High-Performance Vanadium Redox Flow Batteries with Graphite Felt Electrodes
Abstract
:1. Introduction
2. Results and Discussion
2.1. Ex-Situ Tests
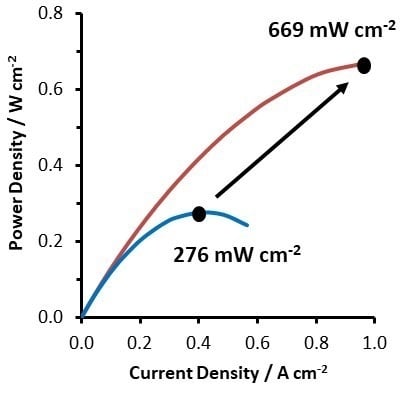
2.2. VRFB Cell Performance
2.3. VRFB Cycling
3. Materials and Methods
3.1. Cell Components
3.2. Electrolyte Solution
3.3. Cell Testing
3.4. Ex-Situ Testing
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix A
Appendix B


References
- Dunn, B.; Kamath, H.; Tarascon, J.-M. Electrical Energy Storage for the Grid: A Battery of Choices. Science 2011, 334, 928–935. [Google Scholar] [CrossRef] [PubMed]
- Deng, D. Li-ion Batteries: Basics, Progress, and Challenges. Energy Sci. Eng. 2015, 3, 385–418. [Google Scholar] [CrossRef]
- Leung, P.; Li, X.; Ponce de León, C.; Berlouis, L.; Low, C.T.J.; Walsh, F.C. Progress in Redox Flow Batteries, Remaining Challenges and Their Applications in Energy Storage. RSC Adv. 2012, 2, 10125–10156. [Google Scholar] [CrossRef]
- Weber, A.Z.; Mench, M.M.; Meyers, J.P.; Ross, P.N.; Gostick, J.T.; Liu, Q. Redox Flow Batteries: A Review. J. Appl. Electrochem. 2011, 41, 1137–1164. [Google Scholar] [CrossRef]
- Pieper, C.; Rubel, H. Revisiting Energy Storage: There Is a Business Case. Boston Consulting Group, 2011. Available online: https://www.bcg.com/documents/file72092.pdf (accessed on 29 November 2017).
- Ulaganathan, M.; Aravindan, V.; Yan, Q.; Madhavi, S.; Skyllas-Kazacos, M.; Lim, T.M. Recent Advancements in All-Vanadium Redox Flow Batteries. Adv. Mater. Interfaces 2016, 3, 1500309. [Google Scholar] [CrossRef]
- Engineering.com. Available online: http://www.engineering.com/DesignerEdge/DesignerEdgeArticles/ArticleID/12312/Massive-800-MegaWatt-hour-Battery-to-Be-Deployed-in-China.aspx (accessed on 29 November 2017).
- Zhou, X.L.; Zhao, T.S.; An, L.; Zeng, Y.K.; Wei, L. Critical Transport Issues for Improving the Performance of Aqueous Redox Flow Batteries. J. Power Sources 2017, 339, 1–12. [Google Scholar] [CrossRef]
- Zhang, M.; Moore, M.; Watson, J.S.; Zawodzinski, T.A.; Counce, R.M. Capital Cost Sensitivity Analysis of an All-Vanadium Redox-Flow Battery. J. Electrochem. Soc. 2012, 159, A1183–A1188. [Google Scholar] [CrossRef]
- Kear, G.; Shah, A.A.; Walsh, F.C. Development of the All-Vanadium Redox Flow Battery for Energy Storage: A Review of Technological, Financial and Policy Aspects. Int. J. Energy Res. 2012, 36, 1105–1120. [Google Scholar] [CrossRef]
- Parasuraman, A.; Lim, T.M.; Menictas, C.; Skyllas-Kazacos, M. Review of Material Research and Development for Vanadium Redox Flow Battery Applications. Electrochim. Acta 2013, 101, 27–40. [Google Scholar] [CrossRef]
- Chakrabarti, M.H.; Brandon, N.P.; Hajimolana, S.A.; Tariq, F.; Yufit, V.; Hashim, M.A.; Hussain, M.A.; Low, C.T.J.; Aravind, P.V. Application of Carbon Materials in Redox Flow Batteries. J. Power Sources 2014, 253, 150–166. [Google Scholar] [CrossRef]
- Kazacos, M.; Skyllas-Kazacos, M. Performance Characteristics of Carbon Plastic Electrodes in the All-Vanadium Redox Cell. J. Electrochem. Soc. 1989, 136, 2759–2760. [Google Scholar] [CrossRef]
- Bhattarai, A.; Wai, N.; Schweiss, R.; Whitehead, A.; Lim, T.M.; Hng, H.H. Advanced Porous Electrodes with Flow Channels for Vanadium Redox Flow Battery. J. Power Sources 2017, 341, 83–90. [Google Scholar] [CrossRef]
- Xu, Q.; Zhao, T.S.; Zhang, C. Performance of a Vanadium Redox Flow Battery with and without Flow Fields. Electrochim. Acta 2014, 142, 61–67. [Google Scholar] [CrossRef]
- Chang, T.-C.; Zhang, J.-P.; Fuh, Y.-K. Electrical, Mechanical and Morphological Properties of Compressed Carbon Felt Electrodes in Vanadium Redox Flow Battery. J. Power Sources 2014, 245, 66–75. [Google Scholar] [CrossRef]
- Brown, L.D.; Neville, T.P.; Jervis, R.; Mason, T.J.; Shearing, P.R.; Brett, D.J.L. The Effect of Felt Compression on the Performance and Pressure Drop of All-Vanadium Redox Flow Batteries. J. Energy Storage 2016, 8, 91–98. [Google Scholar] [CrossRef]
- Park, S.-K.; Shim, J.; Yang, J.H.; Jin, C.-S.; Lee, B.S.; Lee, Y.-S.; Shin, K.-H.; Jeon, J.-D. The Influence of Compressed Carbon Felt Electrodes on the Performance of a Vanadium Redox Flow Battery. Electrochim. Acta 2014, 116, 447–452. [Google Scholar] [CrossRef]
- Oh, K.; Won, S.; Ju, H. Numerical Study of the Effects of Carbon Felt Electrode Compression in All-Vanadium Redox Flow Batteries. Electrochim. Acta 2015, 181, 13–23. [Google Scholar] [CrossRef]
- Chen, D.; Hickner, M.A.; Agar, E.; Kumbur, E.C. Optimizing Membrane Thickness for Vanadium Redox Flow Batteries. J. Membr. Sci. 2013, 437, 108–113. [Google Scholar] [CrossRef]
- Zhang, C.; Zhao, T.S.; Xu, Q.; An, L.; Zhao, G. Effects of Operating Temperature on the Performance of Vanadium Redox Flow Batteries. Appl. Energy 2015, 155, 349–353. [Google Scholar] [CrossRef]
- Aaron, D.S.; Liu, Q.; Tang, Z.; Grim, G.M.; Papandrew, A.B.; Turhan, A.; Zawodzinski, T.A.; Mench, M.M. Dramatic Performance Gains in Vanadium Redox Flow Batteries Through Modified Cell Architecture. J. Power Sources 2012, 206, 450–453. [Google Scholar] [CrossRef]
- Liu, Q.H.; Grim, G.M.; Papandrew, A.B.; Turhan, A.; Zawodzinski, T.A.; Mench, M.M. High Performance Vanadium Redox Flow Batteries with Optimized Electrode Configuration and Membrane Selection. J. Electrochem. Soc. 2012, 159, A1246–A1252. [Google Scholar] [CrossRef]
- Houser, J.; Pezeshki, A.; Clement, J.T.; Aaron, D.; Mench, M.M. Architecture for Improved Mass Transport and System Performance in Redox Flow Batteries. J. Power Sources 2017, 351, 96–105. [Google Scholar] [CrossRef]
- Zhou, X.L.; Zeng, Y.K.; Zhu, X.B.; Wei, L.; Zhao, T.S. A High-Performance Dual-Scale Porous Electrode for Vanadium Redox Flow Batteries. J. Power Sources 2016, 325, 329–336. [Google Scholar] [CrossRef]
- Darling, R.M.; Perry, M.L. The Influence of Electrode and Channel Configurations on Flow Battery Performance. J. Electrochem. Soc. 2014, 161, A1381–A1387. [Google Scholar] [CrossRef]
- Won, S.; Oh, K.; Ju, H. Numerical Studies of Carbon Paper-Based Vanadium Redox Flow Batteries. Electrochim. Acta 2016, 201, 286–299. [Google Scholar] [CrossRef]
- Liu, T.; Li, X.; Xu, C.; Zhang, H. Activated Carbon Fiber Paper Based Electrodes with High Electrocatalytic Activity for Vanadium Flow Batteries with Improved Power Density. ACS Appl. Mater. Interfaces 2017, 9, 4626–4633. [Google Scholar] [CrossRef] [PubMed]
- Dennison, C.R.; Agar, E.; Akuzum, B.; Kumbur, E.C. Enhancing Mass Transport in Redox Flow Batteries by Tailoring Flow Field and Electrode Design. J. Electrochem. Soc. 2016, 163, A5163–A5169. [Google Scholar] [CrossRef]
- Mayrhuber, I.; Dennison, C.R.; Kalra, V.; Kumbur, E.C. Laser-Perforated Carbon Paper Electrodes for Improved Mass-Transport in High Power Density Vanadium Redox Flow Batteries. J. Power Sources 2014, 260, 251–258. [Google Scholar] [CrossRef]
- You, X.; Ye, Q.; Cheng, P. Scale-Up of High Power Density Redox Flow Batteries by Introducing Interdigitated Flow Fields. Int. Commun. Heat Mass Transf. 2016, 75, 7–12. [Google Scholar] [CrossRef]
- Perry, M.L.; Darling, R.M.; Zaffou, R. High Power Density Redox Flow Battery Cells. ECS Trans. 2013, 53, 7–16. [Google Scholar] [CrossRef]
- McCreery, R. Carbon Electrodes: Structural Effects on Electron Transfer Kinetics. In Electroanalytical Chemistry; Bard, A.J., Ed.; Marcel Dekker, Inc.: New York, NY, USA, 1991; Volume 17, pp. 221–374. ISBN 9780824784096-CAT# DK5885. [Google Scholar]
- Tilton, J.N. Fluid and Particle Dynamics. In Perry’s Chemical Engineers’ Handbook, 7th ed.; Green, D.W., Maloney, J.O., Eds.; McGraw-Hill: New York, NY, USA, 1997; ISBN 0-07-049841-5. [Google Scholar]
- Mohammadi, T.; Chieng, S.C.; Skyllas-Kazacos, M. Water Transport Study across Commercial Ion Exchange Membranes in the Vanadium Redox Flow Battery. J. Membr. Sci. 1997, 133, 151–159. [Google Scholar] [CrossRef]








| Cell Number | Membrane | Electrode Thickness/mm | Compression Pressure/bar | % Compression |
|---|---|---|---|---|
| 1 | Nafion 117 | 1.43 | 6.95 | 71 |
| 2 | Nafion 117 | 2.21 | 2.20 | 54 |
| 3 | Nafion 117 | 3.47 | 0.77 | 29 |
| 4 | Nafion 117 | 4.37 | 0.17 | 10 |
| 5 | Nafion 212 | 1.40 | 7.49 | 71 |
| 6 | Nafion 212 | 2.07 | 2.52 | 57 |
| 7 | Nafion 212 | 3.26 | 0.93 | 33 |
| 8 | Nafion 212 | 4.42 | 0.15 | 9 |
| 9 | Nafion 117 | 2.21 | 2.23 | 55 |
| 10 | Nafion 117 | 4.31 | 0.21 | 11 |
| Electrode Material | Electrode Pre-Treatment | % Comp. 1 | Electrolyte | OCV 2/V | Membrane (Thickness 3) | Flow Rate/mL min−1 cm−2 | E at 0.5 A cm−2/V | VEd at 0.5 A cm−2 | Peak Power/mW cm−2 | HFR 4/Ω cm2 | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Paper GDL 10AA | None | - | 1 M V 4 M H2SO4 | 1.68 5 | Nafion 117 (183 μm) | 4 | 0.99 | 0.59 | 557 | 0.50 | [22] |
| Paper GDL 10AA | Thermal | - | 1 M V 5 M H2SO4 | 1.66 5 | Nafion 212 (50.8 μm) | 18 | 1.26 | 0.76 | 767 | 0.219 | [23] |
| Paper GDL 10AA | None | - | 1.7 M V 3.3 M H2SO4 | 1.43 | Nafion 117 (183 μm) | 5.6 | 1.0 | 0.70 | 1290 | - | [24] |
| Felt GFA5 | Thermal | 33% | 1.4 M V 2 M H2SO4 | 1.5 | SFPAE 6 (45 μm) | 10 | 0.47 | 0.31 | 311 | - | [20] |
| Felt GFA5 | None | - | 1 M V 4 M H2SO4 | 1.65 | Nafion 115 (127 μm) | 10 | 0.70 | 0.42 | 350 | - | [21] |
| Felt GFD 4.6EA | None | 71% | 1.6 M V 4 M H2SO4 | 1.38 | Nafion 117 (183 μm) | 3.2 | 0.81 | 0.59 | 429 | 0.753 | Cell 1 |
| Felt GFD 4.6EA | None | 71% | 1.6 M V 4 M H2SO4 | 1.38 | Nafion 212 (50.8 μm) | 3.2 | 0.98 | 0.71 | 669 | 0.374 | Cell 5 |
| Cell | i/mA cm−2 | CE/% | VE/% | EE/% | U/% |
|---|---|---|---|---|---|
| Cell 9, 2.2 bar 55% compressed | 90 | 96.1 | 72.4 | 69.5 | 83.7 |
| 174 | 97.6 | 56.1 | 54.8 | 68.2 | |
| 435 | 96.7 | 28.1 | 27.1 | 43.1 | |
| Cell 10, 0.2 bar 11% compressed | 90 | 97.3 | 67.3 | 65.5 | 70.8 |
| 174 | 96.6 | 50.8 | 49.0 | 49.0 | |
| 435 | - | - | - | - |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Davies, T.J.; Tummino, J.J. High-Performance Vanadium Redox Flow Batteries with Graphite Felt Electrodes. C 2018, 4, 8. https://doi.org/10.3390/c4010008
Davies TJ, Tummino JJ. High-Performance Vanadium Redox Flow Batteries with Graphite Felt Electrodes. C. 2018; 4(1):8. https://doi.org/10.3390/c4010008
Chicago/Turabian StyleDavies, Trevor J., and Joseph J. Tummino. 2018. "High-Performance Vanadium Redox Flow Batteries with Graphite Felt Electrodes" C 4, no. 1: 8. https://doi.org/10.3390/c4010008