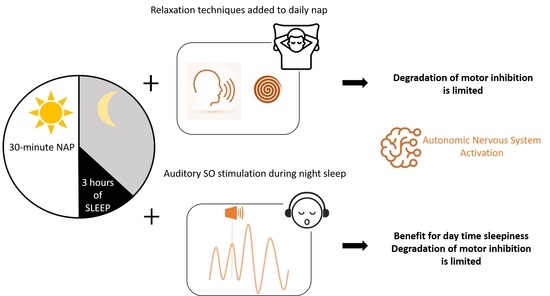
Strategies to Limit Cognitive Impairments under Sleep Restriction: Relationship to Stress Biomarkers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Experimental Design
2.3. Methods
2.3.1. Procedure for Relaxation Techniques in the SR-RT Session
2.3.2. Procedure for Auditory Sleep SO Stimulations in the SR-NS Session
2.3.3. Outcome Measurements and Study Instruments
3. Results
3.1. Participants
3.2. Nighttime and Nap Sleep Assessment
3.3. Subjective Sleepiness (KSS Score)
3.4. Cognitive Performances
3.4.1. Sustained Attention
3.4.2. Inhibition Capacity
3.4.3. Working Memory Capacity
3.5. Salivary Biomarkers Concentrations
3.6. Correlation Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Åkerstedt, T.; Wright, K.P. Sleep Loss and Fatigue in Shift Work and Shift Work Disorder. Sleep Med. Clin. 2009, 4, 257–271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, N.L.; Shattuck, L.G.; Matsangas, P. Longitudinal study of sleep patterns of United States Military Academy cadets. Sleep 2010, 33, 1623–1631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Good, C.H.; Brager, A.J.; Capaldi, V.F.; Mysliwiec, V. Sleep in the United States Military. Neuropsychopharmacology 2020, 45, 176–191. [Google Scholar] [CrossRef] [Green Version]
- Akerstedt, T. Sleepiness as a consequence of shift work. Sleep 1988, 11, 17–34. [Google Scholar] [CrossRef] [PubMed]
- Rabat, A.; Gomez-Merino, D.; Roca-Paixao, L.; Bougard, C.; Van Beers, P.; Dispersyn, G.; Guillard, M.; Bourrilhon, C.; Drogou, C.; Arnal, P.J.; et al. Differential Kinetics in Alteration and Recovery of Cognitive Processes from a Chronic Sleep Restriction in Young Healthy Men. Front. Behav. Neurosci. 2016, 10, 95. [Google Scholar] [CrossRef] [Green Version]
- Lowe, C.J.; Safati, A.; Hall, P.A. The neurocognitive consequences of sleep restriction: A meta-analytic review. Neurosci. Biobehav. Rev. 2017, 80, 586–604. [Google Scholar] [CrossRef]
- Grandner, M.A.; Seixas, A.; Shetty, S.; Shenoy, S. Sleep Duration and Diabetes Risk: Population Trends and Potential Mechanisms. Curr. Diab. Rep. 2016, 16, 106. [Google Scholar] [CrossRef] [Green Version]
- Mantua, J.; Bessey, A.F.; Sowden, W.J. Poor Subjective Sleep Quality Is Associated with Poor Occupational Outcomes in Elite Soldiers. Clocks Sleep 2020, 15, 182–193. [Google Scholar] [CrossRef]
- Martin-Gill, C.; Barger, L.K.; Moore, C.G.; Higgins, J.S.; Teasley, E.M.; Weiss, P.M.; Condle, J.P.; Flickinger, K.L.; Coppler, P.J.; Sequeira, D.J.; et al. Effects of Napping During Shift Work on Sleepiness and Performance in Emergency Medical Services Personnel and Similar Shift Workers: A Systematic Review and Meta-Analysis. Prehosp. Emerg. Care 2018, 22, 47–57. [Google Scholar] [CrossRef]
- Sauvet, F.; Gomez, D.; Rabat, A.; Chennaoui, M. Guide Pratique. In Gestion du Cycle Veille-Sommeil en Milieu Militaire; IRBA (Institut de Recherche Biomédicale des Armées): Brétigny sur Orge, France, 2020; p. 83. [Google Scholar]
- Vgontzas, A.N.; Pejovic, S.; Zoumakis, E.; Lin, H.M.; Bixler, E.O.; Basta, M.; Fang, J.; Sarrigiannidis, A.; Chrousos, G.P. Daytime napping after a night of sleep loss decreases sleepiness, improves performance, and causes beneficial changes in cortisol and interleukin-6 secretion. Am. J. Physiol. Endocrinol. Metab. 2007, 292, E253–E261. [Google Scholar] [CrossRef]
- Faraut, B.; Boudjeltia, K.Z.; Dyzma, M.; Rousseau, A.; David, E.; Stenuit, P.; Franck, T.; Van Antwerpen, P.; Vanhaeverbeek, M.; Kerkhofs, M. Benefits of napping and an extended duration of recovery sleep on alertness and immune cells after acute sleep restriction. Brain Behav. Immun. 2011, 25, 16–24. [Google Scholar] [CrossRef]
- Batéjat, D.M.; Lagarde, D.P. Naps and modafinil as countermeasures for the effects of sleep deprivation on cognitive performance. Aviat. Space Environ. Med. 1999, 70, 493–498. [Google Scholar]
- Bonnet, M.H.; Arand, D.L. Impact of naps and caffeine on extended nocturnal performance. Physiol. Behav. 1994, 56, 103–109. [Google Scholar] [CrossRef]
- Debellemaniere, E.; Gomez-Merino, D.; Erblang, M.; Dorey, R.; Genot, M.; Perreaut-Pierre, E.; Pisani, A.; Rocco, L.; Sauvet, F.; Léger, D.; et al. Using relaxation techniques to improve sleep during naps. Ind. Health 2018, 56, 220–227. [Google Scholar] [CrossRef] [Green Version]
- Debellemaniere, E.; Chambon, S.; Pinaud, C.; Thorey, V.; Dehaene, D.; Léger, D.; Chennaoui, M.; Arnal, P.J.; Galtier, M.N. Performance of an Ambulatory Dry-EEG Device for Auditory Closed-Loop Stimulation of Sleep Slow Oscillations in the Home Environment. Front. Hum. Neurosci. 2018, 12, 88. [Google Scholar] [CrossRef]
- Ngo, H.-V.V.; Martinetz, T.; Born, J.; Mölle, M. Auditory closed-loop stimulation of the sleep slow oscillation enhances memory. Neuron 2013, 78, 545–553. [Google Scholar] [CrossRef] [Green Version]
- Leminen, M.M.; Virkkala, J.; Saure, E.; Paajanen, T.; Zee, P.C.; Santostasi, G.; Hublin, C.; Müller, K.; Porkka-Heiskanen, T.; Huotilainen, M.; et al. Enhanced Memory Consolidation Via Automatic Sound Stimulation During Non-REM Sleep. Sleep 2017, 40, zsx003. [Google Scholar] [CrossRef] [Green Version]
- Besedovsky, L.; Ngo, H.-V.V.; Dimitrov, S.; Gassenmaier, C.; Lehmann, R.; Born, J. Auditory closed-loop stimulation of EEG slow oscillations strengthens sleep and signs of its immune-supportive function. Nat. Commun. 2017, 8, 1984. [Google Scholar] [CrossRef]
- Grimaldi, D.; Papalambros, N.A.; Reid, K.J.; Abbott, S.M.; Malkani, R.G.; Gendy, M.; Iwanaszko, M.; Braun, R.I.; Sanchez, D.J.; Paller, K.A.; et al. Strengthening sleep–autonomic interaction via acoustic enhancement of slow oscillations. Sleep 2019, 42, zsz036. [Google Scholar] [CrossRef]
- Diep, C.; Garcia-Molina, G.; Jasko, J.; Manousakis, J.; Ostrowski, L.; White, D.; Anderson, C. Acoustic enhancement of slow wave sleep on consecutive nights improves alertness and attention in chronically short sleepers. Sleep Med. 2021, 81, 69–79. [Google Scholar] [CrossRef]
- Ozgundondu, B.; Metin, Z.G. Effects of progressive muscle relaxation combined with music on stress, fatigue, and coping styles among intensive care nurses. Intensive Crit. Care Nurs. 2019, 54, 54–63. [Google Scholar] [CrossRef]
- Hashim, H.A.; Zainol, N.A. Changes in emotional distress, short term memory, and sustained attention following 6 and 12 sessions of progressive muscle relaxation training in 10-11 years old primary school children. Psychol. Health Med. 2015, 20, 623–628. [Google Scholar] [CrossRef]
- Obayashi, K. Salivary mental stress proteins. Clin. Chim. Acta 2013, 425, 196–201. [Google Scholar] [CrossRef]
- Buysse, D.J.; Reynolds, C.F.; Monk, T.H.; Berman, S.R.; Kupfer, D.J. The Pittsburgh sleep quality index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989, 28, 193–213. [Google Scholar] [CrossRef]
- Zigmond, A.S.; Snaith, R.P. The Hospital Anxiety and Depression Scale. Acta Psychiatr. Scand. 1983, 67, 361–370. [Google Scholar] [CrossRef] [Green Version]
- Perreaut-Pierre, E. Comprendre et Pratiquer les Techniques d’Optimisation, 2nd ed.; InterEditions: Paris, France, 2012. [Google Scholar]
- Akerstedt, T.; Gillberg, M. Subjective and objective sleepiness in the active individual. Int. J. Neurosci. 1990, 52, 29–37. [Google Scholar] [CrossRef]
- Basner, M.; Dinges, D.F. Maximizing sensitivity of the psychomotor vigilance test (PVT) to sleep loss. Sleep 2011, 34, 581–591. [Google Scholar] [CrossRef]
- Sagaspe, P.; Taillard, J.; Amiéva, H.; Beck, A.; Rascol, O.; Dartigues, J.F.; Capelli, A.; Philip, P. Influence of age, circadian and homeostatic processes on inhibitory motor control: A Go/Nogo task study. PLoS ONE 2012, 7, e39410. [Google Scholar] [CrossRef] [Green Version]
- Braver, T.S.; Cohen, J.D.; Nystrom, L.E.; Jonides, J.; Smith, E.E.; Noll, D.C. A parametric study of prefrontal cortex involvement in human working memory. Neuroimage 1997, 5, 49–62. [Google Scholar] [CrossRef]
- Van Dongen, H.P.A.; Maislin, G.; Mullington, J.M.; Dinges, D.F. The cumulative cost of additional wakefulness: Dose-response effects on neurobehavioral functions and sleep physiology from chronic sleep restriction and total sleep deprivation. Sleep 2003, 26, 117–126. [Google Scholar] [CrossRef]
- Belenky, G.; Wesensten, N.J.; Thorne, D.R.; Thomas, M.L.; Sing, H.C.; Redmond, D.P.; Russo, M.B.; Balkin, T.J. Patterns of performance degradation and restoration during sleep restriction and subsequent recovery: A sleep dose-response study. J. Sleep Res. 2003, 12, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, J.E.A.; Jones, S.A.H.; Eskes, G.A.; Rusak, B. Acute Sleep Restriction Has Differential Effects on Components of Attention. Front. Psychiatry 2018, 9, 499. [Google Scholar] [CrossRef] [PubMed]
- Lo, J.C.; Lee, S.M.; Teo, L.M.; Lim, J.; Gooley, J.J.; Chee, M.W.L. Neurobehavioral Impact of Successive Cycles of Sleep Restriction with and Without Naps in Adolescents. Sleep 2017, 40, zsw042. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Horn, N.R.; Dolan, M.; Elliot, R.; Deakin, J.F.W.; Woodruuf, P.R.W. Response inhibition and impulsivity: An FMRI study. Neuropsychologia 2003, 41, 1959–1966. [Google Scholar] [CrossRef]
- Drummond, S.P.A.; Paulus, M.P.; Tapert, S.F. Effects of two nights sleep deprivation and two nights recovery sleep on response inhibition. J. Sleep Res. 2006, 15, 261–265. [Google Scholar] [CrossRef] [PubMed]
- Davis, M.; Robbins Eshelman, E.; McKay, M. (Eds.) The Relaxation and Stress Reduction Workbook; New Harbinger Publications: Oakland, CA, USA, 1988; p. 312. [Google Scholar]
- Kobayashi, S.; Koitabashi, K. Effects of progressive muscle relaxation on cerebral activity: An fMRI investigation Complement. Ther. Med. 2016, 26, 33–39. [Google Scholar] [CrossRef]
- Weafer, J.; Baggott, M.J.; de Wit, H. Test-retest reliability of behavioral measures of impulsive choice, impulsive action, and inattention. Exp. Clin. Psychopharmacol. 2013, 21, 475–481. [Google Scholar] [CrossRef]
- Borbely, A.A.; Achermann, P. Sleep homeostasis and models of sleep regulation. J. Biol. Rhythm. 1999, 14, 557–568. [Google Scholar] [CrossRef]
- Vgontzas, A.N.; Zoumakis, E.; Bixler, E.O.; Lin, H.M.; Follett, H.; Kales, A.; Chrousos, G.P. Adverse effects of modest sleep restriction on sleepiness, performance, and inflammatory cytokines. J. Clin. Endocrinol. Metab. 2004, 89, 2119–2126. [Google Scholar] [CrossRef] [Green Version]
- Sauvet, F.; Drogou, C.; Bougard, C.; Arnal, P.J.; Dispersyn, G.; Bourrilhon, C.; Rabat, A.; Van Beers, P.; Gomez-Merino, D.; Faraut, B.; et al. Vascular response to 1week of sleep restriction in healthy subjects. A metabolic response? Int. J. Cardiol. 2015, 190, 246–255. [Google Scholar] [CrossRef]
- Van Leeuwen, W.M.A.; Sallinen, M.; Virkkala, J.; Lindholm, H.; Hirvonen, A.; Hublin, C.; Porkka-Heiskanen, T.; Härmä, M. Physiological and autonomic stress responses after prolonged sleep restriction and subsequent recovery sleep in healthy young men. Sleep Biol. Rhythm. 2018, 16, 45–54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prehn-Kristensen, A.; Ngo, H.-V.V.; Lentfer, L.; Berghäuser, J.; Brandes, L.; Schulze, L.; Göder, R.; Mölle, M.; Baving, L. Acoustic closed-loop stimulation during sleep improves consolidation of reward-related memory information in healthy children but not in children with attention-deficit hyperactivity disorder. Sleep 2020, 43, zsaa017. [Google Scholar] [CrossRef]
- Simpson, N.S.; Diolombi, M.; Scott-Sutherland, J.; Yang, H.; Bhatt, V.; Gautam, S.; Mullington, J.; Haack, M. Repeating patterns of sleep restriction and recovery: Do we get used to it? Brain Behav. Immun. 2016, 58, 142–151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teixeira, R.R.; Díaz, M.M.; da Silva Santos, T.V.; Bernardes, J.T.M.; Peixoto, L.G.; Bocanegra, O.L.; Neto, M.B.; Espindola, F.S. Chronic stress induces a hyporeactivity of the autonomic nervous system in response to acute mental stressor and impairs cognitive performance in business executives. PLoS ONE 2015, 10, e0119025. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pajcin, M.; Banks, S.; White, J.M.; Dorrian, J.; Paech, G.M.; Grant, C.; Johnson, K.; Tooley, K.; Fidock, J.; Kamimori, G.H.; et al. Decreased salivary alpha-amylase levels are associated with performance deficits during sleep loss. Psychoneuroendocrinology 2017, 78, 131–141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Engeland, C.G.; Bosch, J.A.; Rohleder, N. Salivary Biomarkers in Psychoneuroimmunology. Curr. Opin. Behav. Sci. 2019, 28, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Takatsuji, K.; Sugimoto, Y.; Ishizaki, S.; Ozaki, Y.; Matsuyama, E.; Yamaguchi, Y. The effects of examination stress on salivary cortisol, immunoglobulin A, and chromogranin A in nursing students. Biomed. Res. 2008, 29, 221–224. [Google Scholar] [CrossRef] [Green Version]
- Bougard, C.; VanBeers, P.; Sauvet, F.; Drogou, C.; Guillard, M.; Dorey, R.; Gomez-Merino, D.; Dauguet, J.; Takillah, S.; Espié, S.; et al. Motorcycling performance and sleepiness during an extended ride on a dynamic simulator: Relationship with stress biomarkers. Physiol. Meas. 2020, 41, 104004. [Google Scholar] [CrossRef]
- Hajali, V.; Andersen, M.; Negah, S.S.; Sheibani, V. Sex differences in sleep and sleep loss-induced cognitive deficits: The influence of gonadal hormones. Horm. Behav. 2019, 108, 50–61. [Google Scholar] [CrossRef] [PubMed]
- Lac, G.; Chamoux, A. Do circannual rhythm of cortisol and testosterone interfere with variations induced by other events? Ann. Endocrinol. 2006, 67, 60–63. [Google Scholar] [CrossRef]







| Condition (C) | Day (D) | C × D | |
|---|---|---|---|
| F(2,20) | F(3,30) | F(6,60) | |
| KSS | 5.11 * | 30.1 *** | 2.12 (p = 0.06) |
| PVT lapses | 1.93 (p = 0.17) | 14.1 *** | 1.05 (p = 0.41) |
| PVT speed | 0.86 (p = 0.44) | 28.7 *** | 0.58 (p = 0.75) |
| Go–noGo errors % | 5.03 * | 10.26 *** | 2.19 (p = 0.06) |
| Go–noGo reaction time | 0.83 (p = 0.45) | 16.6 *** | 1.28 (p = 0.28) |
| 2-back correct responses % | 0.90 (p = 0.42) | 11.2 *** | 0.86 (p = 0.53) |
| Cortisol | 0.55 (p = 0.59) | 6.60 ** | 0.15 (0.99) |
| Alpha-amylase | 2.33 (p = 0.12) | 5.28 ** | 2.47 * |
| Chromogranin A | 3.50 * | 3.52 * | 3.52 ** |
| Variable | KSS | PVT L | PVT S | GnG E | GnG RT | 2-b CR | Cortisol | sAA | CgA | |
|---|---|---|---|---|---|---|---|---|---|---|
| KSS | 1.000 | 0.522 * | −0.429 * | 0.435 * | 0.369 * | −0.325 * | 0.197 | −0.163 | 0.048 | |
| PVT L | 1.000 | −0.592 * | 0.757 * | 0.488 * | −0.503 * | 0.119 | −0.320 * | −0.025 | ||
| PVT S | 1.000 | −0.404 * | −0.561 * | 0.374 * | −0.155 | 0.335 * | −0.010 | |||
| GnG E | 1.000 | 0.490 * | −0.641 * | 0.000 | −0.239 * | 0.090 | ||||
| GnG RT | 1.000 | −0.534 * | 0.184 | −0.070 | 0.286 * | |||||
| 2-b CR | 1.000 | −0.019 | 0.065 | −0.300 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gomez-Merino, D.; Drogou, C.; Debellemaniere, E.; Erblang, M.; Dorey, R.; Guillard, M.; Van Beers, P.; Thouard, M.; Masson, R.; Sauvet, F.; et al. Strategies to Limit Cognitive Impairments under Sleep Restriction: Relationship to Stress Biomarkers. Brain Sci. 2022, 12, 229. https://doi.org/10.3390/brainsci12020229
Gomez-Merino D, Drogou C, Debellemaniere E, Erblang M, Dorey R, Guillard M, Van Beers P, Thouard M, Masson R, Sauvet F, et al. Strategies to Limit Cognitive Impairments under Sleep Restriction: Relationship to Stress Biomarkers. Brain Sciences. 2022; 12(2):229. https://doi.org/10.3390/brainsci12020229
Chicago/Turabian StyleGomez-Merino, Danielle, Catherine Drogou, Eden Debellemaniere, Mégane Erblang, Rodolphe Dorey, Mathias Guillard, Pascal Van Beers, Melanie Thouard, Robin Masson, Fabien Sauvet, and et al. 2022. "Strategies to Limit Cognitive Impairments under Sleep Restriction: Relationship to Stress Biomarkers" Brain Sciences 12, no. 2: 229. https://doi.org/10.3390/brainsci12020229