Scholar] [CrossRef] [PubMed]
- Duvillard, L.; Ortega-Deballon, P.; Bourredjem, A.; Scherrer, M.L.; Mantion, G.; Delhorme, J.B.; Deguelte-Lardiere, S.; Petit, J.M.; Bonithon-Kopp, C.; group, A.S. A case-control study of pre-operative levels of serum neutrophil gelatinase-associated lipocalin and other potential inflammatory markers in colorectal cancer. BMC Cancer 2014, 14, 912. [Google Scholar] [CrossRef] [PubMed]
- Marti, J.; Fuster, J. Prognostic value of serum neutrophil gelatinase-associated lipocalin in metastatic and non-metastatic colorectal cancer: Reply. World J. Surg. 2013, 37, 2729. [Google Scholar] [CrossRef] [PubMed]
- Ozemir, I.A.; Aslan, S.; Eren, T.; Bayraktar, B.; Bilgic, C.; Isbilen, B.; Yalman, H.; Yigitbasi, R.; Alimoglu, O. The Diagnostic and Prognostic Significance of Serum Neutrophil Gelatinase-Associated Lipocalin Levels in Patients with Colorectal Cancer. Chirurgia 2016, 111, 414–421. [Google Scholar] [CrossRef] [PubMed]
- Ricci, S.; Bruzzese, D.; DI Carlo, A. Evaluation of MMP-2, MMP-9, TIMP-1, TIMP-2, NGAL and MMP-9/NGAL complex in urine and sera from patients with bladder cancer. Oncol. Lett. 2015, 10, 2527–2532. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roy, R.; Louis, G.; Loughlin, K.R.; Wiederschain, D.; Kilroy, S.M.; Lamb, C.C.; Zurakowski, D.; Moses, M.A. Tumor-specific urinary matrix metalloproteinase fingerprinting: Identification of high molecular weight urinary matrix metalloproteinase species. Clin. Cancer Res. 2008, 14, 6610–6617. [Google Scholar] [CrossRef] [PubMed]
- Bolignano, D.; Coppolino, G.; Donato, V.; Lacquaniti, A.; Bono, C.; Buemi, M. Neutrophil gelatinase-associated lipocalin (NGAL): A new piece of the anemia puzzle? Med. Sci. Monit. 2010, 16, RA131–RA135. [Google Scholar] [PubMed]
- Kaur, S.; Chakraborty, S.; Baine, M.J.; Mallya, K.; Smith, L.M.; Sasson, A.; Brand, R.; Guha, S.; Jain, M.; Wittel, U.; et al. Potentials of plasma NGAL and MIC-1 as biomarker(s) in the diagnosis of lethal pancreatic cancer. PLoS ONE 2013, 8, e55171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Carlo, A. Evaluation of neutrophil gelatinase-associated lipocalin (NGAL), matrix metalloproteinase-9 (MMP-9) and their complex MMP-9/NGAL in sera and urine of patients with kidney tumors. Oncol. Lett. 2013, 5, 1677–1681. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shalabi, A.; Abassi, Z.; Awad, H.; Halachmi, S.; Moskovitz, B.; Kluger, Y.; Nativ, O. Urinary NGAL and KIM-1: Potential association with histopathologic features in patients with renal cell carcinoma. World J. Urol. 2013, 31, 1541–1545. [Google Scholar] [CrossRef] [PubMed]
- Hiromoto, T.; Noguchi, K.; Yamamura, M.; Zushi, Y.; Segawa, E.; Takaoka, K.; Moridera, K.; Kishimoto, H.; Urade, M. Up-regulation of neutrophil gelatinase-associated lipocalin in oral squamous cell carcinoma: Relation to cell differentiation. Oncol. Rep. 2011, 26, 1415–1421. [Google Scholar] [PubMed]
- Kubben, F.J.; Sier, C.F.; Hawinkels, L.J.; Tschesche, H.; van Duijn, W.; Zuidwijk, K.; van der Reijden, J.J.; Hanemaaijer, R.; Griffioen, G.; Lamers, C.B.; et al. Clinical evidence for a protective role of lipocalin-2 against MMP-9 autodegradation and the impact for gastric cancer. Eur. J. Cancer 2007, 43, 1869–1876. [Google Scholar] [CrossRef] [PubMed]
- Volpe, V.; Raia, Z.; Sanguigno, L.; Somma, D.; Mastrovito, P.; Moscato, F.; Mellone, S.; Leonardi, A.; Pacifico, F. NGAL controls the metastatic potential of anaplastic thyroid carcinoma cells. J. Clin. Endocrinol. Metab. 2013, 98, 228–235. [Google Scholar] [CrossRef] [PubMed]
- Kamiguti, A.S.; Lee, E.S.; Till, K.J.; Harris, R.J.; Glenn, M.A.; Lin, K.; Chen, H.J.; Zuzel, M.; Cawley, J.C. The role of matrix metalloproteinase 9 in the pathogenesis of chronic lymphocytic leukaemia. Br. J. Haematol. 2004, 125, 128–140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, W.C.; Lin, P.M.; Yang, M.Y.; Liu, Y.C.; Chang, C.S.; Chou, W.C.; Hsu, J.F.; Huang, C.T.; Cho, S.F.; Yu, W.H.; et al. Higher lipocalin 2 expression may represent an independent favorable prognostic factor in cytogenetically normal acute myeloid leukemia. Leuk. Lymphoma 2013, 54, 1614–1625. [Google Scholar] [CrossRef] [PubMed]
- Villalva, C.; Sorel, N.; Bonnet, M.L.; Guilhot, J.; Mayeur-Rousse, C.; Guilhot, F.; Chomel, J.C.; Turhan, A.G. Neutrophil gelatinase-associated lipocalin expression in chronic myeloid leukemia. Leuk. Lymphoma 2008, 49, 984–988. [Google Scholar] [CrossRef] [PubMed]
- Leng, X.; Lin, H.; Ding, T.; Wang, Y.; Wu, Y.; Klumpp, S.; Sun, T.; Zhou, Y.; Monaco, P.; Belmont, J.; et al. Lipocalin 2 is required for BCR-ABL-induced tumorigenesis. Oncogene 2008, 27, 6110–6119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monisha, J.; Roy, N.K.; Padmavathi, G.; Banik, K.; Bordoloi, D.; Khwairakpam, A.D.; Arfuso, F.; Chinnathambi, A.; Alahmadi, T.A.; Alharbi, S.A.; et al. NGAL is Downregulated in Oral Squamous Cell Carcinoma and Leads to Increased Survival, Proliferation, Migration and Chemoresistance. Cancers 2018, 10, 228. [Google Scholar] [CrossRef] [PubMed]
- Roli, L.; Pecoraro, V.; Trenti, T. Can NGAL be employed as prognostic and diagnostic biomarker in human cancers? A systematic review of current evidence. Int. J. Boil. Markers 2017, 32, e53–e61. [Google Scholar] [CrossRef] [PubMed]
- Iacobucci, I.; Papayannidis, C.; Lonetti, A.; Ferrari, A.; Baccarani, M.; Martinelli, G. Cytogenetic and molecular predictors of outcome in acute lymphocytic leukemia: Recent developments. Curr. Hematol. Malig. Rep. 2012, 7, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Pleyer, L.; Egle, A.; Hartmann, T.N.; Greil, R. Molecular and cellular mechanisms of CLL: Novel therapeutic approaches. Nat. Rev. Clin. Oncol. 2009, 6, 405–418. [Google Scholar] [CrossRef] [PubMed]
- Meenaghan, T.; Dowling, M.; Kelly, M. Acute leukaemia: Making sense of a complex blood cancer. Br. J. Nurs. 2012, 21, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Burke, B.A.; Carroll, M. BCR-ABL: A multi-faceted promoter of DNA mutation in chronic myelogeneous leukemia. Leukemia 2010, 24, 1105–1112. [Google Scholar] [CrossRef] [PubMed]
- Bixby, D.; Talpaz, M. Seeking the causes and solutions to imatinib-resistance in chronic myeloid leukemia. Leukemia 2011, 25, 7–22. [Google Scholar] [CrossRef] [PubMed]
- Alonci, A.; Allegra, A.; Russo, S.; Penna, G.; Bellomo, G.; D’Angelo, A.; Campo, S.; Cannavo, A.; Centorrino, R.; Musolino, C. Imatinib mesylate therapy induces reduction in neutrophil gelatinase-associated lipocalin serum levels and increase in leptin concentrations in chronic myeloid leukemia patients in molecular remission. Acta Haematol. 2012, 127, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Nadarajan, V.S.; Ang, C.H.; Bee, P.C. Lipocalin-2 is associated with modulation of disease phenotype in a patient with concurrent JAK2-V617F and BCR-ABL mutation. Eur. J. Haematol. 2012, 88, 175–178. [Google Scholar] [CrossRef] [PubMed]
- Miharada, K.; Hiroyama, T.; Sudo, K.; Nagasawa, T.; Nakamura, Y. Lipocalin 2 functions as a negative regulator of red blood cell production in an autocrine fashion. FASEB J. 2005, 19, 1881–1883. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, M.; Xia, L.; Liu, Y.C.; Hochman, T.; Bizzari, L.; Aruch, D.; Lew, J.; Weinberg, R.; Goldberg, J.D.; Hoffman, R. Lipocalin produced by myelofibrosis cells affects the fate of both hematopoietic and marrow microenvironmental cells. Blood 2015, 126, 972–982. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- La Manna, G.; Ghinatti, G.; Tazzari, P.L.; Alviano, F.; Ricci, F.; Capelli, I.; Cuna, V.; Todeschini, P.; Brunocilla, E.; Pagliaro, P.; et al. Neutrophil gelatinase-associated lipocalin increases HLA-G(+)/FoxP3(+) T-regulatory cell population in an in vitro model of PBMC. PLoS ONE 2014, 9, e89497. [Google Scholar] [CrossRef] [PubMed]
- Mougiakakos, D.; Choudhury, A.; Lladser, A.; Kiessling, R.; Johansson, C.C. Regulatory T cells in cancer. Adv. Cancer Res. 2010, 107, 57–117. [Google Scholar] [PubMed]
- Tong, Z.; Wu, X.; Ovcharenko, D.; Zhu, J.; Chen, C.S.; Kehrer, J.P. Neutrophil gelatinase-associated lipocalin as a survival factor. Biochem. J. 2005, 391, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Song, B.; Zhang, H.; Jiang, L.; Chi, Y.; Tian, J.; Du, W.; Yu, B.; Han, Z. Down-regulation of lipocalin 2 suppresses the growth of human lung adenocarcinoma through oxidative stress involving Nrf2/HO-1 signaling. Acta Biochim. Biophys. Sin. 2015, 47, 805–814. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iannetti, A.; Pacifico, F.; Acquaviva, R.; Lavorgna, A.; Crescenzi, E.; Vascotto, C.; Tell, G.; Salzano, A.M.; Scaloni, A.; Vuttariello, E.; et al. The neutrophil gelatinase-associated lipocalin (NGAL), a NF-kappaB-regulated gene, is a survival factor for thyroid neoplastic cells. Proc. Natl. Acad. Sci. USA 2008, 105, 14058–14063. [Google Scholar] [CrossRef] [PubMed]
- Han, M.Y.; Nie, J.W.; Li, Y.Y.; Zhu, Y.Z.; Wu, G. NGAL gene silencing inhibits proliferation and promotes apoptosis of human gastric cancer cells: An in vivo and in vitro study. J. Cell. Biochem. 2017. [Google Scholar] [CrossRef]
- Miyamoto, T.; Kashima, H.; Yamada, Y.; Kobara, H.; Asaka, R.; Ando, H.; Higuchi, S.; Ida, K.; Mvunta, D.H.; Shiozawa, T. Lipocalin 2 Enhances Migration and Resistance against Cisplatin in Endometrial Carcinoma Cells. PLoS ONE 2016, 11, e0155220. [Google Scholar] [CrossRef] [PubMed]
- Bahmani, P.; Halabian, R.; Rouhbakhsh, M.; Roushandeh, A.M.; Masroori, N.; Ebrahimi, M.; Samadikuchaksaraei, A.; Shokrgozar, M.A.; Roudkenar, M.H. Neutrophil gelatinase-associated lipocalin induces the expression of heme oxygenase-1 and superoxide dismutase 1, 2. Cell Stress Chaperones 2010, 15, 395–403. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.K.; Kim, H.J.; Lee, K.J.; Lee, H.J.; Lee, J.S.; Kim, D.G.; Hong, S.W.; Yoon, Y.; Kim, J.S. Inhibition of the proliferation and invasion of hepatocellular carcinoma cells by lipocalin 2 through blockade of JNK and PI3K/Akt signaling. Int. J. Oncol. 2011, 38, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Tong, Z.; Kunnumakkara, A.B.; Wang, H.; Matsuo, Y.; Diagaradjane, P.; Harikumar, K.B.; Ramachandran, V.; Sung, B.; Chakraborty, A.; Bresalier, R.S.; et al. Neutrophil gelatinase-associated lipocalin: A novel suppressor of invasion and angiogenesis in pancreatic cancer. Cancer Res. 2008, 68, 6100–6108. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Hittelman, W.; Lu, T.; Ji, P.; Arlinghaus, R.; Shmulevich, I.; Hamilton, S.R.; Zhang, W. NGAL decreases E-cadherin-mediated cell-cell adhesion and increases cell motility and invasion through Rac1 in colon carcinoma cells. Lab. Investig. 2009, 89, 531–548. [Google Scholar] [CrossRef] [PubMed]
- Nuntagowat, C.; Leelawat, K.; Tohtong, R. NGAL knockdown by siRNA in human cholangiocarcinoma cells suppressed invasion by reducing NGAL/MMP-9 complex formation. Clin. Exp. Metastasis 2010, 27, 295–305. [Google Scholar] [CrossRef] [PubMed]
- Krysan, K.; Cui, X.; Gardner, B.K.; Reckamp, K.L.; Wang, X.; Hong, L.; Walser, T.C.; Rodriguez, N.L.; Pagano, P.C.; Garon, E.B.; et al. Elevated neutrophil gelatinase-associated lipocalin contributes to erlotinib resistance in non-small cell lung cancer. Am. J. Transl. Res. 2013, 5, 481–496. [Google Scholar] [PubMed]
- Zheng, L.T.; Lee, S.; Yin, G.N.; Mori, K.; Suk, K. Down-regulation of lipocalin 2 contributes to chemoresistance in glioblastoma cells. J. Neurochem. 2009, 111, 1238–1251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, D.S.; Wu, C.L.; Ping, S.Y.; Huang, Y.L.; Shen, K.H. NGAL can alternately mediate sunitinib resistance in renal cell carcinoma. J. Urol. 2014, 192, 559–566. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.H.; Chang, G.Q.; Zhang, H.J.; Wang, J.; Lin, Y.N.; Jin, W.N.; Li, H.W.; Gao, W.; Wang, R.J.; Li, Q.H.; et al. Neutrophil gelatinase-associated lipocalin regulates intracellular accumulation of Rh123 in cancer cells. Genes Cells 2012, 17, 205–217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chappell, W.H.; Abrams, S.L.; Montalto, G.; Cervello, M.; Martelli, A.M.; Candido, S.; Libra, M.; Polesel, J.; Talamini, R.; Arlinghaus, R.; et al. Effects of ectopic expression of NGAL on doxorubicin sensitivity. Oncotarget 2012, 3, 1236–1245. [Google Scholar] [CrossRef] [PubMed]
- Tschesche, H.; Zolzer, V.; Triebel, S.; Bartsch, S. The human neutrophil lipocalin supports the allosteric activation of matrix metalloproteinases. Eur. J. Biochem. FEBS 2001, 268, 1918–1928. [Google Scholar] [CrossRef] [Green Version]
- Nielsen, B.S.; Borregaard, N.; Bundgaard, J.R.; Timshel, S.; Sehested, M.; Kjeldsen, L. Induction of NGAL synthesis in epithelial cells of human colorectal neoplasia and inflammatory bowel diseases. Gut 1996, 38, 414–420. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Lee, E.K.; Lee, K.J.; Hong, S.W.; Yoon, Y.; Kim, J.S. Ectopic expression of neutrophil gelatinase-associated lipocalin suppresses the invasion and liver metastasis of colon cancer cells. Int. J. Cancer 2006, 118, 2490–2497. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodvold, J.J.; Mahadevan, N.R.; Zanetti, M. Lipocalin 2 in cancer: When good immunity goes bad. Cancer Lett. 2012, 316, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Skerra, A. Alternative binding proteins: Anticalins—Harnessing the structural plasticity of the lipocalin ligand pocket to engineer novel binding activities. FEBS J. 2008, 275, 2677–2683. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Kinashi, T. Overview of integrin signaling in the immune system. Methods Mol. Boil. 2012, 757, 261–278. [Google Scholar]
- Hvidberg, V.; Jacobsen, C.; Strong, R.K.; Cowland, J.B.; Moestrup, S.K.; Borregaard, N. The endocytic receptor megalin binds the iron transporting neutrophil-gelatinase-associated lipocalin with high affinity and mediates its cellular uptake. FEBS Lett. 2005, 579, 773–777. [Google Scholar] [CrossRef] [PubMed]
- Devireddy, L.R.; Gazin, C.; Zhu, X.; Green, M.R. A cell-surface receptor for lipocalin 24p3 selectively mediates apoptosis and iron uptake. Cell 2005, 123, 1293–1305. [Google Scholar] [CrossRef] [PubMed]
- Marzolo, M.P.; Farfan, P. New insights into the roles of megalin/LRP2 and the regulation of its functional expression. Boil. Res. 2011, 44, 89–105. [Google Scholar] [CrossRef] [PubMed]
- Fang, W.K.; Xu, L.Y.; Lu, X.F.; Liao, L.D.; Cai, W.J.; Shen, Z.Y.; Li, E.M. A novel alternative spliced variant of neutrophil gelatinase-associated lipocalin receptor in oesophageal carcinoma cells. Biochem. J. 2007, 403, 297–303. [Google Scholar] [CrossRef] [PubMed]

| Cancer Type | NGAL | NGAL Complex | Ref. |
|---|---|---|---|
| Breast | serum/tissue | serum/urine | [1,4,10,13,14,21,22,23,24,25,26] |
| Brain | tissue | [1,4,13] | |
| Ovary | serum/urine/tissue | [1,13,25,27] | |
| Endometrium | serum/tissue | serum | [4,13,28,29] |
| Colorectal | plasma/serum/tissue | [1,4,13,30,31,32] | |
| Bladder | serum/urine/tissue | serum/urine | [25,33] |
| Prostate | urine | [34] | |
| Liver | tissue | [4,25,35] | |
| Lung | tissue | [1,2,35] | |
| Pancreas | plasma/serum/tissue | [1,4,25,36] | |
| Kidney | serum/urine/tissue | serum/urine | [1,25,35,37,38] |
| Esophagus | tissue | [4,25,35,39] | |
| Gastric | serum/tissue | [25,35,40] | |
| Thyroid | tissue | [1,41] | |
| ALL | cell | cell | [16,25] |
| CLL | cell | cell | [16,25,42] |
| AML | cell | cell | [16,25,43] |
| CML | plasma/serum/cell | [1,16,42,44,45] |
| Cancer Type | Investigative Technique | Impact of NGAL on Cell Processes | Ref. |
|---|---|---|---|
| Lung (A549) | siRNA anti-NGAL | Survival ↑ Oxidative stress ↓ | [59] |
| Lung (A549, PC9) | siRNA | Proliferation ↑ Oxidative stress ↓ | [60] |
| Lung and liver (A549, HepG2) | siRNA NGAL overexpression | Oxidative stress ↓ | [64] |
| Anaplastic thyroid (FRO) | rhNGAL (*), siRNA anti-NGAL | Survival ↑ | [61] |
| Gastric (MGC-803, SGC-7901) | siRNA | Survival & Proliferation ↑ | [62] |
| Liver (SK-Hep-1) | rhNGAL (R&D) NGAL overexpression | Proliferation & Migration ↓ | [65] |
| Pancreatic (Paca) | siRNA NGAL overexpression | Invasion & Angiogenesis ↓ | [66] |
| Colon (KM12C, HCT116, DLD1) | siRNA NGAL overexpression | Cell-cell adhesion ↓ Migration ↑ | [67] |
| Colon (KM12SM) | NGAL overexpression | Invasion ↓ | [76] |
| Cholangiocarcinoma (RMCCA-1) | siRNA | Migration & Invasion ↑ | [68] |
| Endometrial (HHUA, RL95-2) | siRNA NGAL overexpression | Survival & Migration ↑ Cisplatin resistance ↑ | [63] |
| Squamous cell carcinoma (SAS) | siRNA | Survival & Migration ↓ Cisplatin Resistance ↓ | [46] |
| Non-small-cell lung (H441, H3255) | siRNA NGAL overexpression | Erlotinib Resistance ↑ | [69] |
| Glioblastoma (U87MG, U373MG, T98G) | NGAL overexpression | Carmustine Resistance ↓ Cell Death ↑ | [70] |
| Renal cancer (Caki1, A498, ACHN) | rhNGAL (Pfizer, Sigma) NGAL overexpression | Sunitinib Resistance ↓ | [71] |
| Breast (MDA-MB-23) | rhNGAL (R&D, Sino Biological Inc.) siRNA | Rhodamine-123 Resistance ↓ | [72] |
| Breast (MCF-7) | NGAL overexpression | Proliferation & Angiogenesis ↑ No effect on Doxorubicin Resistance | [24,73] |
| Colorectal (HT-29) | NGAL overexpression | No effect on Doxorubicin Resistance | [73] |
| AML (THP1, OCI-AML3) | NGAL overexpression | Cytarabine Resistance ↓ Cell death ↑ | [43] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bauvois, B.; Susin, S.A. Revisiting Neutrophil Gelatinase-Associated Lipocalin (NGAL) in Cancer: Saint or Sinner? Cancers 2018, 10, 336. https://doi.org/10.3390/cancers10090336
Bauvois B, Susin SA. Revisiting Neutrophil Gelatinase-Associated Lipocalin (NGAL) in Cancer: Saint or Sinner? Cancers. 2018; 10(9):336. https://doi.org/10.3390/cancers10090336
Chicago/Turabian StyleBauvois, Brigitte, and Santos A. Susin. 2018. "Revisiting Neutrophil Gelatinase-Associated Lipocalin (NGAL) in Cancer: Saint or Sinner?" Cancers 10, no. 9: 336. https://doi.org/10.3390/cancers10090336