Advanced Nanomaterials-Based Electrochemical Biosensors for Catecholamines Detection: Challenges and Trends
Abstract
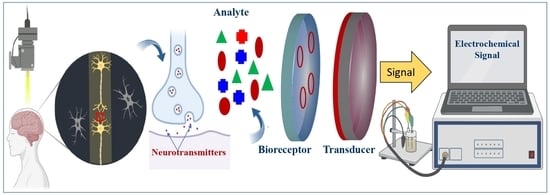
:1. Introduction
2. Catecholamines Significance in Biological System
3. Nanomaterials Enhancing Electrochemical Biosensors for Catecholamines Detection
3.1. Carbon Nanomaterials Based Electrochemical Sensors
| NTs | Sample | Catalyst/Transducer | Technique Used | Linear Range (μM) | Detection Limit (nM) | Ref. |
|---|---|---|---|---|---|---|
| DA | Human serum | MWCNTs-ZnO/GCE | CV, DPV | 0.01–30 | 3.2 | [59] |
| DA, 5-HT | PBS | Curcumin oxidized carbon nanotubes/GCE | LSV | 0–170 10–130 | 0.010 0.011 | [60] |
| EP | Real water | MWCNTs-molybdenum disulphide/GCE | CV | 9.9–137.9 | 0.003 | [61] |
| DA, EP | Synthetic urine | Oxidized capsaicin-MWCNTs/GCE | CV Amperometry | 5–75 5–115 | 0.0072 0.0015 | [62] |
| EP | Urine and pharmaceutical sample | Chitosan-functionalized carbon nanotubes/GCE | CV, DPV | 0.05–10 | 30 | [63] |
| DA | Human blood serum | CaCO3-PANi-rGO/GCE | DPV | 0.1–14 | 100 | [64] |
| DA, UA | PBS buffer | Thermally rGO/GCE | CV, DPV | 5–42 | 120 150 | [65] |
| DA | Human urine | rGO-tungsten trioxide/ GCE | CV, Amperometry | 0.3–1245 | 306 | [66] |
| EP | PBS buffer | rGO-MoS2-Fe3O4/GCE | CV, DPV | 0–11 | 137 | [67] |
| EP | Human serum | 2D nickel oxide-rGO/GCE | CV, DPV | 50–500 | 1000 | [68] |
| EP | Urine | rGO-Ti3C2Tx MXene/Indium tin oxide | DPV | 1–60 | 3.5 | [69] |
| DA | PBS | GO-CuAlO2/GCE | LSV | 0.92–10 | 15 | [70] |
| EP | Human serum | Au-Pd-rGO/GCE | CV, DPV | 0.001–1000 | 12 | [71] |
3.2. Metal Nanoparticles-Based Sensors
| NTs | Transducer | Probe | Detection Method | Linear Range (μM) | LOD (μM) | Ref. |
|---|---|---|---|---|---|---|
| DA | PGE | Citrate-stabilized gold nanoparticles @polydopamine) | SWV | 0.5–7.0 | 0.53 | [90] |
| DA | GCE | Copper nanoparticles | CV, DPV | 0.05–5.0 | 0.04 | [91] |
| DA | GCE | Carbon quantum dots and copper oxide | SWV | 1–180 | 25.4 | [92] |
| DA | Diamond anoporous | AuNPs and Nafion | SWV | 3–100 | 0.068 | [93] |
| DA | GCE | Gold-decorated porous silicon-poly(3-hexylthiophene) | Amperometry | 1–460 | 0.63 | [94] |
| DA, UA | CPE | Cu-based metal-organic frameworks | DVP | 0.05–500 | 0.03 0.07 | [95] |
| DA | GCE | Copper organic framework@halloysite nanotubes-rGO | DPV | 0.1–130 | 0.015 | [96] |
| DA | GCE | Carbon-titanium nitride nanoparticles | DPV | 0.1–250 | 0.03 | [97] |
| DA | GCE | Palladium nanoparticles decorated nickel-based metal–organic framework | CV, DPV | 0.001–100 | 0.01 | [98] |
| DA | GCE | Nitrogen-doped titanium dioxide-AgNPs-GQD | CV, DPV | 0.003–300 | 0.001 | [99] |
| DA | FTO | Nanoplatelets of zinc oxide embedded polyvinyl alcohol | EIS | 0.020–3000 | 0.005 | [100] |
| DA | GCE | Cobalt phthalocyanine-nitrogen-doped GQD | Amperometry | 100–1000 | 0.12 | [101] |
| DA | Carbon spheres | Sodium tungsten bronzes nanoparticles | DPV | 0.004–106.4 | 0.001 | [102] |
| EP, NE | CPE | Cu quantum dot@ SH-nanoparticles immobilized on CuMOF | DPV | 0.2–34,285 | 1.6 0.5 | [103] |
| NE | GCE | Graphene quantum dots decorated AuNPs | DPV | 0.5–7.5 | 0.15 | [104] |
| DA, EP | CPE | Nickel telluride | SWV | 4–31 | 0.15 0.35 | [105] |
MOF and COF-Based Sensor
3.3. Polymer Film Based Electrochemical Sensors
| Catecho-Lamine | Transducer | Catalyst | Technique Used | Linear Range (μM) | Detection Limit (nM) | Ref. |
|---|---|---|---|---|---|---|
| DA | GCE | Poly paraphenylene diamine | DPV | 0.038–4.76 | 0.094 | [120] |
| DA, UA | GCE | polypyrrole matrix supported iron | CV | 10–900 | 321 348 | [121] |
| DA | GCE | polyaniline-WO3 | CV, DPV | 20–300 | 139 | [122] |
| DA | CPE | Polymelamine-AuNPs | CV, DPV | 0.2–11 | 67 | [123] |
| DA | LSGE | Overoxidized polypyrrole (PPyox) | CV, DPV | 0.010–10 | 7 | [82] |
| EP, 5-HT | CPE | Poly Victoria blue B | DPV | 1–80 | 330 980 | [117] |
| DA | GCE | Poly-tryptophan | DPV | 0.2–100 | 60 | [124] |
| DA, UA, AA | GCE | Copper monoamino-phthalocyanine-acrylate polymer | DPV | 0.01–10 | 0.7 2.5 5 | [125] |
4. DNA Aptamer-Based Catecholamine Biosensors
| NTs | Biosensor Structure | Interferents | Sample | Measurement | Linear Range (nM) | LOD (nM) | Ref. |
|---|---|---|---|---|---|---|---|
| EP | Aptamer based Organic electrochemical transistors | DA, Cysteine, AA and tryptophan | PBS solution | Amperometry | 0.9–90 × 103 | 0.9 | [136] |
| DA | Aptamer-AuNPs-rGO/GCE | AA, UA, EP and cathechol | Human serum | DPV | 1–100 | 47 | [137] |
| DA | Aptamer-Copper aluminate-rGO-TEPA/SPE | UA, AA, and glucose | Human serum | DPV | 0.05–10 × 103 | 0.017 | [138] |
| DA | Aptamer-CeMOF/GCE | AA, BSA, and bilirubin | Clinical serum | SWV | 0.5–100 | 0.06 | [133] |
| DA | Aptamer-GCSC-GO/GCE | DOPA, AA, HVA | Human serum | DPV | 1–1000 | 0.75 | [139] |
| DA | Aptmer-Gold nanostructure/Au electrode | AA, UA, Catechol, EP, and NE | Clinical serum | DPV | 0.163–20 | 0.01 | [140] |
| EP | Aptamer-Methylene blue/GCE | AA, UA, and levodopa | SH-SY5Y cells | CV, DPV | 200–10 × 103 | 67 | [141] |
5. Enzyme Based Catecholamine Biosensors
6. Nano/Microelectrode-Based Catecholamine Monitoring
7. Advantages and Challenges of Electrochemical Catecholamines Detection
8. Other Strategies for Catecholamines Monitoring
8.1. Colorimetry and Spectrophotometry
8.2. Surface-Enhanced Raman Spectroscopy (SERS)
8.3. Fluorescence Spectrometry
8.4. Electrochemiluminescence (ECL) Spectrometry
8.5. Surface Plasmon Resonance (SPR)
9. Conclusions and Future Trends
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| NTs | Neurotransmitters |
| CNS | Central nervous system |
| DA | Dopamine |
| Ep | Epinephrine |
| NE | Norepinephrine |
| 5-HT | Serotonin |
| UA | Uric Acid |
| AA | Ascorbic acid |
| GCE | Glassy carbon electrode |
| CPE | Carbon paste electrode |
| PGE | Pencil graphite electrode |
| ITO | Indium Tin Oxide |
| SWV | Square wave voltammetry |
| DPV | Differential pulse voltammetry |
| CV | Cyclic voltammetry |
| EIS | Electrochemical impedance spectroscopy |
| LSV | Linear sweep voltammogram. |
| CuNPs | Copper nanoparticles |
| RC | Renewable carbon |
| CNTs | Carbon nanotubes |
| SWCNTs | Single-Walled Carbon Nanotubes |
| CQDs | Carbon quantum dots |
| CuO | Copper oxide |
| PANi | Polyaniline |
| GO | Graphene oxide |
| rGO: | Reduced graphene oxide |
| PBS | Phosphate-buffered saline |
| FTO | Fluorine-doped tin oxide |
| MoS2 | Molybdenum disulfide |
| LSGE | Laser-scribed graphene electrode |
| ZnO | Zinc oxide |
| LOD | Limit of detection |
| Ce-MOF | Cerium metal-organic framework |
| Cu-MOF | Copper metal-organic framework |
| GCSC | Grass carp skin collagen |
| FET | Field-effect transistor |
| TEPA | Tetraethy lenepentamine |
| CuAlO2 | Copper aluminate |
| POC | Point of care |
| IoT | Internet of Things |
| AI | Artificial intelligence |
| 3-APTMS | 3-aminopropyltrimethoxysilane |
| 3-GPTMS | 3-glycidoxypropyltrimethoxysilane |
References
- Munzuroğlu, M.; Danışman, B.; Akçay, G.; Yelli, İ.; Aslan, M.; Derin, N. Effects of Biotin Deficiency on Short Term Memory: The Role of Glutamate, Glutamic Acid, Dopamine and Protein Kinase A. Brain Res. 2022, 1792, 148031. [Google Scholar] [CrossRef] [PubMed]
- Lončar, A.; Negrojević, L.; Dimitrić-Marković, J.; Dimić, D. The Reactivity of Neurotransmitters and Their Metabolites towards Various Nitrogen-Centered Radicals: Experimental, Theoretical, and Biotoxicity Evaluation. Comput. Biol. Chem. 2021, 95, 107573. [Google Scholar] [CrossRef] [PubMed]
- Roondhe, B.; Jha, P.K. Neurotransmitter-Functionalized Boron Nitride Nanoribbons as Biological Cargo Carriers: Analysis by Density Functional Theory. ACS Appl. Nano Mater. 2019, 2, 1552–1561. [Google Scholar] [CrossRef]
- Xia, X.; Wang, Y.; Qin, Y.; Zhao, S.; Zheng, J.C. Exosome: A Novel Neurotransmission Modulator or Non-Canonical Neurotransmitter? Ageing Res. Rev. 2022, 74, 101558. [Google Scholar] [CrossRef]
- Xie, W.; Yin, Y.; Gu, R.; Xu, J.; Su, X.; Wang, Y.; Liu, R.; Liu, X.; Huang, J. Label-Free and Highly Selective MOFs-Based Dopamine Detection in Urine of Parkinson’s Patients. Chem. Eng. J. 2022, 443, 136371. [Google Scholar] [CrossRef]
- Savransky, A.; Chiappelli, J.; Du, X.; Carino, K.; Kvarta, M.; Bruce, H.; Kochunov, P.; Goldwaser, E.; Tan, Y.; Hare, S.; et al. Association of Working Memory and Elevated Overnight Urinary Norepinephrine in Patients with Schizophrenia. J. Psychiatr. Res. 2021, 137, 89–95. [Google Scholar] [CrossRef]
- Pandey, S.N.; Rangra, N.K.; Singh, S.; Arora, S.; Gupta, V. Evolving Role of Natural Products from Traditional Medicinal Herbs in the Treatment of Alzheimer’s Disease. ACS Chem. Neurosci. 2021, 12, 2718–2728. [Google Scholar] [CrossRef]
- Bacchella, C.; Dell’Acqua, S.; Nicolis, S.; Monzani, E.; Casella, L. The Reactivity of Copper Complexes with Neuronal Peptides Promoted by Catecholamines and Its Impact on Neurodegeneration. Coord. Chem. Rev. 2022, 471, 214756. [Google Scholar] [CrossRef]
- Umek, N. The Effects of Biologically Important Divalent and Trivalent Metal Cations on the Cyclization Step of Dopamine Autoxidation Reaction: A Quantum Chemical Study. Arab. J. Chem. 2022, 15, 104153. [Google Scholar] [CrossRef]
- Thomas, R.; Pooventhiran, T.; Bakht, M.A.; Alzahrani, A.Y.; Salem, M.A. Study of Interaction between Different Solvents and Neurotransmitters Dopamine, l-Adrenaline, and l-Noradrenaline Using LED, QTAIM and AIMD. J. Mol. Liq. 2022, 368, 120708. [Google Scholar] [CrossRef]
- Harris, E.P.; Villalobos-Manriquez, F.; Melo, T.G.; Clarke, G.; O’Leary, O.F. Stress during Puberty Exerts Sex-Specific Effects on Depressive-like Behavior and Monoamine Neurotransmitters in Adolescence and Adulthood. Neurobiol. Stress 2022, 21, 100494. [Google Scholar] [CrossRef] [PubMed]
- Gubbi, S.; Nazari, M.A.; Taieb, D.; Klubo-Gwiezdzinska, J.; Pacak, K. Catecholamine Physiology and Its Implications in Patients with COVID-19. Lancet Diabetes Endocrinol. 2020, 8, 978–986. [Google Scholar] [CrossRef] [PubMed]
- Derakhshan, M.; Ansarian, H.R.; Ghomshei, M. Possible Effect of Epinephrine in Minimizing COVID-19 Severity: A Review. J. Int. Med. Res. 2020, 48, 300060520958594. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Li, C.; Xu, S.; Yang, J.; Wan, H.; He, Y. LC-MS/MS Combined with Blood-Brain Dual Channel Microdialysis for Simultaneous Determination of Active Components of Astragali Radix-Safflower Combination and Neurotransmitters in Rats with Cerebral Ischemia Reperfusion Injury: Application in Pharmacokinetic and Pharmacodynamic Study. Phytomedicine 2022, 106, 154432. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.-J.; Wang, N.; Zhang, M.-J.; Liu, S.-S.; Yu, H.; Tang, M.-H.; Liu, Z.-Y. Simultaneous Determination of Five Amino Acid Neurotransmitters in Rat and Porcine Blood and Brain by Two-Dimensional Liquid Chromatography. J. Chromatogr. B 2021, 1163, 122507. [Google Scholar] [CrossRef]
- Chen, H.; Xie, H.; Xiao, T.; Wang, Z.; Ni, X.; Deng, S.; Lu, H.; Hu, J.; Li, L.; Wen, Y.; et al. Development of Mass Spectrometry-Based Relatively Quantitative Targeted Method for Amino Acids and Neurotransmitters: Applications in the Diagnosis of Major Depression. J. Pharm. Biomed. Anal. 2021, 194, 113773. [Google Scholar] [CrossRef]
- Šolínová, V.; Žáková, L.; Jiráček, J.; Kašička, V. Pressure Assisted Partial Filling Affinity Capillary Electrophoresis Employed for Determination of Binding Constants of Human Insulin Hexamer Complexes with Serotonin, Dopamine, Arginine, and Phenol. Anal. Chim. Acta 2019, 1052, 170–178. [Google Scholar] [CrossRef]
- Ramya, M.; Senthil Kumar, P.; Rangasamy, G.; Uma shankar, V.; Rajesh, G.; Nirmala, K.; Saravanan, A.; Krishnapandi, A. A Recent Advancement on the Applications of Nanomaterials in Electrochemical Sensors and Biosensors. Chemosphere 2022, 308, 136416. [Google Scholar] [CrossRef]
- Lakard, S.; Pavel, I.-A.; Lakard, B. Electrochemical Biosensing of Dopamine Neurotransmitter: A Review. Biosensors 2021, 11, 179. [Google Scholar] [CrossRef]
- Lakshmanakumar, M.; Nesakumar, N.; Kulandaisamy, A.J.; Rayappan, J.B.B. Principles and Recent Developments in Optical and Electrochemical Sensing of Dopamine: A Comprehensive Review. Measurement 2021, 183, 109873. [Google Scholar] [CrossRef]
- Su, Y.; Bian, S.; Sawan, M. Real-Time in Vivo Detection Techniques for Neurotransmitters: A Review. Analyst 2020, 145, 6193–6210. [Google Scholar] [CrossRef] [PubMed]
- Kaur, H.; Siwal, S.S.; Saini, R.V.; Singh, N.; Thakur, V.K. Significance of an Electrochemical Sensor and Nanocomposites: Toward the Electrocatalytic Detection of Neurotransmitters and Their Importance within the Physiological System. ACS Nanosci. Au 2022. [Google Scholar] [CrossRef]
- Aslanoglou, D.; Bertera, S.; Sánchez-Soto, M.; Benjamin Free, R.; Lee, J.; Zong, W.; Xue, X.; Shrestha, S.; Brissova, M.; Logan, R.W.; et al. Dopamine Regulates Pancreatic Glucagon and Insulin Secretion via Adrenergic and Dopaminergic Receptors. Transl Psychiatry 2021, 11, 59. [Google Scholar] [CrossRef]
- Post, M.R.; Lee, W.-L.; Guo, J.; Sames, D.; Sulzer, D. Development of a Dual Fluorescent and Magnetic Resonance False Neurotransmitter That Reports Accumulation and Release from Dopaminergic Synaptic Vesicles. ACS Chem. Neurosci. 2021, 12, 4546–4553. [Google Scholar] [CrossRef] [PubMed]
- Benes, F.M. Carlsson and the Discovery of Dopamine. Trends Pharm. Sci. 2001, 22, 46–47. [Google Scholar] [CrossRef]
- Liu, X.; Liu, J. Biosensors and Sensors for Dopamine Detection. VIEW 2021, 2, 20200102. [Google Scholar] [CrossRef]
- Feng, P.; Chen, Y.; Zhang, L.; Qian, C.-G.; Xiao, X.; Han, X.; Shen, Q.-D. Near-Infrared Fluorescent Nanoprobes for Revealing the Role of Dopamine in Drug Addiction. ACS Appl. Mater. Interfaces 2018, 10, 4359–4368. [Google Scholar] [CrossRef]
- Nasa, K.; Kurnia, I.; Hartati, Y.W.; Einaga, Y. Low-Interference Norepinephrine Signal on Dopamine Detection Using Nafion-Coated Boron Doped Diamond Electrodes. Biosens. Bioelectron. 2023, 220, 114892. [Google Scholar] [CrossRef]
- Krishna, V.M.; Somanathan, T.; Manikandan, E.; Tadi, K.K.; Uvarajan, S. Neurotransmitter Dopamine Enhanced Sensing Detection Using Fibre-Like Carbon Nanotubes by Chemical Vapor Deposition Technique. J. Nanosci. Nanotechnol. 2018, 18, 5380–5389. [Google Scholar] [CrossRef]
- Chou, S.-H.; Chen, Y.-J.; Liao, C.-P.; Pan, C.-L. A Role for Dopamine in C. Elegans Avoidance Behavior Induced by Mitochondrial Stress. Neurosci. Res. 2022, 178, 87–92. [Google Scholar] [CrossRef]
- Kim, S.G.; Lee, J.S. Ruthenium Nanoparticle-Immobilized Porous Carbon Nanofibers for Nonenzymatic Dopamine Sensing. ACS Appl. Nano Mater. 2021, 4, 13683–13691. [Google Scholar] [CrossRef]
- Oshaghi, M.; Kourosh-Arami, M.; Roozbehkia, M. Role of Neurotransmitters in Immune-Mediated Inflammatory Disorders: A Crosstalk between the Nervous and Immune Systems. Neurol. Sci. 2022, 44, 99–113. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, K.T.; Makhijani, V.H.; Boyt, K.M.; Cogan, E.S.; Pati, D.; Pina, M.M.; Bravo, I.M.; Locke, J.L.; Jones, S.R.; Besheer, J.; et al. Stress-Induced Alterations of Norepinephrine Release in the Bed Nucleus of the Stria Terminalis of Mice. ACS Chem. Neurosci. 2019, 10, 1908–1914. [Google Scholar] [CrossRef] [PubMed]
- Snyder, A.E.; Silberman, Y. Corticotropin Releasing Factor and Norepinephrine Related Circuitry Changes in the Bed Nucleus of the Stria Terminalis in Stress and Alcohol and Substance Use Disorders. Neuropharmacology 2021, 201, 108814. [Google Scholar] [CrossRef] [PubMed]
- Gu, P.; Li, L.; Fu, A.; Song, H.; Zhao, B.; Wei, L.; Ji, L.; Li, W.; Zhang, R.; Wang, Q.; et al. High-Performance Fluorescence Probe for Fast and Specific Visualization of Norepinephrine in Vivo and Depression-like Mice. Bioorganic Chem. 2023, 131, 106306. [Google Scholar] [CrossRef]
- Shao, H.; Li, C.-S. Epinephrine in Out-of-Hospital Cardiac Arrest: Helpful or Harmful? Chin. Med. J. 2017, 130, 2112–2116. [Google Scholar] [CrossRef]
- Prasad, B.B.; Prasad, A.; Tiwari, M.P.; Madhuri, R. Multiwalled Carbon Nanotubes Bearing ‘Terminal Monomeric Unit’ for the Fabrication of Epinephrine Imprinted Polymer-Based Electrochemical Sensor. Biosens. Bioelectron. 2013, 45, 114–122. [Google Scholar] [CrossRef]
- Ziegler, M.G.; Elayan, H.; Milic, M.; Sun, P.; Gharaibeh, M. Epinephrine and the Metabolic Syndrome. Curr Hypertens Rep 2012, 14, 1–7. [Google Scholar] [CrossRef]
- Altuntas, D.B.; Ören, T.; Anik, U. Centri-Voltammetric Detection of Epinephrine. Anal. Methods 2016, 8, 6872–6876. [Google Scholar] [CrossRef]
- Mathew, R.J.; Ho, B.T.; Francis, D.J.; Taylor, D.L.; Weinman, M.L. Catecholamines and Anxiety. Acta Psychiatr. Scand. 1982, 65, 142–147. [Google Scholar] [CrossRef]
- van Zijderveld, G.A.; Veltman, D.J.; van Dyck, R.; van Doornen, L.J. Epinephrine-Induced Panic Attacks and Hyperventilation. J. Psychiatr. Res. 1999, 33, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Martinho, R.; Oliveira, A.; Correia, G.; Marques, M.; Seixas, R.; Serrão, P.; Moreira-Rodrigues, M. Epinephrine May Contribute to the Persistence of Traumatic Memories in a Post-Traumatic Stress Disorder Animal Model. Front. Mol. Neurosci. 2020, 13, 588802. [Google Scholar] [CrossRef] [PubMed]
- Eivazzadeh-Keihan, R.; Bahojb Noruzi, E.; Chidar, E.; Jafari, M.; Davoodi, F.; Kashtiaray, A.; Ghafori Gorab, M.; Masoud Hashemi, S.; Javanshir, S.; Ahangari Cohan, R.; et al. Applications of Carbon-Based Conductive Nanomaterials in Biosensors. Chem. Eng. J. 2022, 442, 136183. [Google Scholar] [CrossRef]
- Afsarimanesh, N.; Nag, A.; e Alahi, M.E.; Sarkar, S.; Mukhopadhyay, S.; Sabet, G.S.; Altinsoy, M.E. A Critical Review of the Recent Progress on Carbon Nanotubes-Based Nanogenerators. Sens. Actuators A Phys. 2022, 344, 113743. [Google Scholar] [CrossRef]
- Thakur, N.; Das Adhikary, S.; Kumar, M.; Mehta, D.; Padhan, A.K.; Mandal, D.; Nagaiah, T.C. Ultrasensitive and Highly Selective Electrochemical Detection of Dopamine Using Poly(Ionic Liquids)–Cobalt Polyoxometalate/CNT Composite. ACS Omega 2018, 3, 2966–2973. [Google Scholar] [CrossRef] [PubMed]
- Thakur, N.; Gupta, D.; Mandal, D.; Nagaiah, T.C. Ultrasensitive Electrochemical Biosensors for Dopamine and Cholesterol: Recent Advances, Challenges and Strategies. Chem. Commun. 2021, 57, 13084–13113. [Google Scholar] [CrossRef]
- Durairaj, V.; Wester, N.; Etula, J.; Laurila, T.; Lehtonen, J.; Rojas, O.J.; Pahimanolis, N.; Koskinen, J. Multiwalled Carbon Nanotubes/Nanofibrillar Cellulose/Nafion Composite-Modified Tetrahedral Amorphous Carbon Electrodes for Selective Dopamine Detection. J. Phys. Chem. C 2019, 123, 24826–24836. [Google Scholar] [CrossRef] [Green Version]
- Numan, A.; Shahid, M.; Omar, F.S.; Rafique, S.; Bashir, S.; Ramesh, K.; Ramesh, S. Binary Nanocomposite Based on Co3O4 Nanocubes and Multiwalled Carbon Nanotubes as an Ultrasensitive Platform for Amperometric Determination of Dopamine. Microchim Acta. 2017, 184, 2739–2748. [Google Scholar] [CrossRef]
- Farahani, A. Developing a Point-of-Care System for Determination of Dopamine, Ascorbic and Uric Acids in Biological Fluids Using a Screen-Printed Electrode Modified by Three Dimensional Graphene/Carbon Nanotube Hybrid. Int. J. Electrochem. Sci. 2019, 14, 6195–6208. [Google Scholar] [CrossRef]
- Yang, X.; Zhao, P.; Xie, Z.; Ni, M.; Wang, C.; Yang, P.; Xie, Y.; Fei, J. Selective Determination of Epinephrine Using Electrochemical Sensor Based on Ordered Mesoporous Carbon / Nickel Oxide Nanocomposite. Talanta 2021, 233, 122545. [Google Scholar] [CrossRef]
- Kiranmai, S.; Kuchi, C.; Sravani, B.; Ƚuczak, T.; Kim, M.J.; Madhavi, G.; Veera Manohara Reddy, Y. Construction of Ultrasensitive Electrochemical Sensor Using TiO2-Reduced Graphene Oxide Nanofibers Nanocomposite for Epinephrine Detection. Surf. Interfaces 2022, 35, 102455. [Google Scholar] [CrossRef]
- Joseph, T.; Thomas, T.; Thomas, J.; Thomas, N. The Effect of Different GO Reduction Strategies on the Lower Level Electrochemical Determination of Epinephrine and Serotonin in Quaternary Mixtures. J. Electroanal. Chem. 2021, 901, 115760. [Google Scholar] [CrossRef]
- Sen, K.; Ali, S.; Singh, D.; Singh, K.; Gupta, N. Development of Metal Free Melamine Modified Graphene Oxide for Electrochemical Sensing of Epinephrine. FlatChem 2021, 30, 100288. [Google Scholar] [CrossRef]
- Suriyaprakash, J.; Gupta, N.; Wu, L.; Shan, L. Engineering of All Solution/Substrate Processable Biosensors for the Detection of Epinephrine as Low as PM with Rapid Readout. Chem. Eng. J. 2022, 436, 135254. [Google Scholar] [CrossRef]
- Thondaiman, P.; Manikandan, R.; Raj, C.J.; Savariraj, A.D.; Moulton, S.E.; Kim, B.C. Boron and Nitrogen Doped Graphene Quantum Dots on a Surface Modified Cu Mesh for the Determination of Dopamine and Epinephrine. Synth. Met. 2021, 278, 116831. [Google Scholar] [CrossRef]
- Buleandră, M.; Popa, D.E.; David, I.G.; Ciucu, A.A. A Simple and Efficient Cyclic Square Wave Voltammetric Method for Simultaneous Determination of Epinephrine and Norepinephrine Using an Activated Pencil Graphite Electrode. Microchem. J. 2021, 160, 105621. [Google Scholar] [CrossRef]
- Suriyaprakash, J.; Bala, K.; Shan, L.; Wu, L.; Gupta, N. Molecular Engineered Carbon-Based Sensor for Ultrafast and Specific Detection of Neurotransmitters. ACS Appl. Mater. Interfaces 2021, 13, 60878–60893. [Google Scholar] [CrossRef]
- Olejnik, A.; Ficek, M.; Szkodo, M.; Stanisławska, A.; Karczewski, J.; Ryl, J.; Dołęga, A.; Siuzdak, K.; Bogdanowicz, R. Tailoring Diffusional Fields in Zwitterion/Dopamine Copolymer Electropolymerized at Carbon Nanowalls for Sensitive Recognition of Neurotransmitters. ACS Nano 2022, 16, 13183–13198. [Google Scholar] [CrossRef]
- Ni, M.; Chen, J.; Wang, C.; Wang, Y.; Huang, L.; Xiong, W.; Zhao, P.; Xie, Y.; Fei, J. A High-Sensitive Dopamine Electrochemical Sensor Based on Multilayer Ti3C2 MXene, Graphitized Multi-Walled Carbon Nanotubes and ZnO Nanospheres. Microchem. J. 2022, 178, 107410. [Google Scholar] [CrossRef]
- Nayak, S.P.; Prathyusha, V.; Kumar, J.K.K. Eco-Friendly Surface Modification of Oxidized Carbon Nanotubes with Curcumin for Simultaneous Electrochemical Detection of Dopamine and Serotonin. Mater. Chem. Phys. 2022, 287, 126293. [Google Scholar] [CrossRef]
- Kumar, S.; Awasthi, A.; Sharma, M.D.; Singh, K.; Singh, D. Functionalized Multiwall Carbon Nanotube-Molybdenum Disulphide Nanocomposite Based Electrochemical Ultrasensitive Detection of Neurotransmitter Epinephrine. Mater. Chem. Phys. 2022, 290, 126656. [Google Scholar] [CrossRef]
- da Silva, L.V.; dos Santos, N.D.; de Almeida, A.K.A.; dos Santos, D.D.E.R.; Santos, A.C.F.; França, M.C.; Lima, D.J.P.; Lima, P.R.; Goulart, M.O.F. A New Electrochemical Sensor Based on Oxidized Capsaicin/Multi-Walled Carbon Nanotubes/Glassy Carbon Electrode for the Quantification of Dopamine, Epinephrine, and Xanthurenic, Ascorbic and Uric Acids. J. Electroanal. Chem. 2021, 881, 114919. [Google Scholar] [CrossRef]
- Koteshwara Reddy, K.; Satyanarayana, M.; Yugender Goud, K.; Vengatajalabathy Gobi, K.; Kim, H. Carbon Nanotube Ensembled Hybrid Nanocomposite Electrode for Direct Electrochemical Detection of Epinephrine in Pharmaceutical Tablets and Urine. Mater. Sci. Eng. C 2017, 79, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Deffo, G.; Basumatary, M.; Hussain, N.; Hazarika, R.; Kalita, S.; Njanja, E.; Puzari, P. Eggshell Nano-CaCO3 Decorated PANi/RGO Composite for Sensitive Determination of Ascorbic Acid, Dopamine, and Uric Acid in Human Blood Serum and Urine. Mater. Today Commun. 2022, 33, 104357. [Google Scholar] [CrossRef]
- Gaidukevic, J.; Aukstakojyte, R.; Barkauskas, J.; Niaura, G.; Murauskas, T.; Pauliukaite, R. A Novel Electrochemical Sensor Based on Thermally Reduced Graphene Oxide for the Sensitive Determination of Dopamine. Appl. Surf. Sci. 2022, 592, 153257. [Google Scholar] [CrossRef]
- Anbumannan, V.; Kumar, R.T.R.; Suresh, K. Enhanced Electrochemical Detection of Dopamine by Graphene Oxide/Tungsten Trioxide Nanocomposite. Mater. Sci. Semicond. Process. 2021, 127, 105696. [Google Scholar] [CrossRef]
- Kalia, S.; Rana, D.S.; Thakur, N.; Singh, D.; Kumar, R.; Singh, R.K. Two-Dimensional Layered Molybdenum Disulfide (MoS2)-Reduced Graphene Oxide (RGO) Heterostructures Modified with Fe3O4 for Electrochemical Sensing of Epinephrine. Mater. Chem. Phys. 2022, 287, 126274. [Google Scholar] [CrossRef]
- Ramu, A.G.; Umar, A.; Ibrahim, A.A.; Algadi, H.; Ibrahim, Y.S.A.; Wang, Y.; Hanafiah, M.M.; Shanmugam, P.; Choi, D. Synthesis of Porous 2D Layered Nickel Oxide-Reduced Graphene Oxide (NiO-RGO) Hybrid Composite for the Efficient Electrochemical Detection of Epinephrine in Biological Fluid. Environ. Res. 2021, 200, 111366. [Google Scholar] [CrossRef]
- Li, Z.; Guo, Y.; Yue, H.; Gao, X.; Huang, S.; Zhang, X.; Yu, Y.; Zhang, H.; Zhang, H. Electrochemical Determination of Epinephrine Based on Ti3C2Tx MXene-Reduced Graphene Oxide/ITO Electrode. J. Electroanal. Chem. 2021, 895, 115425. [Google Scholar] [CrossRef]
- Subramaniam, T.; Kesavan, G.; Venkatachalam, G. Development of CuAlO2-Encapsulated Reduced Graphene Oxide Nanocomposites: An Efficient and Selective Electrocatalyst for Detection of Neurodegenerative Disorders. ACS Appl. Bio Mater. 2020, 3, 7769–7778. [Google Scholar] [CrossRef]
- Dong, W.; Ren, Y.; Bai, Z.; Jiao, J.; Chen, Y.; Han, B.; Chen, Q. Synthesis of Tetrahexahedral Au-Pd Core–Shell Nanocrystals and Reduction of Graphene Oxide for the Electrochemical Detection of Epinephrine. J. Colloid Interface Sci. 2018, 512, 812–818. [Google Scholar] [CrossRef]
- Luhana, C.; Moyo, I.; Tshenkeng, K.; Mashazi, P. In-Sera Selectivity Detection of Catecholamine Neurotransmitters Using Covalent Composite of Cobalt Phthalocyanine and Aminated Graphene Quantum Dots. Microchem. J. 2022, 180, 107605. [Google Scholar] [CrossRef]
- Li, J.; Huang, X.; Shi, W.; Jiang, M.; Tian, L.; Su, M.; Wu, J.; Liu, Q.; Yu, C.; Gu, H. Pt Nanoparticle Decorated Carbon Nanotubes Nanocomposite Based Sensing Platform for the Monitoring of Cell-Secreted Dopamine. Sens. Actuators B Chem. 2021, 330, 129311. [Google Scholar] [CrossRef]
- Rajarathinam, T.; Thirumalai, D.; Kwon, M.; Lee, S.; Jayaraman, S.; Paik, H.; Lee, J.; Chang, S.-C. Screen-Printed Carbon Electrode Modified with de-Bundled Single-Walled Carbon Nanotubes for Voltammetric Determination of Norepinephrine in Ex Vivo Rat Tissue. Bioelectrochemistry 2022, 146, 108155. [Google Scholar] [CrossRef]
- Verde, M.; Lippiello, P.; Singh, S.; Miniaci, M.C.; Cinti, S. A Frugal Printed Electrochemical Architecture to Monitor Dopamine Release in Mice Brain: Organ-on-Screen-Printed Approach. Biosens. Bioelectron. X 2022, 12, 100225. [Google Scholar] [CrossRef]
- Negahdary, M.; Akira Ameku, W.; Gomes Santos, B.; dos Santos Lima, I.; Gomes de Oliveira, T.; Carvalho França, M.; Angnes, L. Recent Electrochemical Sensors and Biosensors for Toxic Agents Based on Screen-Printed Electrodes Equipped with Nanomaterials. Microchem. J. 2023, 185, 108281. [Google Scholar] [CrossRef]
- Musa, A.M.; Kiely, J.; Luxton, R.; Honeychurch, K.C. Recent Progress in Screen-Printed Electrochemical Sensors and Biosensors for the Detection of Estrogens. TrAC Trends Anal. Chem. 2021, 139, 116254. [Google Scholar] [CrossRef]
- Lakhera, P.; Chaudhary, V.; Jha, A.; Singh, R.; Kush, P.; Kumar, P. Recent Developments and Fabrication of the Different Electrochemical Biosensors Based on Modified Screen Printed and Glassy Carbon Electrodes for the Early Diagnosis of Diverse Breast Cancer Biomarkers. Mater. Today Chem. 2022, 26, 101129. [Google Scholar] [CrossRef]
- Liu, J.; Ji, H.; Lv, X.; Zeng, C.; Li, H.; Li, F.; Qu, B.; Cui, F.; Zhou, Q. Laser-Induced Graphene (LIG)-Driven Medical Sensors for Health Monitoring and Diseases Diagnosis. Microchim Acta 2022, 189, 54. [Google Scholar] [CrossRef]
- Bahri, M.; Amin Elaguech, M.; Nasraoui, S.; Djebbi, K.; Kanoun, O.; Qin, P.; Tlili, C.; Wang, D. Laser-Induced Graphene Electrodes for Highly Sensitive Detection of DNA Hybridization via Consecutive Cytosines (PolyC)-DNA-Based Electrochemical Biosensors. Microchem. J. 2023, 185, 108208. [Google Scholar] [CrossRef]
- Xu, G.; Jarjes, Z.A.; Wang, H.-W.; Phillips, A.R.J.; Kilmartin, P.A.; Travas-Sejdic, J. Detection of Neurotransmitters by Three-Dimensional Laser-Scribed Graphene Grass Electrodes. ACS Appl. Mater. Interfaces 2018, 10, 42136–42145. [Google Scholar] [CrossRef] [PubMed]
- Berni, A.; Ait Lahcen, A.; Salama, K.N.; Amine, A. 3D-Porous Laser-Scribed Graphene Decorated with Overoxidized Polypyrrole as an Electrochemical Sensing Platform for Dopamine. J. Electroanal. Chem. 2022, 919, 116529. [Google Scholar] [CrossRef]
- Uppachai, P.; Srijaranai, S.; Poosittisak, S.; Md Isa, I.; Mukdasai, S. Supramolecular Electrochemical Sensor for Dopamine Detection Based on Self-Assembled Mixed Surfactants on Gold Nanoparticles Deposited Graphene Oxide. Molecules 2020, 25, 2528. [Google Scholar] [CrossRef] [PubMed]
- Ouellette, M.; Mathault, J.; Niyonambaza, S.D.; Miled, A.; Boisselier, E. Electrochemical Detection of Dopamine Based on Functionalized Electrodes. Coatings 2019, 9, 496. [Google Scholar] [CrossRef] [Green Version]
- Thamilselvan, A.; Manivel, P.; Rajagopal, V.; Nesakumar, N.; Suryanarayanan, V. Improved Electrocatalytic Activity of Au@Fe3O4 Magnetic Nanoparticles for Sensitive Dopamine Detection. Colloids Surf. B Biointerfaces 2019, 180, 1–8. [Google Scholar] [CrossRef]
- Lim, T.; Zhang, H. Multilayer Carbon Nanotube/Gold Nanoparticle Composites on Gallium-Based Liquid Metals for Electrochemical Biosensing. ACS Appl. Nano Mater. 2021, 4, 12690–12701. [Google Scholar] [CrossRef]
- Zhan, S.; Xu, C.; Chen, J.; Xiao, Q.; Zhou, Z.; Xing, Z.; Gu, C.; Yin, Z.; Liu, H. A Novel Epinephrine Biosensor Based on Gold Nanoparticles Coordinated Polydopamine-Functionalized Acupuncture Needle Microelectrode. Electrochim. Acta 2023, 437, 141468. [Google Scholar] [CrossRef]
- Sabar, M.; Amara, U.; Riaz, S.; Hayat, A.; Nasir, M.; Nawaz, M.H. Fabrication of MoS2 Enwrapped Carbon Cloth as Electrochemical Probe for Non-Enzymatic Detection of Dopamine. Mater. Lett. 2022, 308, 131233. [Google Scholar] [CrossRef]
- Wu, P.; Huang, Y.; Zhao, X.; Lin, D.; Xie, L.; Li, Z.; Zhu, Z.; Zhao, H.; Lan, M. MnFe2O4/MoS2 Nanocomposite as Oxidase-like for Electrochemical Simultaneous Detection of Ascorbic Acid, Dopamine and Uric Acid. Microchem. J. 2022, 181, 107780. [Google Scholar] [CrossRef]
- Karatas, E.; Ozden, D.S.; Yilmaz, M.; Yazan, Z.; Piskin, E. A Sensitive Nanocomposite Design via Polydopamine Mediated Au and Ag Nanoparticles: Voltammetric Assay for Dopamine in Biological Samples. Thin Solid Film. 2022, 756, 139354. [Google Scholar] [CrossRef]
- Trindade, C.M.B.; Silva, M.K.L.; Cesarino, I. Copper Nanostructures Anchored on Renewable Carbon as Electrochemical Platform for the Detection of Dopamine, Fluoxetine and Escitalopram. Sens. Actuators Rep. 2022, 4, 100107. [Google Scholar] [CrossRef]
- Elugoke, S.E.; Fayemi, O.E.; Adekunle, A.S.; Mamba, B.B.; Nkambule, T.T.I.; Ebenso, E.E. Electrochemical Sensor for the Detection of Dopamine Using Carbon Quantum Dots/Copper Oxide Nanocomposite Modified Electrode. FlatChem 2022, 33, 100372. [Google Scholar] [CrossRef]
- Li, H.; Deng, Z.; Jiao, Z.; Zhu, R.; Ma, L.; Zhou, K.; Yu, Z.; Wei, Q. Engineering a Au-NPs/Nafion Modified Nanoporous Diamond Sensing Interface for Reliable Voltammetric Quantification of Dopamine in Human Serum. Chem. Eng. J. 2022, 446, 136927. [Google Scholar] [CrossRef]
- Ahmed, J.; Faisal, M.; Alsareii, S.A.; Jalalah, M.; Harraz, F.A. A Novel Gold-Decorated Porous Silicon-Poly(3-Hexylthiophene) Ternary Nanocomposite as a Highly Sensitive and Selective Non-Enzymatic Dopamine Electrochemical Sensor. J. Alloy. Compd. 2022, 931, 167403. [Google Scholar] [CrossRef]
- Azizpour Moallem, Q.; Beitollahi, H. Electrochemical Sensor for Simultaneous Detection of Dopamine and Uric Acid Based on a Carbon Paste Electrode Modified with Nanostructured Cu-Based Metal-Organic Frameworks. Microchem. J. 2022, 177, 107261. [Google Scholar] [CrossRef]
- Manoj, D.; Rajendran, S.; Hoang, T.K.A.; Ansar, S.; Joo, S.-W.; Vasseghian, Y.; Soto-Moscoso, M. In-Situ Growth of 3D Cu-MOF on 1D Halloysite Nanotubes/Reduced Graphene Oxide Nanocomposite for Simultaneous Sensing of Dopamine and Paracetamol. J. Ind. Eng. Chem. 2022, 112, 287–295. [Google Scholar] [CrossRef]
- Wang, C.; Li, W.; Long, Y. Molten Salt-Assisted Synthesis of C-TiN Nanocomposites for Sensitive Dopamine Determination. Mater. Lett. 2022, 329, 133234. [Google Scholar] [CrossRef]
- Kavya, K.V.; Muthu, D.; Pattappan, D.; Vargheese, S.; Gokila, N.; Sivaramkumar, M.S.; Rajendra Kumar, R.T.; Haldorai, Y. Palladium Nanoparticles Decorated Ni-MOF Nanocomposite as an Electrochemical Platform for the Selective Detection of Dopamine. Mater. Lett. 2022, 306, 130926. [Google Scholar] [CrossRef]
- Nosratzehi, F.; Halakoei, H.; Rostami, M.; Sorouri, A.; Adib, K.; Rahimi-Nasrabadi, M.; Ehrlich, H. A Glassy Carbon Electrode Modified with N-TiO2@AgNPs@GQDs for Electrochemical Determination of Dopamine. Diam. Relat. Mater. 2022, 127, 109120. [Google Scholar] [CrossRef]
- Emadoddin, M.; Mozaffari, S.A.; Ebrahimi, F. An Antifouling Impedimetric Sensor Based on Zinc Oxide Embedded Polyvinyl Alcohol Nanoplatelets for Wide Range Dopamine Determination in the Presence of High Concentration Ascorbic Acid. J. Pharm. Biomed. Anal. 2021, 205, 114278. [Google Scholar] [CrossRef]
- Ndebele, N.; Sen, P.; Nyokong, T. Electrochemical Detection of Dopamine Using Phthalocyanine-Nitrogen-Doped Graphene Quantum Dot Conjugates. J. Electroanal. Chem. 2021, 886, 115111. [Google Scholar] [CrossRef]
- Fan, L.; Xin, Y.; Xu, Y.; Zhang, X.; Cheng, X.; Liu, L.; Song, H.; Gao, S.; Huo, L. Carbon Nanospheres Modified with WO2-NaxWO3 Nanoparticles for Highly Sensitive Electrochemical Detection of Dopamine. Microchem. J. 2021, 170, 106770. [Google Scholar] [CrossRef]
- Fallah, F.; Shishehbore, M.R.; Sheibani, A. Fabrication of a Novel Sensor Based on Cu Quantum Dot and SH-SiO2 Nanoparticles Supported on Copper-Based Metal Organic Framework (Cu QD-SH-SiO2@Cu-MOF) and Its Application for the Simultaneous Determination of Norepinephrine, Piroxicam and Epinephrine. Talanta 2023, 252, 123776. [Google Scholar] [CrossRef]
- Fajardo, A.; Tapia, D.; Pizarro, J.; Segura, R.; Jara, P. Determination of Norepinephrine Using a Glassy Carbon Electrode Modified with Graphene Quantum Dots and Gold Nanoparticles by Square Wave Stripping Voltammetry. J. Appl. Electrochem. 2019, 49, 423–432. [Google Scholar] [CrossRef]
- de Fatima Ulbrich, K.; Winiarski, J.P.; Jost, C.L.; Maduro de Campos, C.E. Mechanochemical Synthesis of a Ni3-XTe2 Nanocrystalline Composite and Its Application for Simultaneous Electrochemical Detection of Dopamine and Adrenaline. Compos. Part B Eng. 2020, 183, 107649. [Google Scholar] [CrossRef]
- Gao, L.-L.; Gao, E.-Q. Metal–Organic Frameworks for Electrochemical Sensors of Neurotransmitters. Coord. Chem. Rev. 2021, 434, 213784. [Google Scholar] [CrossRef]
- Cohen, S.M. Postsynthetic Methods for the Functionalization of Metal–Organic Frameworks. Chem. Rev. 2012, 112, 970–1000. [Google Scholar] [CrossRef]
- Xu, Y.; Li, Q.; Xue, H.; Pang, H. Metal-Organic Frameworks for Direct Electrochemical Applications. Coord. Chem. Rev. 2018, 376, 292–318. [Google Scholar] [CrossRef]
- Morozan, A.; Jaouen, F. Metal Organic Frameworks for Electrochemical Applications. Energy Environ. Sci. 2012, 5, 9269–9290. [Google Scholar] [CrossRef]
- Palakollu, V.N.; Chen, D.; Tang, J.-N.; Wang, L.; Liu, C. Recent Advancements in Metal-Organic Frameworks Composites Based Electrochemical (Bio)Sensors. Mikrochim Acta 2022, 189, 161. [Google Scholar] [CrossRef]
- Liu, K.; Chen, Y.; Dong, X.; Huang, H. Simultaneous Voltammetric Determination of Dopamine and Uric Acid Based on MOF-235 Nanocomposite. Inorg. Chem. Commun. 2022, 142, 109584. [Google Scholar] [CrossRef]
- Guo, H.; Liu, B.; Pan, Z.; Sun, L.; Peng, L.; Chen, Y.; Wu, N.; Wang, M.; Yang, W. Electrochemical Determination of Dopamine and Uric Acid with Covalent Organic Frameworks and Ox-MWCNT Co-Modified Glassy Carbon Electrode. Colloids Surf. A Physicochem. Eng. Asp. 2022, 648, 129316. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, S.; Wu, D.; Shen, J.; Wei, Y.; Wang, C. Amino Bearing Core-Shell Structured Magnetic Covalent Organic Framework Nanospheres: Preparation, Postsynthetic Modification with Phenylboronic Acid and Enrichment of Monoamine Neurotransmitters in Human Urine. Anal. Chim. Acta 2020, 1093, 61–74. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, N.; Soni, S.; Agrawal, P.; Balhara, Y.P.S.; Jain, U. Recent Advancement in Nanosensors for Neurotransmitters Detection: Present and Future Perspective. Process Biochem. 2020, 91, 241–259. [Google Scholar] [CrossRef]
- Zhao, R.; Li, D.; Yin, N.; Guo, Z.; Wang, D.; Yao, X. The High Sensitive and Selective Detection of Dopamine Based on Its Electropolymerization by Electrochemical Surface Plasmon Resonance. Sens. Actuators B Chem. 2022, 370, 132401. [Google Scholar] [CrossRef]
- Ma, J.; Wu, W.; Xiao, X.; Feng, Y.; Hao, Y.; Zhang, J.; Liu, C.; Zhang, P.; Chen, J.; Zeng, R.; et al. New Insight into Electropolymerization of Melamine. II: Low Onset Potential Deposition of Polymelamine with Trace Active Bromine. Electrochim. Acta 2022, 410, 139991. [Google Scholar] [CrossRef]
- Banu, R.; Kumara Swamy, B.E.; Jayaprakash, G.K.; Sharma, S.C. Simultaneous Resolution of Serotonin and Epinephrine at Poly (Victoria Blue B) Amplified Carbon Paste Electrode: A Voltammetric Study with Density Functional Theory Evidences. Inorg. Chem. Commun. 2022, 144, 109627. [Google Scholar] [CrossRef]
- Fatma, S.; Prasad, B.B.; Jaiswal, S.; Singh, R.; Singh, K. Electrochemical Simultaneous Analysis of Dopamine and Epinephrine Using Double Imprinted One MoNomer Acryloylated Graphene Oxide-Carbon Black Composite Polymer. Biosens. Bioelectron. 2019, 135, 36–44. [Google Scholar] [CrossRef]
- Kaya, H.K.; Cinar, S.; Altundal, G.; Bayramlı, Y.; Unaleroglu, C.; Kuralay, F. A Novel Design Thia-Bilane Structure-Based Molecular Imprinted Electrochemical Sensor for Sensitive and Selective Dopamine Determination. Sens. Actuators B Chem. 2021, 346, 130425. [Google Scholar] [CrossRef]
- Rajeshwari, V.; Vedhi, C.; Fernando, J. Dopamine Sensor Based on Coreshell Poly Paraphenylene Diamine/ Titanium Dioxide/ Multiwalled Carbon Nanotube Nanocomposite. Mater. Today Proc. 2022, 68, 287–293. [Google Scholar] [CrossRef]
- Qu, K.; Qiu, Y.; Li, J. Electro-Catalytic Behavior by Polypyrrole-Derived Carbon Supported Iron for Simultaneous Electrochemical Sensing of Dopamine and Uric Acid. J. Electroanal. Chem. 2022, 910, 116188. [Google Scholar] [CrossRef]
- Vanitha, C.; Sanmugam, A.; Yogananth, A.; Rajasekar, M.; Kuppusamy, P.G.; Devasagayam, G. A Facile Synthesis of Polyaniline-WO3 Hybrid Nanocomposite for Enhanced Dopamine Detection. Mater. Lett. 2022, 328, 133149. [Google Scholar] [CrossRef]
- Harsini, M.; Widyaningrum, B.A.; Fitriany, E.; Paramita, D.R.A.; Farida, A.N.; Baktir, A.; Kurniawan, F.; Sakti, S.C.W. Electrochemical Synthesis of Polymelamine/Gold Nanoparticle Modified Carbon Paste Electrode as Voltammetric Sensor of Dopamine. Chin. J. Anal. Chem. 2022, 50, 100052. [Google Scholar] [CrossRef]
- Gong, Q.; Han, H.; Wang, Y.; Yao, C.; Yang, H.; Qiao, J. An Electrochemical Sensor for Dopamine Detection Based on the Electrode of a Poly-Tryptophan-Functionalized Graphene Composite. New Carbon Mater. 2020, 35, 34–41. [Google Scholar] [CrossRef]
- Fredj, Z.; Ali, M.B.; Abbas, M.N.; Dempsey, E. Simultaneous Determination of Ascorbic Acid, Uric Acid and Dopamine Using Silver Nanoparticles and Copper Monoamino-Phthalocyanine Functionalised Acrylate Polymer. Anal. Methods 2020, 12, 3883–3891. [Google Scholar] [CrossRef]
- Casadio, S.; Lowdon, J.W.; Betlem, K.; Ueta, J.T.; Foster, C.W.; Cleij, T.J.; van Grinsven, B.; Sutcliffe, O.B.; Banks, C.E.; Peeters, M. Development of a Novel Flexible Polymer-Based Biosensor Platform for the Thermal Detection of Noradrenaline in Aqueous Solutions. Chem. Eng. J. 2017, 315, 459–468. [Google Scholar] [CrossRef]
- Zhang, J.; Guo, X.-T.; Zhou, J.-P.; Liu, G.-Z.; Zhang, S.-Y. Electrochemical Preparation of Surface Molecularly Imprinted Poly(3-Aminophenylboronic Acid)/MWCNTs Nanocomposite for Sensitive Sensing of Epinephrine. Mater. Sci. Eng. C 2018, 91, 696–704. [Google Scholar] [CrossRef]
- Liu, W.; Cui, F.; Li, H.; Wang, S.; Zhuo, B.; Wang, S. Three-Dimensional Hybrid Networks of Molecularly Imprinted Poly(9-Carbazoleacetic Acid) and MWCNTs for Simultaneous Voltammetric Determination of Dopamine and Epinephrine in Plasma Sample. Sens. Actuators B Chem. 2020, 323, 128669. [Google Scholar] [CrossRef]
- Zhao, X.; Cui, Y.; He, Y.; Wang, S.; Wang, J. Synthesis of Multi-Mode Quantum Dots Encoded Molecularly Imprinted Polymers Microspheres and Application in Quantitative Detection for Dopamine. Sens. Actuators B Chem. 2020, 304, 127265. [Google Scholar] [CrossRef]
- Lu, Z.; Li, Y.; Liu, T.; Wang, G.; Sun, M.; Jiang, Y.; He, H.; Wang, Y.; Zou, P.; Wang, X.; et al. A Dual-Template Imprinted Polymer Electrochemical Sensor Based on AuNPs and Nitrogen-Doped Graphene Oxide Quantum Dots Coated on NiS2/Biomass Carbon for Simultaneous Determination of Dopamine and Chlorpromazine. Chem. Eng. J. 2020, 389, 124417. [Google Scholar] [CrossRef]
- Nakatsuka, N.; Andrews, A.M. Differentiating Siblings: The Case of Dopamine and Norepinephrine. ACS Chem. Neurosci. 2017, 8, 218–220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moraldo, C.; Vuille-dit-Bille, E.; Shkodra, B.; Kloter, T.; Nakatsuka, N. Aptamer-Modified Biosensors to Visualize Neurotransmitter Flux. J. Neurosci. Methods 2022, 365, 109386. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; You, X.; Li, Y.; Zuo, Y.; Wang, W.; Li, D.; Huang, S.; Hu, H.; Yuan, F.; Shao, F.; et al. A Novel Electrochemical Aptasensor for Serum Dopamine Detection Based on Methylene Blue-Integrated m-PdNFs Signal Material. Sens. Actuators B Chem. 2022, 354, 131233. [Google Scholar] [CrossRef]
- Gao, Z.; Wu, G.; Song, Y.; Li, H.; Zhang, Y.; Schneider, M.J.; Qiang, Y.; Kaszas, J.; Weng, Z.; Sun, H.; et al. Multiplexed Monitoring of Neurochemicals via Electrografting-Enabled Site-Selective Functionalization of Aptamers on Field-Effect Transistors. Anal. Chem. 2022, 94, 8605–8617. [Google Scholar] [CrossRef]
- Wu, G.; Zhang, N.; Matarasso, A.; Heck, I.; Li, H.; Lu, W.; Phaup, J.G.; Schneider, M.J.; Wu, Y.; Weng, Z.; et al. Implantable Aptamer-Graphene Microtransistors for Real-Time Monitoring of Neurochemical Release in Vivo. Nano Lett. 2022, 22, 3668–3677. [Google Scholar] [CrossRef] [PubMed]
- Saraf, N.; Woods, E.R.; Peppler, M.; Seal, S. Highly Selective Aptamer Based Organic Electrochemical Biosensor with Pico-Level Detection. Biosens. Bioelectron. 2018, 117, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Shen, M.; Kan, X. Aptamer and Molecularly Imprinted Polymer: Synergistic Recognition and Sensing of Dopamine. Electrochim. Acta 2021, 367, 137433. [Google Scholar] [CrossRef]
- Wei, S.; Gu, M.; Xiao, H.; Cao, L.; Zhao, F.; Chen, Z. Electrochemical DNA Aptamer Platform Based on CuAlO2/RGO-TEPA@AuPt Nanocomposites for Dopamine Detection. Mater. Today Chem. 2022, 26, 101248. [Google Scholar] [CrossRef]
- Wei, B.; Zhong, H.; Wang, L.; Liu, Y.; Xu, Y.; Zhang, J.; Xu, C.; He, L.; Wang, H. Facile Preparation of a Collagen-Graphene Oxide Composite: A Sensitive and Robust Electrochemical Aptasensor for Determining Dopamine in Biological Samples. Int. J. Biol. Macromol. 2019, 135, 400–406. [Google Scholar] [CrossRef] [PubMed]
- Taheri, R.A.; Eskandari, K.; Negahdary, M. An Electrochemical Dopamine Aptasensor Using the Modified Au Electrode with Spindle-Shaped Gold Nanostructure. Microchem. J. 2018, 143, 243–251. [Google Scholar] [CrossRef]
- Tang, C.; Zou, Z.; Liang, T.; Yuan, C.; Gao, J.; Tang, K.; Li, C.M. Methylene Blue Intercalated Aptamer to Amplify Signals toward Sensitively Electrochemical Detection of Dopamine Released from Living Parkinson’s Disease Model Cells. Sens. Actuators Rep. 2022, 4, 100080. [Google Scholar] [CrossRef]
- Sethuraman, V.; Sridhar, T.M.; Sasikumar, R. Development of an Electrochemical Biosensor for Determination of Dopamine by Gold Modified Poly(Thiophene-3-Boronic Acid)-Polyphenol Oxidase Modified Electrode. Mater. Lett. 2021, 302, 130387. [Google Scholar] [CrossRef]
- Srivastava, A.; Mishra, G.; Singh, J.; Pandey, M.D. A Highly Efficient Nanostructured Au@La2O3 Based Platform for Dopamine Detection. Mater. Lett. 2022, 308, 131231. [Google Scholar] [CrossRef]
- Wu, R.; Yu, S.; Chen, S.; Dang, Y.; Wen, S.-H.; Tang, J.; Zhou, Y.; Zhu, J.-J. A Carbon Dots-Enhanced Laccase-Based Electrochemical Sensor for Highly Sensitive Detection of Dopamine in Human Serum. Anal. Chim. Acta 2022, 1229, 340365. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Wang, D.P.; Guo, C.; Liu, Y.; Rao, Q.; Lou, F.; Li, Q.; Dong, Y.; Li, Q.; Yang, H.B.; et al. Single-Atom Ruthenium Biomimetic Enzyme for Simultaneous Electrochemical Detection of Dopamine and Uric Acid. Anal. Chem. 2021, 93, 4916–4923. [Google Scholar] [CrossRef] [PubMed]
- Decarli, N.O.; Zapp, E.; de Souza, B.S.; Santana, E.R.; Winiarski, J.P.; Vieira, I.C. Biosensor Based on Laccase-Halloysite Nanotube and Imidazolium Zwitterionic Surfactant for Dopamine Determination. Biochem. Eng. J. 2022, 186, 108565. [Google Scholar] [CrossRef]
- Gopal, P.; Narasimha, G.; Reddy, T.M. Development, Validation and Enzyme Kinetic Evaluation of Multi Walled Carbon Nano Tubes Mediated Tyrosinase Based Electrochemical Biosensing Platform for the Voltammetric Monitoring of Epinephrine. Process Biochem. 2020, 92, 476–485. [Google Scholar] [CrossRef]
- Alvarado-Ramírez, L.; Rostro-Alanis, M.; Rodríguez-Rodríguez, J.; Sosa-Hernández, J.E.; Melchor-Martínez, E.M.; Iqbal, H.M.N.; Parra-Saldívar, R. Enzyme (Single and Multiple) and Nanozyme Biosensors: Recent Developments and Their Novel Applications in the Water-Food-Health Nexus. Biosensors 2021, 11, 410. [Google Scholar] [CrossRef]
- Mustafa, F.; Andreescu, S. Paper-Based Enzyme Biosensor for One-Step Detection of Hypoxanthine in Fresh and Degraded Fish. ACS Sens. 2020, 5, 4092–4100. [Google Scholar] [CrossRef]
- Hatamie, A.; He, X.; Zhang, X.-W.; Oomen, P.E.; Ewing, A.G. Advances in Nano/Microscale Electrochemical Sensors and Biosensors for Analysis of Single Vesicles, a Key Nanoscale Organelle in Cellular Communication. Biosens. Bioelectron. 2023, 220, 114899. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, X.; Zhang, X.; Wang, Y.; Pei, W. Implantable Intracortical Microelectrodes: Reviewing the Present with a Focus on the Future. Microsyst Nanoeng 2023, 9, 7. [Google Scholar] [CrossRef] [PubMed]
- Marinesco, S. Micro- and Nano-Electrodes for Neurotransmitter Monitoring. Curr. Opin. Electrochem. 2021, 29, 100746. [Google Scholar] [CrossRef]
- Deng, Z.; Zhao, L.; Mu, H.; Jiang, L.; Xi, W.; Xu, X.; Zheng, W. High Selective Property of Gelatin/MWCNTs Functionalized Carbon Fiber Microelectrode: Toward Real-Time Monitoring of Ascorbate. J. Electroanal. Chem. 2022, 914, 116315. [Google Scholar] [CrossRef]
- Liang, H.; Zhu, M.; Ye, H.; Zeng, C.; Wang, S.; Niu, Y. Carbon Fiber Microelectrode Array Loaded with the Diazonium Salt-Single-Walled Carbon Nanotubes Composites for the Simultaneous Monitoring of Dopamine and Serotonin in Vivo. Anal. Chim. Acta 2021, 1186, 339086. [Google Scholar] [CrossRef] [PubMed]
- Hu, K.; Le Vo, K.L.; Hatamie, A.; Ewing, A.G. Quantifying Intracellular Single Vesicular Catecholamine Concentration with Open Carbon Nanopipettes to Unveil the Effect of L-DOPA on Vesicular Structure. Angew. Chem. Int. Ed. Engl. 2022, 61, e202113406. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Hu, K.; Wang, D.; Zubi, Y.; Lee, S.T.; Puthongkham, P.; Mirkin, M.V.; Venton, B.J. Cavity Carbon-Nanopipette Electrodes for Dopamine Detection. Anal. Chem. 2019, 91, 4618–4624. [Google Scholar] [CrossRef]
- Peltola, E.; Wester, N.; Holt, K.B.; Johansson, L.-S.; Koskinen, J.; Myllymäki, V.; Laurila, T. Nanodiamonds on Tetrahedral Amorphous Carbon Significantly Enhance Dopamine Detection and Cell Viability. Biosens. Bioelectron. 2017, 88, 273–282. [Google Scholar] [CrossRef] [Green Version]
- Laborda, E.; Molina, A.; Martínez-Ortiz, F.; Compton, R.G. Electrode Modification Using Porous Layers. Maximising the Analytical Response by Choosing the Most Suitable Voltammetry: Differential Pulse vs Square Wave vs Linear Sweep Voltammetry. Electrochim. Acta 2012, 73, 3–9. [Google Scholar] [CrossRef]
- Heydari-Badrooei, E.; Ensafi, A.A. Nanomaterials-Based Biosensing Strategies for Biomarkers Diagnosis, a Review. Biosens. Bioelectron. X 2022, 100245. [Google Scholar] [CrossRef]
- McCarty, G.S.; Dunaway, L.E.; Denison, J.D.; Sombers, L.A. Neurotransmitter Readily Escapes Detection at the Opposing Microelectrode Surface in Typical Amperometric Measurements of Exocytosis at Single Cells. Anal. Chem. 2022, 94, 9548–9556. [Google Scholar] [CrossRef]
- Luka, G.S.; Najjaran, H.; Hoorfar, M. On-Chip-Based Electrochemical Biosensor for the Sensitive and Label-Free Detection of Cryptosporidium. Sci. Rep. 2022, 12, 6957. [Google Scholar] [CrossRef] [PubMed]
- Molinnus, D.; Hardt, G.; Käver, L.; Willenberg, H.S.; Kröger, J.-C.; Poghossian, A.; Keusgen, M.; Schöning, M.J. Chip-Based Biosensor for the Detection of Low Adrenaline Concentrations to Support Adrenal Venous Sampling. Sens. Actuators B Chem. 2018, 272, 21–27. [Google Scholar] [CrossRef]
- Senel, M.; Dervisevic, E.; Alhassen, S.; Dervisevic, M.; Alachkar, A.; Cadarso, V.J.; Voelcker, N.H. Microfluidic Electrochemical Sensor for Cerebrospinal Fluid and Blood Dopamine Detection in a Mouse Model of Parkinson’s Disease. Anal. Chem. 2020, 92, 12347–12355. [Google Scholar] [CrossRef] [PubMed]
- Salmon, I.; Grebenyuk, S.; Fattah, A.R.A.; Rustandi, G.; Pilkington, T.; Verfaillie, C.; Ranga, A. Engineering Neurovascular Organoids with 3D Printed Microfluidic Chips. Lab Chip 2022, 22, 1615–1629. [Google Scholar] [CrossRef]
- Jin, X.; Liu, C.; Xu, T.; Su, L.; Zhang, X. Artificial Intelligence Biosensors: Challenges and Prospects. Biosens. Bioelectron. 2020, 165, 112412. [Google Scholar] [CrossRef]
- Li, Z.Y.; Zhu, L.Q.; Guo, L.Q.; Ren, Z.Y.; Xiao, H.; Cai, J.C. Mimicking Neurotransmitter Activity and Realizing Algebraic Arithmetic on Flexible Protein-Gated Oxide Neuromorphic Transistors. ACS Appl. Mater. Interfaces 2021, 13, 7784–7791. [Google Scholar] [CrossRef]
- Huang, G.; Chen, X.; Li, N.; Xie, T.; Guo, Y.; Fu, Y.; Jiao, T. A Convenient Synthesis of Gold Nanoparticles in Spirulina Extract for Rapid Visual Detection of Dopamine in Human Urine. Colloids Surf. A Physicochem. Eng. Asp. 2022, 650, 129675. [Google Scholar] [CrossRef]
- Qin, X.; Yuan, C.-L.; Shi, R.; Wang, S.-Z.; Wang, Y.-L. Colorimetric Detection of Dopamine Based on Iodine-Mediated Etching of Gold Nanorods. Chin. J. Anal. Chem. 2021, 49, 60–67. [Google Scholar] [CrossRef]
- Rostami, S.; Mehdinia, A.; Niroumand, R.; Jabbari, A. Enhanced LSPR Performance of Graphene Nanoribbons-Silver Nanoparticles Hybrid as a Colorimetric Sensor for Sequential Detection of Dopamine and Glutathione. Anal. Chim. Acta 2020, 1120, 11–23. [Google Scholar] [CrossRef]
- Alizadeh, N.; Ghasemi, S.; Salimi, A.; Sham, T.-K.; Hallaj, R. CuO Nanorods as a Laccase Mimicking Enzyme for Highly Sensitive Colorimetric and Electrochemical Dual Biosensor: Application in Living Cell Epinephrine Analysis. Colloids Surf. B Biointerfaces 2020, 195, 111228. [Google Scholar] [CrossRef]
- Wang, J.; Du, R.; Liu, W.; Yao, L.; Ding, F.; Zou, P.; Wang, Y.; Wang, X.; Zhao, Q.; Rao, H. Colorimetric and Fluorometric Dual-Signal Determination of Dopamine by the Use of Cu-Mn-O Microcrystals and C-Dots. Sens. Actuators B Chem. 2019, 290, 125–132. [Google Scholar] [CrossRef]
- Zhu, W.; Cheng, Y.; Yan, S.; Chen, X.; Wang, C.; Lu, X. A General Cation-Exchange Strategy for Constructing Hierarchical TiO2/CuInS2/CuS Hybrid Nanofibers to Boost Their Peroxidase-like Activity toward Sensitive Detection of Dopamine. Microchem. J. 2022, 183, 108090. [Google Scholar] [CrossRef]
- Nishan, U.; Sabba, U.; Rahim, A.; Asad, M.; Shah, M.; Iqbal, A.; Iqbal, J.; Muhammad, N. Ionic Liquid Tuned Titanium Dioxide Nanostructures as an Efficient Colorimetric Sensing Platform for Dopamine Detection. Mater. Chem. Phys. 2021, 262, 124289. [Google Scholar] [CrossRef]
- Chellasamy, G.; Ankireddy, S.R.; Lee, K.-N.; Govindaraju, S.; Yun, K. Smartphone-Integrated Colorimetric Sensor Array-Based Reader System and Fluorometric Detection of Dopamine in Male and Female Geriatric Plasma by Bluish-Green Fluorescent Carbon Quantum Dots. Mater. Today Bio 2021, 12, 100168. [Google Scholar] [CrossRef]
- Wang, J.; Hu, Y.; Zhou, Q.; Hu, L.; Fu, W.; Wang, Y. Peroxidase-like Activity of Metal–Organic Framework [Cu(PDA)(DMF)] and Its Application for Colorimetric Detection of Dopamine. ACS Appl. Mater. Interfaces 2019, 11, 44466–44473. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xue, Y.; Zhao, Q.; Wang, S.; Sun, J.; Yang, X. Colorimetric Assay for Acetylcholinesterase Activity and Inhibitor Screening Based on Metal–Organic Framework Nanosheets. Anal. Chem. 2022, 94, 16345–16352. [Google Scholar] [CrossRef] [PubMed]
- Ren, R.; Cai, G.; Yu, Z.; Zeng, Y.; Tang, D. Metal-Polydopamine Framework: An Innovative Signal-Generation Tag for Colorimetric Immunoassay. Anal. Chem. 2018, 90, 11099–11105. [Google Scholar] [CrossRef] [PubMed]
- Jafarinejad, S.; Ghazi-Khansari, M.; Ghasemi, F.; Sasanpour, P.; Hormozi-Nezhad, M.R. Colorimetric Fingerprints of Gold Nanorods for Discriminating Catecholamine Neurotransmitters in Urine Samples. Sci. Rep. 2017, 7, 8266. [Google Scholar] [CrossRef] [Green Version]
- Godoy-Reyes, T.M.; Costero, A.M.; Gaviña, P.; Martínez-Máñez, R.; Sancenón, F. A Colorimetric Probe for the Selective Detection of Norepinephrine Based on a Double Molecular Recognition with Functionalized Gold Nanoparticles. ACS Appl. Nano Mater. 2019. [Google Scholar] [CrossRef]
- Ilgar, M.; Baytemir, G.; Taşaltın, N.; Güllülü, S.; Yeşilyurt, İ.S.; Karakuş, S. Multifunctional Maca Extract Coated CuO Nanoparticles with Antimicrobial and Dopamine Sensing Activities: A Dual Electrochemical – Smartphone Colorimetric Detection System. J. Photochem. Photobiol. A Chem. 2022, 431, 114075. [Google Scholar] [CrossRef]
- Han, X.X.; Rodriguez, R.S.; Haynes, C.L.; Ozaki, Y.; Zhao, B. Surface-Enhanced Raman Spectroscopy. Nat Rev Methods Prim. 2022, 1, 1–17. [Google Scholar] [CrossRef]
- Zhou, B.; Li, X.; Tang, X.; Li, P.; Yang, L.; Liu, J. Highly Selective and Repeatable Surface-Enhanced Resonance Raman Scattering Detection for Epinephrine in Serum Based on Interface Self-Assembled 2D Nanoparticles Arrays. ACS Appl. Mater. Interfaces 2017, 9, 7772–7779. [Google Scholar] [CrossRef] [PubMed]
- Dowek, A.; Berge, M.; Prognon, P.; Legrand, F.-X.; Larquet, E.; Tfayli, A.; Lê, L.M.M.; Caudron, E. Discriminative and Quantitative Analysis of Norepinephrine and Epinephrine by Surface-Enhanced Raman Spectroscopy with Gold Nanoparticle Suspensions. Anal. Bioanal. Chem. 2022, 414, 1163–1176. [Google Scholar] [CrossRef] [PubMed]
- Ansah, I.B.; Lee, W.-C.; Mun, C.; Rha, J.-J.; Jung, H.S.; Kang, M.; Park, S.-G.; Kim, D.-H. In Situ Electrochemical Surface Modification of Au Electrodes for Simultaneous Label-Free SERS Detection of Ascorbic Acid, Dopamine and Uric Acid. Sens. Actuators B Chem. 2022, 353, 131196. [Google Scholar] [CrossRef]
- Shi, J.; Li, J.; Liang, A.; Jiang, Z. Highly Catalysis MOFCe Supported Ag Nanoclusters Coupled with Specific Aptamer for SERS Quantitative Assay of Trace Dopamine. Talanta 2022, 245, 123468. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liu, X.; Liu, Y.; Gao, R.; Wu, X.; Gao, X. Highly Sensitive Detection of Dopamine Based on Gold Nanoflowers Enhanced-Tb(III) Fluorescence. Talanta 2022, 249, 123700. [Google Scholar] [CrossRef]
- Das, D.; Dutta, R.K. Ethylene Glycol and Alanine Anhydride Based Nitrogen Doped Fluorescent Carbon Nanoparticles as Probe for Detection of Epinephrine, nor-Epinephrine and Dopamine. Dye. Pigment. 2022, 203, 110314. [Google Scholar] [CrossRef]
- Jafarinejad, S.; Bigdeli, A.; Ghazi-Khansari, M.; Sasanpour, P.; Hormozi-Nezhad, M.R. Identification of Catecholamine Neurotransmitters Using a Fluorescent Electronic Tongue. ACS Chem. Neurosci. 2020, 11, 25–33. [Google Scholar] [CrossRef]
- An, J.; Shi, Y.; Fang, J.; Hu, Y.; Liu, Y. Multichannel Ratiometric Fluorescence Sensor Arrays for Rapid Visual Monitoring of Epinephrine, Norepinephrine, and Levodopa. Chem. Eng. J. 2021, 425, 130595. [Google Scholar] [CrossRef]
- Hegde, M.; Pai, P.; Shetty, M.G.; Babitha, K.S. Gold Nanoparticle Based Biosensors for Rapid Pathogen Detection: A Review. Environ. Nanotechnol. Monit. Manag. 2022, 18, 100756. [Google Scholar] [CrossRef]
- Peng, J.; Zhou, N.; Zhong, Y.; Su, Y.; Zhao, L.; Chang, Y.-T. Gold Nanoparticle-Based Detection of Dopamine Based on Fluorescence Resonance Energy Transfer between a 4-(4-Dialkylaminostyryl)Pyridinium Derived Fluorophore and Citrate-Capped Gold Nanoparticles. Mikrochim Acta 2019, 186, 618. [Google Scholar] [CrossRef]
- Mitra, M.D.; Pandey, P.C. Functional Trialkoxysilane Mediated Controlled Synthesis of Fluorescent Gold Nanoparticles and Fluoremetric Sensing of Dopamine. Opt. Mater. 2022, 132, 112810. [Google Scholar] [CrossRef]
- Zhou, T.; Su, Z.; Tu, Y.; Yan, J. Determination of Dopamine Based on Its Enhancement of Gold-Silver Nanocluster Fluorescence. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 252, 119519. [Google Scholar] [CrossRef] [PubMed]
- Devi, R.; Gogoi, S.; Dutta, H.S.; Saikia, P.J.; Singhal, A.; Khan, R. Boronic Acid-Functionalized Tungsten Disulfide Quantum Dots as a Fluorescence Probe for Sensitive Detection of Dopamine. Biosens. Bioelectron. X 2022, 11, 100168. [Google Scholar] [CrossRef]
- Zeng, S.; Wang, S.; Xie, X.; Yang, S.; Fan, J.; Nie, Z.; Huang, Y.; Wang, H. Live-Cell Imaging of Neurotransmitter Release with a Cell-Surface-Anchored DNA-Nanoprism Fluorescent Sensor. Anal. Chem. 2020, 92, 15194–15201. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Han, Q.; Liu, P.; Zhang, G.; Song, L.; Zou, X.; Fu, Y. A Superstable Luminescent Lanthanide Metal Organic Gel Utilized in an Electrochemiluminescence Sensor for Epinephrine Detection with a Narrow Potential Sweep Range. ACS Sens. 2021, 6, 252–258. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Qin, D.; Meng, S.; Wu, Y.; Luo, Z.; Deng, B. Cathodic Electrochemiluminescence Based on Resonance Energy Transfer between Sulfur Quantum Dots and Dopamine Quinone for the Detection of Dopamine. Microchem. J. 2022, 181, 107776. [Google Scholar] [CrossRef]
- Zhu, L.; Fu, W.; Chen, J.; Li, S.; Xie, X.; Zhang, Z.; Liu, J.; Zhou, L.; Su, B.; Chen, X. A Fully Integrated and Handheld Electrochemiluminescence Device for Detection of Dopamine in Bio-Samples. Sens. Actuators B Chem. 2022, 366, 131972. [Google Scholar] [CrossRef]
- Wang, N.; Ao, H.; Xiao, W.; Chen, W.; Li, G.; Wu, J.; Ju, H. Confined Electrochemiluminescence Imaging Microarray for High-Throughput Biosensing of Single Cell-Released Dopamine. Biosens. Bioelectron. 2022, 201, 113959. [Google Scholar] [CrossRef]
- Li, R.; Zhang, D.; Li, X.; Qi, H. Sensitive and Selective Electrogenerated Chemiluminescence Aptasensing Method for the Determination of Dopamine Based on Target-Induced Conformational Displacement. Bioelectrochemistry 2022, 146, 108148. [Google Scholar] [CrossRef]
- Ning, H.; Liu, F.; Zhang, T.; Zhao, Y.; Li, Y.; Zhao, Z.; Liu, C.; Zhang, W.; Wang, H.; Li, F. A Signal-Amplification Electrochemiluminescence Sensor Based on Layer-by-Layer Assembly of Perylene Diimide Derivatives for Dopamine Detection at Low Potential. Anal. Chim. Acta 2022, 1214, 339963. [Google Scholar] [CrossRef]
- Kumar, D.; Kumar Sharma, G.; Kumar, M. Surface Plasmon Resonance Sensor for Chemical and Bio-Sensing Application: A Review. Mater. Today Proc. 2022. [Google Scholar] [CrossRef]
- Pathak, A.; Gupta, B.D. Ultra-Selective Fiber Optic SPR Platform for the Sensing of Dopamine in Synthetic Cerebrospinal Fluid Incorporating Permselective Nafion Membrane and Surface Imprinted MWCNTs-PPy Matrix. Biosens. Bioelectron. 2019, 133, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Xiong, X.; Tan, Y.; Mubango, E.; Shi, C.; Regenstein, J.M.; Yang, Q.; Hong, H.; Luo, Y. Rapid Freshness and Survival Monitoring Biosensors of Fish: Progress, Challenge, and Future Perspective. Trends Food Sci. Technol. 2022, 129, 61–73. [Google Scholar] [CrossRef]
- Asefpour Vakilian, K.; Massah, J. A Portable Nitrate Biosensing Device Using Electrochemistry and Spectroscopy. IEEE Sens. J. 2018, 18, 3080–3089. [Google Scholar] [CrossRef]
- Ridhuan, N.S.; Abdul Razak, K.; Lockman, Z. Fabrication and Characterization of Glucose Biosensors by Using Hydrothermally Grown ZnO Nanorods. Sci. Rep. 2018, 8, 13722. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Wang, J. Optical Biosensors: An Exhaustive and Comprehensive Review. Analyst 2020, 145, 1605–1628. [Google Scholar] [CrossRef]










Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fredj, Z.; Sawan, M. Advanced Nanomaterials-Based Electrochemical Biosensors for Catecholamines Detection: Challenges and Trends. Biosensors 2023, 13, 211. https://doi.org/10.3390/bios13020211
Fredj Z, Sawan M. Advanced Nanomaterials-Based Electrochemical Biosensors for Catecholamines Detection: Challenges and Trends. Biosensors. 2023; 13(2):211. https://doi.org/10.3390/bios13020211
Chicago/Turabian StyleFredj, Zina, and Mohamad Sawan. 2023. "Advanced Nanomaterials-Based Electrochemical Biosensors for Catecholamines Detection: Challenges and Trends" Biosensors 13, no. 2: 211. https://doi.org/10.3390/bios13020211