Hydrogel Actuators and Sensors for Biomedical Soft Robots: Brief Overview with Impending Challenges
Abstract
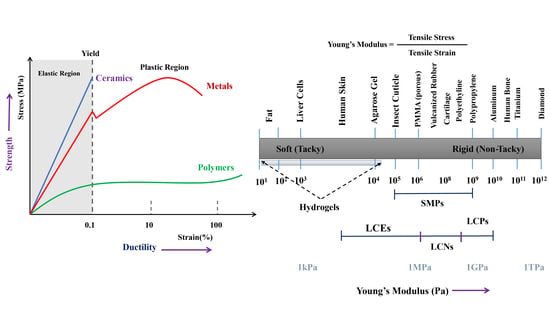
:1. Introduction
2. Hydrogel-Based Soft Actuators
2.1. Thermo-Sensitive Hydrogel Actuators
2.2. Electro-Responsive Hydrogel Actuators
2.3. Magneto-Responsive Hydrogel Actuators
2.4. Biomimetic Hydrogel Actuators
Key Limitations
2.5. Miscellaneous
3. Hydrogel-Based Biomedical Sensors
3.1. Glucose Sensors
3.2. Touch/Stress/Stretch Sensors
3.3. Ionic Strength Sensing for pH Ions
3.4. Temperature Sensors
3.5. Living Hydrogel Sensors
3.6. Biochemical Sensing Mechanisms
4. Different Hydrogel Structures: An Overview from a Mechanical Standpoint
4.1. Single-Phase Gels
4.2. Multi-Phase Gels
4.3. Double-Network Gels
5. Mechanical Properties: Robust, Tougher, and Stretchable Hydrogels
6. Rapid Prototyping Tools: Hydrogel-Based 3D Printing for Biomedical Soft Robots
7. Self-Healing Hydrogels for Soft Robotics
8. Conclusions and Future Challenges
Author Contributions
Funding
Conflicts of Interest
References
- Haque, M.A.; Kurokawa, T.; Gong, J.P. Super tough double network hydrogels and their application as biomaterials. Polymer 2012, 53, 1805–1822. [Google Scholar] [CrossRef]
- Zhang, Y.S.; Khademhosseini, A. Advances in engineering hydrogels. Science 2017, 356, eaaf3627. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.Y.; Zhao, X.; Illeperuma, W.R.; Chaudhuri, O.; Oh, K.H.; Mooney, D.J.; Vlassak, J.J.; Suo, Z. Highly stretchable and tough hydrogels. Nature 2012, 489, 133. [Google Scholar] [CrossRef] [PubMed]
- Calvert, P. Hydrogels for soft machines. Adv. Mater. 2009, 21, 743–756. [Google Scholar] [CrossRef]
- Haraguchi, K.; Takehisa, T. Nanocomposite hydrogels: A unique organic–inorganic network structure with extraordinary mechanical, optical, and swelling/de-swelling properties. Adv. Mater. 2002, 14, 1120–1124. [Google Scholar] [CrossRef]
- Yuk, H.; Lin, S.; Ma, C.; Takaffoli, M.; Fang, N.X.; Zhao, X. Hydraulic hydrogel actuators and robots optically and sonically camouflaged in water. Nat. Commun. 2017, 8, 14230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stanton, M.; Samitier, J.; Sanchez, S. Bioprinting of 3D hydrogels. Lab Chip 2015, 15, 3111–3115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ionov, L. Biomimetic hydrogel-based actuating systems. Adv. Funct. Mater. 2013, 23, 4555–4570. [Google Scholar] [CrossRef]
- Peppas, N.A.; Hilt, J.Z.; Khademhosseini, A.; Langer, R. Hydrogels in biology and medicine: From molecular principles to bionanotechnology. Adv. Mater. 2006, 18, 1345–1360. [Google Scholar] [CrossRef]
- Qiu, Y.; Park, K. Environment-sensitive hydrogels for drug delivery. Adv. Drug Deliv. Rev. 2001, 53, 321–339. [Google Scholar] [CrossRef]
- Hamidi, M.; Azadi, A.; Rafiei, P. Hydrogel nanoparticles in drug delivery. Adv. Drug Deliv. Rev. 2008, 60, 1638–1649. [Google Scholar] [CrossRef] [PubMed]
- Uhrich, K.E.; Cannizzaro, S.M.; Langer, R.S.; Shakesheff, K.M. Polymeric systems for controlled drug release. Chem. Rev. 1999, 99, 3181–3198. [Google Scholar] [CrossRef] [PubMed]
- Langer, R. New methods of drug delivery. Science 1990, 249, 1527–1533. [Google Scholar] [CrossRef] [PubMed]
- Slaughter, B.V.; Khurshid, S.S.; Fisher, O.Z.; Khademhosseini, A.; Peppas, N.A. Hydrogels in regenerative medicine. Adv. Mater. 2009, 21, 3307–3329. [Google Scholar] [CrossRef] [PubMed]
- Bettinger, C.J.; Borenstein, J.T.; Langer, R. Microfabrication techniques in scaffold development. In Nanotechnology and Regenerative Engineering: The Scaffold; CRC Press: Boca Raton, FL, USA, 2014; p. 103. [Google Scholar]
- Lee, K.Y.; Mooney, D.J. Hydrogels for tissue engineering. Chem. Rev. 2001, 101, 1869–1880. [Google Scholar] [CrossRef] [PubMed]
- Drury, J.L.; Mooney, D.J. Hydrogels for tissue engineering: Scaffold design variables and applications. Biomaterials 2003, 24, 4337–4351. [Google Scholar] [CrossRef]
- Khademhosseini, A.; Langer, R. A decade of progress in tissue engineering. Nat. Protoc. 2016, 11, 1775. [Google Scholar] [CrossRef] [PubMed]
- Leijten, J.; Seo, J.; Yue, K.; Trujillo-de Santiago, G.; Tamayol, A.; Ruiz-Esparza, G.U.; Shin, S.R.; Sharifi, R.; Noshadi, I.; Álvarez, M.M.; et al. Spatially and temporally controlled hydrogels for tissue engineering. Mater. Sci. Eng. R Rep. 2017, 119, 1–35. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, A.S. Hydrogels for biomedical applications. Adv. Drug Deliv. Rev. 2012, 64, 18–23. [Google Scholar] [CrossRef]
- Burczak, K.; Gamian, E.; Kochman, A. Long-term in vivo performance and biocompatibility of poly(vinyl alcohol) hydrogel macrocapsules for hybrid-type artificial pancreas. Biomaterials 1996, 17, 2351–2356. [Google Scholar] [CrossRef]
- Anderson, D.G.; Appel, E.A.; Dong, Y.; Langer, R.S.; Tang, B.C.; Veiseh, O.; Wang, W.; Webber, M.J.; Xue, K. Polymers, Hydrogels, and Uses Thereof. U.S. Patent Application No. 15/078,685, 29 September 2016. [Google Scholar]
- Webber, M.J.; Appel, E.A.; Meijer, E.; Langer, R. Supramolecular biomaterials. Nat. Mater. 2016, 15, 13. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.; Liu, Z.; Liu, Y.; Bentley, W.E.; Payne, G.F. Catechol-based hydrogel for chemical information processing. Biomimetics 2017, 2, 11. [Google Scholar] [CrossRef]
- Banerjee, H.; Tse, Z.T.H.; Ren, H. Soft robotics with compliance and adaptation for biomedical applications and forthcoming challenges. Int. J. Robot. Autom. 2018, 33. [Google Scholar] [CrossRef]
- Ilievski, F.; Mazzeo, A.D.; Shepherd, R.F.; Chen, X.; Whitesides, G.M. Soft robotics for chemists. Angew. Chem. 2011, 123, 1930–1935. [Google Scholar] [CrossRef] [Green Version]
- Rossiter, J.; Winfield, J.; Ieropoulos, I. Here today, gone tomorrow: Biodegradable soft robots. In Electroactive Polymer Actuators and Devices (EAPAD) 2016; International Society for Optics and Photonics: Washington, DC, USA, 2016; Volume 9798, p. 97981S. [Google Scholar]
- Tolley, M.T.; Shepherd, R.F.; Mosadegh, B.; Galloway, K.C.; Wehner, M.; Karpelson, M.; Wood, R.J.; Whitesides, G.M. A resilient, untethered soft robot. Soft Robot. 2014, 1, 213–223. [Google Scholar] [CrossRef]
- Shepherd, R.F.; Ilievski, F.; Choi, W.; Morin, S.A.; Stokes, A.A.; Mazzeo, A.D.; Chen, X.; Wang, M.; Whitesides, G.M. Multigait soft robot. Proc. Natl. Acad. Sci. USA 2011, 108, 20400–20403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Colas, A.; Curtis, J. Silicone biomaterials: History and chemistry. Biomater. Sci. 2004, 2, 80–85. [Google Scholar]
- De Buyl, F. Silicone sealants and structural adhesives. Int. J. Adhes. Adhes. 2001, 21, 411–422. [Google Scholar] [CrossRef]
- Boonstra, B.; Cochrane, H.; Dannenberg, E. Reinforcement of silicone rubber by particulate silica. Rubber Chem. Technol. 1975, 48, 558–576. [Google Scholar] [CrossRef]
- Naji, A.; Harmand, M.F. Cytocompatibility of two coating materials, amorphous alumina and silicon carbide, using human differentiated cell cultures. Biomaterials 1991, 12, 690–694. [Google Scholar] [CrossRef]
- Kue, R.; Sohrabi, A.; Nagle, D.; Frondoza, C.; Hungerford, D. Enhanced proliferation and osteocalcin production by human osteoblast-like MG63 cells on silicon nitride ceramic discs. Biomaterials 1999, 20, 1195–1201. [Google Scholar] [CrossRef]
- Hou, X. Design, Fabrication, Properties and Applications of Smart and Advanced Materials; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Kotzar, G.; Freas, M.; Abel, P.; Fleischman, A.; Roy, S.; Zorman, C.; Moran, J.M.; Melzak, J. Evaluation of MEMS materials of construction for implantable medical devices. Biomaterials 2002, 23, 2737–2750. [Google Scholar] [CrossRef]
- Discher, D.E.; Janmey, P.; Wang, Y.L. Tissue cells feel and respond to the stiffness of their substrate. Science 2005, 310, 1139–1143. [Google Scholar] [CrossRef] [PubMed]
- Otake, M.; Kagami, Y.; Inaba, M.; Inoue, H. Motion design of a starfish-shaped gel robot made of electro-active polymer gel. Robot. Auton. Syst. 2002, 40, 185–191. [Google Scholar] [CrossRef]
- Tobita, H.; Hamielec, A.E. Control of network structure in free-radical crosslinking copolymerization. Polymer 1992, 33, 3647–3657. [Google Scholar] [CrossRef]
- Hild, G.; Okasha, R. Kinetic investigation of the free radical crosslinking copolymerization in the pre-gel state, 1. Styrene/m-and p-divinylbenzene systems. Macromol. Chem. Phys. 1985, 186, 93–110. [Google Scholar] [CrossRef]
- Araci, I.E.; Su, B.; Quake, S.R.; Mandel, Y. An implantable microfluidic device for self-monitoring of intraocular pressure. Nat. Med. 2014, 20, 1074. [Google Scholar] [CrossRef] [PubMed]
- Bryant, S.J.; Anseth, K.S. Hydrogel properties influence ECM production by chondrocytes photoencapsulated in poly(ethylene glycol) hydrogels. J. Biomed. Mater. Res. Part A 2002, 59, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Ifkovits, J.L.; Tous, E.; Minakawa, M.; Morita, M.; Robb, J.D.; Koomalsingh, K.J.; Gorman, J.H.; Gorman, R.C.; Burdick, J.A. Injectable hydrogel properties influence infarct expansion and extent of postinfarction left ventricular remodeling in an ovine model. Proc. Natl. Acad. Sci. USA 2010, 107, 11507–11512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okay, O. General properties of hydrogels. In Hydrogel Sensors and Actuators; Springer: Berlin, Germany, 2009; pp. 1–14. [Google Scholar]
- Hong, Y.; Song, H.; Gong, Y.; Mao, Z.; Gao, C.; Shen, J. Covalently crosslinked chitosan hydrogel: Properties of in vitro degradation and chondrocyte encapsulation. Acta Biomater. 2007, 3, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Warrick, E.; Pierce, O.; Polmanteer, K.; Saam, J. Silicone elastomer developments 1967–1977. Rubber Chem. Technol. 1979, 52, 437–525. [Google Scholar] [CrossRef]
- Han, Y.; Kiat-amnuay, S.; Powers, J.M.; Zhao, Y. Effect of nano-oxide concentration on the mechanical properties of a maxillofacial silicone elastomer. J. Prosthet. Dent. 2008, 100, 465–473. [Google Scholar] [CrossRef]
- Ibrahim, N.A.; Hadithon, K.A.; Abdan, K. Effect of fiber treatment on mechanical properties of kenaf fiber-ecoflex composites. J. Reinf. Plast. Compos. 2010, 29, 2192–2198. [Google Scholar] [CrossRef]
- Yamamoto, M.; Witt, U.; Skupin, G.; Beimborn, D.; Müller, R.J. Biodegradable Aliphatic-Aromatic Polyesters: “Ecoflex®”. Biopolym. Online 2005. [Google Scholar] [CrossRef]
- Shepherd, R.F.; Stokes, A.A.; Nunes, R.; Whitesides, G.M. Soft machines that are resistant to puncture and that self seal. Adv. Mater. 2013, 25, 6709–6713. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Yang, Y.; Chen, Y.; Majidi, C.; Iida, F.; Askounis, E.; Pei, Q. Controllable and reversible tuning of material rigidity for robot applications. Mater. Today 2018. [Google Scholar] [CrossRef]
- Morales, D.; Palleau, E.; Dickey, M.D.; Velev, O.D. Electro-actuated hydrogel walkers with dual responsive legs. Soft Matter 2014, 10, 1337–1348. [Google Scholar] [CrossRef] [PubMed]
- Richter, A. Hydrogels for actuators. In Hydrogel Sensors and Actuators; Springer: Berlin, Germany, 2009; pp. 221–248. [Google Scholar]
- Ashley, S. Artificial muscles. Sci. Am. 2003, 289, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Takashima, Y.; Hatanaka, S.; Otsubo, M.; Nakahata, M.; Kakuta, T.; Hashidzume, A.; Yamaguchi, H.; Harada, A. Expansion–contraction of photoresponsive artificial muscle regulated by host–guest interactions. Nat. Commun. 2012, 3, 1270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mao, L.; Hu, Y.; Piao, Y.; Chen, X.; Xian, W.; Piao, D. Structure and character of artificial muscle model constructed from fibrous hydrogel. Curr. Appl. Phys. 2005, 5, 426–428. [Google Scholar] [CrossRef]
- Ismail, Y.A.; Martínez, J.G.; Al Harrasi, A.S.; Kim, S.J.; Otero, T.F. Sensing characteristics of a conducting polymer/hydrogel hybrid microfiber artificial muscle. Sens. Actuators B Chem. 2011, 160, 1180–1190. [Google Scholar] [CrossRef]
- Bassil, M.; Davenas, J.; Tahchi, M.E. Electrochemical properties and actuation mechanisms of polyacrylamide hydrogel for artificial muscle application. Sens. Actuators B Chem. 2008, 134, 496–501. [Google Scholar] [CrossRef]
- Hsu, S.H.; Chen, C.W.; Hung, K.C.; Tsai, Y.C.; Li, S. Thermo-Responsive Polyurethane Hydrogels Based on Poly (ε-caprolactone) Diol and Amphiphilic Polylactide-Poly(Ethylene Glycol) Block Copolymers. Polymers 2016, 8, 252. [Google Scholar] [CrossRef]
- Suntornnond, R.; Tan, E.Y.S.; An, J.; Chua, C.K. A mathematical model on the resolution of extrusion bioprinting for the development of new bioinks. Materials 2016, 9, 756. [Google Scholar] [CrossRef] [PubMed]
- Nikouei, N.S.; Ghasemi, N.; Lavasanifar, A. Temperature/pH responsive hydrogels based on poly(ethylene glycol) and functionalized poly(e-caprolactone) block copolymers for controlled delivery of macromolecules. Pharm. Res. 2016, 33, 358–366. [Google Scholar] [CrossRef] [PubMed]
- Joshi, A.; Chaudhari, R.; Jayant, R.D. On-Demand Controlled Drug Delivery. In Advances in Personalized Nanotherapeutics; Springer: Berlin, Germany, 2017; pp. 131–156. [Google Scholar]
- Muniz, E.C.; Geuskens, G. Compressive elastic modulus of polyacrylamide hydrogels and semi-IPNs with poly(N-isopropylacrylamide). Macromolecules 2001, 34, 4480–4484. [Google Scholar] [CrossRef]
- Zhang, X.; Pint, C.L.; Lee, M.H.; Schubert, B.E.; Jamshidi, A.; Takei, K.; Ko, H.; Gillies, A.; Bardhan, R.; Urban, J.J.; et al. Optically-and thermally-responsive programmable materials based on carbon nanotube-hydrogel polymer composites. Nano Lett. 2011, 11, 3239–3244. [Google Scholar] [CrossRef] [PubMed]
- Kishi, R.; Ichijo, H.; Hirasa, O. Thermo-responsive devices using poly(vinyl methyl ether) hydrogels. J. Intell. Mater. Syst. Struct. 1993, 4, 533–537. [Google Scholar] [CrossRef]
- Chiarelli, P.; Ragni, P. Thermally driven hydrogel actuator for controllable flow rate pump in long-term drug delivery. Biomed. Appl. Electroact. Polym. Actuators 2009, 89–99. [Google Scholar] [CrossRef]
- Okuzaki, H. Progress and current status of materials and properties of soft actuators. In Soft Actuators; Springer: Berlin, Germany, 2014; pp. 3–18. [Google Scholar]
- Nguyen, K.T.; West, J.L. Photopolymerizable hydrogels for tissue engineering applications. Biomaterials 2002, 23, 4307–4314. [Google Scholar] [CrossRef]
- Oh, J.K.; Drumright, R.; Siegwart, D.J.; Matyjaszewski, K. The development of microgels/nanogels for drug delivery applications. Prog. Polym. Sci. 2008, 33, 448–477. [Google Scholar] [CrossRef]
- Li, A.; Khosla, A.; Drewbrook, C.; Gray, B.L. Fabrication and testing of thermally responsive hydrogel-based actuators using polymer heater elements for flexible microvalves. In Microfluidics, BioMEMS, and Medical Microsystems IX; International Society for Optics and Photonics: Washington, DC, USA, 2011; Volume 7929, p. 79290G. [Google Scholar]
- Yu, C.; Mutlu, S.; Selvaganapathy, P.; Mastrangelo, C.H.; Svec, F.; Fréchet, J.M. Flow control valves for analytical microfluidic chips without mechanical parts based on thermally responsive monolithic polymers. Anal. Chem. 2003, 75, 1958–1961. [Google Scholar] [CrossRef] [PubMed]
- Shoji, S.; Esashi, M. Microflow devices and systems. J. Micromech. Microeng. 1994, 4, 157. [Google Scholar] [CrossRef]
- Li, A.; Lee, J.; Gray, B.L.; Li, P.C. Fabrication and testing of hydrogel-based microvalves for flow control in flexible lab-on-a-chip systems. In Microfluidics, BioMEMS, and Medical Microsystems X; International Society for Optics and Photonics: Washington, DC, USA, 2012; Volume 8251, p. 82510Z. [Google Scholar]
- Wang, J.; Chen, Z.; Mauk, M.; Hong, K.S.; Li, M.; Yang, S.; Bau, H.H. Self-actuated, thermo-responsive hydrogel valves for lab on a chip. Biomed. Microdevices 2005, 7, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chen, Z.; Corstjens, P.L.; Mauk, M.G.; Bau, H.H. A disposable microfluidic cassette for DNA amplification and detection. Lab Chip 2006, 6, 46–53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, R.H.; Yu, Q.; Beebe, D.J. Fabrication and characterization of hydrogel-based microvalves. J. Microelectromech. Syst. 2002, 11, 45–53. [Google Scholar] [CrossRef]
- Eddington, D.T.; Beebe, D.J. Flow control with hydrogels. Adv. Drug Deliv. Rev. 2004, 56, 199–210. [Google Scholar] [CrossRef] [PubMed]
- Harmon, M.E.; Tang, M.; Frank, C.W. A microfluidic actuator based on thermoresponsive hydrogels. Polymer 2003, 44, 4547–4556. [Google Scholar] [CrossRef]
- Kim, Y.S.; Liu, M.; Ishida, Y.; Ebina, Y.; Osada, M.; Sasaki, T.; Hikima, T.; Takata, M.; Aida, T. Thermoresponsive actuation enabled by permittivity switching in an electrostatically anisotropic hydrogel. Nat. Mater. 2015, 14, 1002. [Google Scholar] [CrossRef] [PubMed]
- Wang, E.; Desai, M.S.; Lee, S.W. Light-controlled graphene-elastin composite hydrogel actuators. Nano Lett. 2013, 13, 2826–2830. [Google Scholar] [CrossRef] [PubMed]
- Murdan, S. Electro-responsive drug delivery from hydrogels. J. Control. Release 2003, 92, 1–17. [Google Scholar] [CrossRef]
- Guiseppi-Elie, A. Electroconductive hydrogels: Synthesis, characterization and biomedical applications. Biomaterials 2010, 31, 2701–2716. [Google Scholar] [CrossRef] [PubMed]
- Justin, G.; Guiseppi-Elie, A. Electroconductive blends of poly(HEMA-co-PEGMA-co-HMMAco-SPMA) and poly(Py-co-PyBA): In vitro biocompatibility. J. Bioact. Compat. Polym. 2010, 25, 121–140. [Google Scholar] [CrossRef]
- Kavanagh, A.; Byrne, R.; Diamond, D.; Fraser, K.J. Stimuli Responsive Ionogels for Sensing Applications—An Overview. Membranes 2012, 2, 16–39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brahim, S.; Narinesingh, D.; Guiseppi-Elie, A. Interferent Suppression Using a Novel Polypyrrole-Containing Hydrogel in Amperometric Enzyme Biosensors. Electroanalysis 2002, 14, 627–633. [Google Scholar] [CrossRef]
- Brahim, S.; Narinesingh, D.; Guiseppi-Elie, A. Amperometric determination of cholesterol in serum using a biosensor of cholesterol oxidase contained within a polypyrrole–hydrogel membrane. Anal. Chim. Acta 2001, 448, 27–36. [Google Scholar] [CrossRef]
- Brahim, S.; Narinesingh, D.; Guiseppi-Elie, A. Polypyrrole-hydrogel composites for the construction of clinically important biosensors. Biosens. Bioelectron. 2002, 17, 53–59. [Google Scholar] [CrossRef]
- Guimard, N.K.; Gomez, N.; Schmidt, C.E. Conducting polymers in biomedical engineering. Prog. Polym. Sci. 2007, 32, 876–921. [Google Scholar] [CrossRef]
- Bai, H.; Sheng, K.; Zhang, P.; Li, C.; Shi, G. Graphene oxide/conducting polymer composite hydrogels. J. Mater. Chem. 2011, 21, 18653–18658. [Google Scholar] [CrossRef]
- Green, R.A.; Baek, S.; Poole-Warren, L.A.; Martens, P.J. Conducting polymer-hydrogels for medical electrode applications. Sci. Technol. Adv. Mater. 2010, 11, 014107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gong, J.P.; Katsuyama, Y.; Kurokawa, T.; Osada, Y. Double-network hydrogels with extremely high mechanical strength. Adv. Mater. 2003, 15, 1155–1158. [Google Scholar] [CrossRef]
- Du, P.; Liu, H.C.; Yi, C.; Wang, K.; Gong, X. Polyaniline-modified oriented graphene hydrogel film as the free-standing electrode for flexible solid-state supercapacitors. ACS Appl. Mater. Interfaces 2015, 7, 23932–23940. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Qiu, L.; Cheng, C.; Wu, Y.; Ma, Z.F.; Li, D. Ordered gelation of chemically converted graphene for next-generation electroconductive hydrogel films. Angew. Chem. Int. Ed. 2011, 50, 7325–7328. [Google Scholar] [CrossRef] [PubMed]
- Shiga, T.; Hirose, Y.; Okada, A.; Kurauchi, T. Electrically driven polymer gel finger working in the air. J. Intell. Mater. Syst. Struct. 1993, 4, 553–557. [Google Scholar] [CrossRef]
- Cianchetti, M.; Laschi, C.; Menciassi, A.; Dario, P. Biomedical applications of soft robotics. Nat. Rev. Mater. 2018, 3, 143–153. [Google Scholar] [CrossRef]
- Di Domenico, E.D.; Hobot, C.M.; Stokes, K.B.; Coury, A.J.; Doan, P.D.; Sandstrom, R.D. Medical Electrical Lead with Polymeric Monolithic Controlled Release Device and Method of Manufacture. U.S. Patent Application No. 4,972,848, 27 November 1990. [Google Scholar]
- Cortese, R.; Theeuwes, F. Osmotic Device with Hydrogel Driving Member. U.S. Patent Application No. 4,327,725, 4 May 1982. [Google Scholar]
- Liu, Y.; Servant, A.; Guy, O.; Al-Jamal, K.T.; Williams, P.; Hawkins, K.; Kostarelos, K. An electric-field responsive microsystem for controllable miniaturised drug delivery applications. Sens. Actuators B Chem. 2012, 175, 100–105. [Google Scholar] [CrossRef]
- O’Grady, M.L. Conductive Polymers for Biological Applications; Harvard University: Cambridge, MA, USA, 2010. [Google Scholar]
- Kim, H.I.; Park, S.J.; Kim, S.I.; Kim, N.G.; Kim, S.J. Electroactive polymer hydrogels composed of polyacrylic acid and poly(vinyl sulfonic acid) copolymer for application of biomaterial. Synth. Met. 2005, 155, 674–676. [Google Scholar] [CrossRef]
- Kim, S.J.; Kim, H.I.; Park, S.J.; Kim, S.I. Shape change characteristics of polymer hydrogel based on polyacrylic acid/poly(vinyl sulfonic acid) in electric fields. Sens. Actuators A Phys. 2004, 115, 146–150. [Google Scholar] [CrossRef]
- Shiga, T.; Hirose, Y.; Okada, A.; Kurauchi, T. Bending of poly(vinyl alcohol)–poly(sodium acrylate) composite hydrogel in electric fields. J. Appl. Polym. Sci. 1992, 44, 249–253. [Google Scholar] [CrossRef]
- Zhang, M.; Atkinson, K.R.; Baughman, R.H. Multifunctional carbon nanotube yarns by downsizing an ancient technology. Science 2004, 306, 1358–1361. [Google Scholar] [CrossRef] [PubMed]
- Xia, Z. Biomimetic Principles and Design of Advanced Engineering Materials; John Wiley & Sons: Hoboken, NJ, USA, 2016. [Google Scholar]
- Kwon, G.H.; Park, J.Y.; Kim, J.Y.; Frisk, M.L.; Beebe, D.J.; Lee, S.H. Biomimetic soft multifunctional miniature aquabots. Small 2008, 4, 2148–2153. [Google Scholar] [CrossRef] [PubMed]
- Palleau, E.; Morales, D.; Dickey, M.D.; Velev, O.D. Reversible patterning and actuation of hydrogels by electrically assisted ionoprinting. Nat. Commun. 2013, 4, 2257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hines, L.; Petersen, K.; Lum, G.Z.; Sitti, M. Soft Actuators for Small-Scale Robotics. Adv. Mater. 2017, 29. [Google Scholar] [CrossRef] [PubMed]
- Ren, H.; Banerjee, H. A Preface in Electromagnetic Robotic Actuation and Sensing in Medicine. In Electromagnetic Actuation and Sensing in Medical Robotics; Springer: Berlin, Germany, 2018; pp. 1–10. [Google Scholar]
- Banerjee, H.; Shen, S.; Ren, H. Magnetically Actuated Minimally Invasive Microbots for Biomedical Applications. In Electromagnetic Actuation and Sensing in Medical Robotics; Springer: Berlin, Germany, 2018; pp. 11–41. [Google Scholar]
- Fuhrer, R.; Athanassiou, E.K.; Luechinger, N.A.; Stark, W.J. Crosslinking Metal Nanoparticles into the Polymer Backbone of Hydrogels Enables Preparation of Soft, Magnetic Field-Driven Actuators with Muscle-Like Flexibility. Small 2009, 5, 383–388. [Google Scholar] [CrossRef] [PubMed]
- Ramanujan, R.; Lao, L. The mechanical behavior of smart magnet–hydrogel composites. Smart Mater. Struct. 2006, 15, 952. [Google Scholar] [CrossRef]
- Anal, A.K. Stimuli-induced pulsatile or triggered release delivery systems for bioactive compounds. Recent Pat. Endocr. Metab. Immune Drug Discov. 2007, 1, 83–90. [Google Scholar] [CrossRef]
- Fusco, S.; Chatzipirpiridis, G.; Sivaraman, K.M.; Ergeneman, O.; Nelson, B.J.; Pané, S. Chitosan electrodeposition for microrobotic drug delivery. Adv. Healthc. Mater. 2013, 2, 1037–1044. [Google Scholar] [CrossRef] [PubMed]
- Pessot, G.; Schümann, M.; Gundermann, T.; Odenbach, S.; Löwen, H.; Menzel, A.M. Tunable dynamic moduli of magnetic elastomers: From characterization by X-ray micro-computed tomography to mesoscopic modeling. J. Phys. Condens. Matter 2018, 30, 125101. [Google Scholar] [CrossRef] [PubMed]
- Harne, R.L.; Deng, Z.; Dapino, M.J. Adaptive magnetoelastic metamaterials: A new class of magnetorheological elastomers. J. Intell. Mater. Syst. Struct. 2017. [Google Scholar] [CrossRef]
- Banerjee, H.; Ren, H. Electromagnetically Responsive Soft-Flexible Robots and Sensors for Biomedical Applications and Impending Challenges. In Electromagnetic Actuation and Sensing in Medical Robotics; Springer: Berlin, Germany, 2018; pp. 43–72. [Google Scholar]
- Hwang, D.K.; Dendukuri, D.; Doyle, P.S. Microfluidic-based synthesis of non-spherical magnetic hydrogel microparticles. Lab Chip 2008, 8, 1640–1647. [Google Scholar] [CrossRef] [PubMed]
- Yuet, K.P.; Hwang, D.K.; Haghgooie, R.; Doyle, P.S. Multifunctional superparamagnetic Janus particles. Langmuir 2009, 26, 4281–4287. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Huang, G.; Zhang, X.; Li, B.; Chen, Y.; Lu, T.; Lu, T.J.; Xu, F. Magnetic hydrogels and their potential biomedical applications. Adv. Funct. Mater. 2013, 23, 660–672. [Google Scholar] [CrossRef]
- Barbucci, R.; Giani, G.; Fedi, S.; Bottari, S.; Casolaro, M. Biohydrogels with magnetic nanoparticles as crosslinker: characteristics and potential use for controlled antitumor drug-delivery. Acta Biomater. 2012, 8, 4244–4252. [Google Scholar] [CrossRef] [PubMed]
- Barbucci, R.; Pasqui, D.; Giani, G.; De Cagna, M.; Fini, M.; Giardino, R.; Atrei, A. A novel strategy for engineering hydrogels with ferromagnetic nanoparticles as crosslinkers of the polymer chains. Potential applications as a targeted drug delivery system. Soft Matter 2011, 7, 5558–5565. [Google Scholar] [CrossRef]
- Satarkar, N.S.; Johnson, D.; Marrs, B.; Andrews, R.; Poh, C.; Gharaibeh, B.; Saito, K.; Anderson, K.W.; Hilt, J.Z. Hydrogel-MWCNT nanocomposites: Synthesis, characterization, and heating with radiofrequency fields. J. Appl. Polym. Sci. 2010, 117, 1813–1819. [Google Scholar] [CrossRef]
- Zhang, Y.; Bai, Y.; Yan, B. Functionalized carbon nanotubes for potential medicinal applications. Drug Discov. Today 2010, 15, 428–435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, H.W.; Sakar, M.S.; Petruska, A.J.; Pané, S.; Nelson, B.J. Soft micromachines with programmable motility and morphology. Nat. Commun. 2016, 7, 12263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, X.; Kim, J.; Cezar, C.A.; Huebsch, N.; Lee, K.; Bouhadir, K.; Mooney, D.J. Active scaffolds for on-demand drug and cell delivery. Proc. Natl. Acad. Sci. USA 2011, 108, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Harrington, M.J.; Razghandi, K.; Ditsch, F.; Guiducci, L.; Rueggeberg, M.; Dunlop, J.W.; Fratzl, P.; Neinhuis, C.; Burgert, I. Origami-like unfolding of hydro-actuated ice plant seed capsules. Nat. Commun. 2011, 2, 337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burgert, I.; Fratzl, P. Actuation systems in plants as prototypes for bioinspired devices. Phil. Trans. R. Soc. Lond. A Math. Phys. Eng. Sci. 2009, 367, 1541–1557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fratzl, P.; Barth, F.G. Biomaterial systems for mechanosensing and actuation. Nature 2009, 462, 442. [Google Scholar] [CrossRef] [PubMed]
- Ionov, L. Actuating Hydrogel Thin Films. In Responsive Polymer Surfaces: Dynamics in Surface Topography; Wiley: Hoboken, NJ, USA, 2017. [Google Scholar]
- Ionov, L. Actively-moving materials based on stimuli-responsive polymers. J. Mater. Chem. 2010, 20, 3382–3390. [Google Scholar] [CrossRef]
- Tokarev, I.; Minko, S. Stimuli-responsive hydrogel thin films. Soft Matter 2009, 5, 511–524. [Google Scholar] [CrossRef]
- Yang, F.; Williams, C.G.; Wang, D.A.; Lee, H.; Manson, P.N.; Elisseeff, J. The effect of incorporating RGD adhesive peptide in polyethylene glycol diacrylate hydrogel on osteogenesis of bone marrow stromal cells. Biomaterials 2005, 26, 5991–5998. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Shu, X.Z.; Prestwich, G.D. Osteochondral defect repair with autologous bone marrow–derived mesenchymal stem cells in an injectable, in situ, cross-linked synthetic extracellular matrix. Tissue Eng. 2006, 12, 3405–3416. [Google Scholar] [CrossRef] [PubMed]
- Ji, C.; Khademhosseini, A.; Dehghani, F. Enhancing cell penetration and proliferation in chitosan hydrogels for tissue engineering applications. Biomaterials 2011, 32, 9719–9729. [Google Scholar] [CrossRef] [PubMed]
- Rafat, M.; Li, F.; Fagerholm, P.; Lagali, N.S.; Watsky, M.A.; Munger, R.; Matsuura, T.; Griffith, M. PEG-stabilized carbodiimide crosslinked collagen–chitosan hydrogels for corneal tissue engineering. Biomaterials 2008, 29, 3960–3972. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.; Chu, C.R.; Payne, K.A.; Marra, K.G. Injectable in situ forming biodegradable chitosan–hyaluronic acid based hydrogels for cartilage tissue engineering. Biomaterials 2009, 30, 2499–2506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suh, J.K.F.; Matthew, H.W. Application of chitosan-based polysaccharide biomaterials in cartilage tissue engineering: A review. Biomaterials 2000, 21, 2589–2598. [Google Scholar] [PubMed]
- Liu, Z.; Calvert, P. Multilayer hydrogels as muscle-like actuators. Adv. Mater. 2000, 12, 288–291. [Google Scholar] [CrossRef]
- Nelson, B.J.; Sakar, S.; Fusco, S. Self folding microrobots for biomedical applications. In Proceedings of the 2014 IEEE International Conference on Robotics and Automation (ICRA), Hong Kong, China, 31 May–7 June 2014. [Google Scholar]
- Fu, F.; Shang, L.; Chen, Z.; Yu, Y.; Zhao, Y. Bioinspired living structural color hydrogels. Sci. Robot. 2018, 3, eaar8580. [Google Scholar] [CrossRef]
- Hong, Y.; Huber, A.; Takanari, K.; Amoroso, N.J.; Hashizume, R.; Badylak, S.F.; Wagner, W.R. Mechanical properties and in vivo behavior of a biodegradable synthetic polymer microfiber–extracellular matrix hydrogel biohybrid scaffold. Biomaterials 2011, 32, 3387–3394. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zolfagharian, A.; Kouzani, A.Z.; Khoo, S.Y.; Gibson, I.; Kaynak, A. 3D printed hydrogel soft actuators. In Proceedings of the 2016 IEEE Region 10 Conference (TENCON), Singapore, 22–25 November 2016; pp. 2272–2277. [Google Scholar]
- Bakarich, S.E.; Gorkin, R.; Spinks, G.M. 4D printing with mechanically robust, thermally actuating hydrogels. Macromol. Rapid Commun. 2015, 36, 1211–1217. [Google Scholar] [CrossRef] [PubMed]
- Calvert, P. Gel sensors and actuators. MRS Bull. 2008, 33, 207–212. [Google Scholar] [CrossRef]
- Yang, C.; Wang, W.; Yao, C.; Xie, R.; Ju, X.J.; Liu, Z.; Chu, L.Y. Hydrogel walkers with electro-driven motility for cargo transport. Sci. Rep. 2015, 5, 13622. [Google Scholar] [CrossRef] [PubMed]
- Kwon, G.H.; Choi, Y.Y.; Park, J.Y.; Woo, D.H.; Lee, K.B.; Kim, J.H.; Lee, S.H. Electrically-driven hydrogel actuators in microfluidic channels: Fabrication, characterization, and biological application. Lab Chip 2010, 10, 1604–1610. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Lee, J.M.; Yeong, W.Y. Smart hydrogels for 3D bioprinting. Int. J. Bioprint. 2015, 1, 3–14. [Google Scholar] [CrossRef] [Green Version]
- Kozlovskaya, V.; Chen, J.; Tedjo, C.; Liang, X.; Campos-Gomez, J.; Oh, J.; Saeed, M.; Lungu, C.T.; Kharlampieva, E. pH-responsive hydrogel cubes for release of doxorubicin in cancer cells. J. Mater. Chem. B 2014, 2, 2494–2507. [Google Scholar] [CrossRef]
- Zhu, W.; Li, J.; Leong, Y.J.; Rozen, I.; Qu, X.; Dong, R.; Wu, Z.; Gao, W.; Chung, P.H.; Wang, J.; et al. 3D-printed artificial microfish. Adv. Mater. 2015, 27, 4411–4417. [Google Scholar] [CrossRef] [PubMed]
- Santulli, C.; Patel, S.; Jeronimidis, G.; Davis, F.; Mitchell, G. Development of smart variable stiffness actuators using polymer hydrogels. Smart Mater. Struct. 2005, 14, 434. [Google Scholar] [CrossRef]
- Jiang, Z.C.; Xiao, Y.Y.; Kang, Y.; Pan, M.; Li, B.J.; Zhang, S. Shape memory polymers based on supramolecular interactions. ACS Appl. Mater. Interfaces 2017, 9, 20276–20293. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.T.; Leisk, G.G.; Trimmer, B. GoQBot: A caterpillar-inspired soft-bodied rolling robot. Bioinspir. Biomim. 2011, 6, 026007. [Google Scholar] [CrossRef] [PubMed]
- Gul, J.Z.; Sajid, M.; Rehman, M.M.; Siddiqui, G.U.; Shah, I.; Kim, K.H.; Lee, J.W.; Choi, K.H. 3D printing for soft robotics—A review. Sci. Technol. Adv. Mater. 2018, 19, 243–262. [Google Scholar] [CrossRef] [PubMed]
- Gul, J.Z.; Yang, B.S.; Yang, Y.J.; Chang, D.E.; Choi, K.H. In situ UV curable 3D printing of multi-material tri-legged soft bot with spider mimicked multi-step forward dynamic gait. Smart Mater. Struct. 2016, 25, 115009. [Google Scholar] [CrossRef] [Green Version]
- Paik, J.K.; Wood, R.J. A bidirectional shape memory alloy folding actuator. Smart Mater. Struct. 2012, 21, 065013. [Google Scholar] [CrossRef]
- Buenger, D.; Topuz, F.; Groll, J. Hydrogels in sensing applications. Prog. Polym. Sci. 2012, 37, 1678–1719. [Google Scholar] [CrossRef]
- Topuz, F.; Buenger, D.; Tanaka, D.; Groll, J. Hydrogels in biosensing applications. In Comprehensive Biomaterials; Elsevier: New York, NY, USA, 2011. [Google Scholar]
- Tian, E.; Wang, J.; Zheng, Y.; Song, Y.; Jiang, L.; Zhu, D. Colorful humidity sensitive photonic crystal hydrogel. J. Mater. Chem. 2008, 18, 1116–1122. [Google Scholar] [CrossRef]
- Arregui, F.J.; Ciaurriz, Z.; Oneca, M.; Matıas, I.R. An experimental study about hydrogels for the fabrication of optical fiber humidity sensors. Sens. Actuators B Chem. 2003, 96, 165–172. [Google Scholar] [CrossRef]
- Herber, S.; Bomer, J.; Olthuis, W.; Bergveld, P.; van den Berg, A. A miniaturized carbon dioxide gas sensor based on sensing of pH-sensitive hydrogel swelling with a pressure sensor. Biomed. Microdevices 2005, 7, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Richter, A.; Paschew, G.; Klatt, S.; Lienig, J.; Arndt, K.F.; Adler, H.J.P. Review on hydrogel-based pH sensors and microsensors. Sensors 2008, 8, 561–581. [Google Scholar] [CrossRef] [PubMed]
- Tavakoli, J.; Tang, Y. Hydrogel Based Sensors for Biomedical Applications: An Updated Review. Polymers 2017, 9, 364. [Google Scholar] [CrossRef]
- Qu, F.; Zhang, Y.; Rasooly, A.; Yang, M. Electrochemical biosensing platform using hydrogel prepared from ferrocene modified amino acid as highly efficient immobilization matrix. Anal. Chem. 2014, 86, 973–976. [Google Scholar] [CrossRef] [PubMed]
- Edmondson, R.; Broglie, J.J.; Adcock, A.F.; Yang, L. Three-dimensional cell culture systems and their applications in drug discovery and cell-based biosensors. Assay Drug Dev. Technol. 2014, 12, 207–218. [Google Scholar] [CrossRef] [PubMed]
- Reddy, S.M.; Phan, Q.T.; El-Sharif, H.; Govada, L.; Stevenson, D.; Chayen, N.E. Protein crystallization and biosensor applications of hydrogel-based molecularly imprinted polymers. Biomacromolecules 2012, 13, 3959–3965. [Google Scholar] [CrossRef] [PubMed]
- Russell, R.J.; Pishko, M.V.; Gefrides, C.C.; McShane, M.J.; Cote, G.L. A fluorescence-based glucose biosensor using concanavalin A and dextran encapsulated in a poly(ethylene glycol) hydrogel. Anal. Chem. 1999, 71, 3126–3132. [Google Scholar] [CrossRef] [PubMed]
- Zhan, W.; Seong, G.H.; Crooks, R.M. Hydrogel-based microreactors as a functional component of microfluidic systems. Anal. Chem. 2002, 74, 4647–4652. [Google Scholar] [CrossRef] [PubMed]
- Koh, W.G.; Pishko, M.V. Fabrication of cell-containing hydrogel microstructures inside microfluidic devices that can be used as cell-based biosensors. Anal. Bioanal. Chem. 2006, 385, 1389–1397. [Google Scholar] [CrossRef] [PubMed]
- Quinn, J.G.; O’Neill, S.; Doyle, A.; McAtamney, C.; Diamond, D.; MacCraith, B.D.; O’Kennedy, R. Development and application of surface plasmon resonance-based biosensors for the detection of cell–ligand interactions. Anal. Biochem. 2000, 281, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Shang, J.; Yan, J.; Zhang, Z.; Huang, X.; Maturavongsadit, P.; Song, B.; Jia, Y.; Ma, T.; Li, D.; Xu, K.; et al. A hydrogel-based glucose affinity microsensor. Sens. Actuators B Chem. 2016, 237, 992–998. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, X.; James, T.D. Glucose sensing in supramolecular chemistry. Chem. Rev. 2015, 115, 8001–8037. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Losego, M.D.; Braun, P.V. Hydrogel-based glucose sensors: Effects of phenylboronic acid chemical structure on response. Chem. Mater. 2013, 25, 3239–3250. [Google Scholar] [CrossRef]
- Dou, Q.; Hu, D.; Gao, H.; Zhang, Y.; Yetisen, A.K.; Butt, H.; Wang, J.; Nie, G.; Dai, Q. High performance boronic acid-containing hydrogel for biocompatible continuous glucose monitoring. RSC Adv. 2017, 7, 41384–41390. [Google Scholar] [CrossRef] [Green Version]
- Pham, X.H.; Shim, S.; Kim, T.H.; Hahm, E.; Kim, H.M.; Rho, W.Y.; Jeong, D.H.; Lee, Y.S.; Jun, B.H. Glucose detection using 4-mercaptophenyl boronic acid-incorporated silver nanoparticles-embedded silica-coated graphene oxide as a SERS substrate. BioChip J. 2017, 11, 46–56. [Google Scholar] [CrossRef]
- Yu, Y.; Nguyen, T.; Tathireddy, P.; Young, D.J.; Roundy, S. Wireless hydrogel-based glucose sensor for future implantable applications. In Proceedings of the 2016 IEEE SENSORS, Orlando, FL, USA, 30 October–3 November 2016; pp. 1–3. [Google Scholar]
- Zhang, C.; Cano, G.G.; Braun, P.V. Linear and fast hydrogel glucose sensor materials enabled by volume resetting agents. Adv. Mater. 2014, 26, 5678–5683. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.; Czarnik, A.W. Fluorescent Chemosensors of Carbohydrates. A Means of Chemically Commcating the Binding of Polyols in Water Based on Chelation-enhanced Quenching. In LEOS’92 Conference Proceedings, Proceedings of the 1992 Annual Meeting of IEEE Lasers and Electro-Optics Society Boston, MA, USA, 16–19 November 1992; IEEE: Piscataway, NJ, USA, 1992; pp. 276–277. [Google Scholar]
- Kaur, G.; Lin, N.; Fang, H.; Wang, B. Boronic acid-based fluorescence sensors for glucose monitoring. In Glucose Sensing; Springer: Berlin, Germany, 2006; pp. 377–397. [Google Scholar]
- Zhai, D.; Liu, B.; Shi, Y.; Pan, L.; Wang, Y.; Li, W.; Zhang, R.; Yu, G. Highly sensitive glucose sensor based on Pt nanoparticle/polyaniline hydrogel heterostructures. ACS Nano 2013, 7, 3540–3546. [Google Scholar] [CrossRef] [PubMed]
- Mano, N.; Yoo, J.E.; Tarver, J.; Loo, Y.L.; Heller, A. An electron-conducting cross-linked polyaniline-based redox hydrogel, formed in one step at pH 7.2, wires glucose oxidase. J. Am. Chem. Soc. 2007, 129, 7006–7007. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Li, B.; Deng, Q.; Dong, S. Amperometric glucose biosensor based on sol-gel organic-inorganic hybrid material. Anal. Chem. 1998, 70, 3170–3174. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.L.; Xu, J.J.; Du, Y.; Chen, H.Y. A glucose biosensor based on chitosan–glucose oxidase–gold nanoparticles biocomposite formed by one-step electrodeposition. Anal. Biochem. 2004, 334, 284–289. [Google Scholar] [CrossRef] [PubMed]
- Qiu, J.D.; Huang, J.; Liang, R.P. Nanocomposite film based on graphene oxide for high performance flexible glucose biosensor. Sens. Actuators B Chem. 2011, 160, 287–294. [Google Scholar] [CrossRef]
- Reiter, S.; Habermüller, K.; Schuhmann, W. A reagentless glucose biosensor based on glucose oxidase entrapped into osmium-complex modified polypyrrole films. Sens. Actuators B Chem. 2001, 79, 150–156. [Google Scholar] [CrossRef]
- Shibata, H.; Heo, Y.J.; Okitsu, T.; Matsunaga, Y.; Kawanishi, T.; Takeuchi, S. Injectable hydrogel microbeads for fluorescence-based in vivo continuous glucose monitoring. Proc. Natl. Acad. Sci. USA 2010, 107, 17894–17898. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Endo, T.; Ikeda, R.; Yanagida, Y.; Hatsuzawa, T. Stimuli-responsive hydrogel–silver nanoparticles composite for development of localized surface plasmon resonance-based optical biosensor. Anal. Chim. Acta 2008, 611, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Bornhoeft, L.R.; Biswas, A.; McShane, M.J. Composite hydrogels with engineered microdomains for optical glucose sensing at low oxygen conditions. Biosensors 2017, 7, 8. [Google Scholar] [CrossRef] [PubMed]
- Kajisa, T.; Sakata, T. Glucose-responsive hydrogel electrode for biocompatible glucose transistor. Sci. Technol. Adv. Mater. 2017, 18, 26–33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, C. Bioinspired touch sensors for medical applications. In Proceedings of the 2013 IEEE SENSORS, Baltimore, MD, USA, 3–6 November 2013; pp. 1–4. [Google Scholar]
- Sarwar, M.S.; Dobashi, Y.; Preston, C.; Wyss, J.K.; Mirabbasi, S.; Madden, J.D.W. Bend, stretch, and touch: Locating a finger on an actively deformed transparent sensor array. Sci. Adv. 2017, 3, e1602200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Orthner, M.; Buetefisch, S.; Magda, J.; Rieth, L.; Solzbacher, F. Development, fabrication, and characterization of hydrogel based piezoresistive pressure sensors with perforated diaphragms. Sens. Actuators A Phys. 2010, 161, 29–38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, C.C.; Lee, H.H.; Oh, K.H.; Sun, J.Y. Highly stretchable, transparent ionic touch panel. Science 2016, 353, 682–687. [Google Scholar] [CrossRef] [PubMed]
- Larson, C.; Peele, B.; Li, S.; Robinson, S.; Totaro, M.; Beccai, L.; Mazzolai, B.; Shepherd, R. Highly stretchable electroluminescent skin for optical signaling and tactile sensing. Science 2016, 351, 1071–1074. [Google Scholar] [CrossRef] [PubMed]
- Edwards, L.; McCrimmon, P.; Watson, R.T. Hydrogel-Based Tactile-Feedback Touch Screen. U.S. Patent Application No. 12/253,776, 22 April 2010. [Google Scholar]
- Sawahata, K.; Gong, J.P.; Osada, Y. Soft and wet touch-sensing system made of hydrogel. Macromol. Rapid Commun. 1995, 16, 713–716. [Google Scholar] [CrossRef]
- Tai, Y.; Mulle, M.; Ventura, I.A.; Lubineau, G. A highly sensitive, low-cost, wearable pressure sensor based on conductive hydrogel spheres. Nanoscale 2015, 7, 14766–14773. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Wei, J.; Xu, B.; Liu, X.; Wang, H.; Wang, W.; Wang, Q.; Liu, W. A robust, highly stretchable supramolecular polymer conductive hydrogel with self-healability and thermo-processability. Sci. Rep. 2017, 7, 41566. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, Y.; He, W.; Cao, T.; Guo, H.; Zhang, Y.; Li, Q.; Shao, Z.; Cui, Y.; Zhang, X. Elastic, conductive, polymeric hydrogels and sponges. Sci. Rep. 2014, 4, 5792. [Google Scholar] [CrossRef] [PubMed]
- Gerlach, G.; Guenther, M.; Sorber, J.; Suchaneck, G.; Arndt, K.F.; Richter, A. Chemical and pH sensors based on the swelling behavior of hydrogels. Sens. Actuators B Chem. 2005, 111, 555–561. [Google Scholar] [CrossRef]
- Trinh, Q.T.; Gerlach, G.; Sorber, J.; Arndt, K.F. Hydrogel-based piezoresistive pH sensors: Design, simulation and output characteristics. Sens. Actuators B Chem. 2006, 117, 17–26. [Google Scholar] [CrossRef]
- Reese, C.E.; Baltusavich, M.E.; Keim, J.P.; Asher, S.A. Development of an intelligent polymerized crystalline colloidal array colorimetric reagent. Anal. Chem. 2001, 73, 5038–5042. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Go, G.; Ko, S.Y.; Park, J.O.; Park, S. Magnetic actuated pH-responsive hydrogel-based soft micro-robot for targeted drug delivery. Smart Mater. Struct. 2016, 25, 027001. [Google Scholar] [CrossRef]
- Xu, L.; Qiu, L.; Sheng, Y.; Sun, Y.; Deng, L.; Li, X.; Bradley, M.; Zhang, R. Biodegradable pH-responsive hydrogels for controlled dual-drug release. J. Mater. Chem. B 2018, 6, 510–517. [Google Scholar] [CrossRef]
- Guenther, M.; Gerlach, G.; Corten, C.; Kuckling, D.; Muller, M.; Shi, Z.; Sorber, J.; Arndt, K.F. Application of Polyelectrolytic Temperature-Responsive Hydrogels in Chemical Sensors. In Macromolecular Symposia; Wiley: Hoboken, NJ, USA, 2007; Volume 254, pp. 314–321. [Google Scholar]
- Maruyama, H.; Masuda, T.; Honda, A.; Arai, F. Local temperature measurement using specrta shift of quantum-dot hydrogel sensor. In Proceedings of the 2011 11th IEEE Conference on Nanotechnology (IEEE-NANO), Portland, OR, USA, 15–18 August 2011; pp. 319–322. [Google Scholar]
- Borisov, S.M.; Wolfbeis, O.S. Temperature-sensitive europium(III) probes and their use for simultaneous luminescent sensing of temperature and oxygen. Anal. Chem. 2006, 78, 5094–5101. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Shi, C.; Yang, X.; Shen, B.; Sun, Y.; Chen, Y.; Xu, X.; Sun, H.; Yu, K.; Yang, B.; et al. pH-and temperature-sensitive hydrogel nanoparticles with dual photoluminescence for bioprobes. ACS Nano 2016, 10, 5856–5863. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, K.Q. Photonic crystal structures with tunable structure color as colorimetric sensors. Sensors 2013, 13, 4192–4213. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Tang, T.C.; Tham, E.; Yuk, H.; Lin, S.; Lu, T.K.; Zhao, X. Stretchable living materials and devices with hydrogel–elastomer hybrids hosting programmed cells. Proc. Natl. Acad. Sci. USA 2017, 114, 2200–2205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wolfbeis, O.S. Materials for fluorescence-based optical chemical sensors. J. Mater. Chem. 2005, 15, 2657–2669. [Google Scholar] [CrossRef]
- Wichterle, O.; Lim, D. Hydrophilic gels for biological use. Nature 1960, 185, 117. [Google Scholar] [CrossRef]
- Hoffman, A.; Schmer, G.; Harris, C.; Kraft, W. Covalent binding of biomolecules to radiation-grafted hydrogels on inert polymer surfaces. ASAIO J. 1972, 18, 10–16. [Google Scholar] [CrossRef]
- Ratner, B.D.; Hoffman, A.S. Synthetic Hydrogels for Biomedical Applications; ACS Publications: Washington, DC, USA, 1976. [Google Scholar]
- Huglin, M. Hydrogels in medicine and pharmacy Edited by NA Peppas, CRC Press Inc., Boca Raton, Florida, 1986 (Vol. l), 1987 (Vols. 2 and 3). Vol. 1 Fundamentals, pp. vii+ 180, £72.00, ISBN 0-8493-5546-X; Vol. 2 Polymers, pp. vii+ 171, £72.00, ISBN 0-8493-5547-8; Vol. 3 Properties and Applications, pp. vii+ 195, £8000, ISBN 0-8493-5548-6. Br. Polym. J. 1989, 21, 184. [Google Scholar]
- Park, H.; Park, K.; Shalaby, W.S. Biodegradable Hydrogels for Drug Delivery; CRC Press: Boca Raton, FL, USA, 2011. [Google Scholar]
- Harland, R.S.; Prud’homme, R.K. Polyelectrolyte Gels: Properties, Preparation, and Applications; ACS Publications: Washington, DC, USA, 1992. [Google Scholar]
- Ulbrich, K.; Subr, V.; Podpěrová, P.; Burešová, M. Synthesis of novel hydrolytically degradable hydrogels for controlled drug release. J. Control. Release 1995, 34, 155–165. [Google Scholar] [CrossRef]
- Park, K. Controlled Drug Delivery: Challenges and Strategies; American Chemical Society: Washington, DC, USA, 1997. [Google Scholar]
- Harris, Z.; Zalipsky, S. Poly(ethylene Glycol); American Chemical Society: Washington, DC, USA, 1997. [Google Scholar]
- Dong, L.; Hoffman, A.; Yan, Q. Macromolecular penetration through hydrogels. J. Biomater. Sci. Polym 1994, 5, 473–484. [Google Scholar] [CrossRef]
- Kuo, C.; Ma, P. Diffusivity of three-dimensional, ionically crosslinked alginate hydrogels. Polymer 2000, 41, 1661–1662. [Google Scholar]
- Sun, J.E. Hydrophobic Payload Encapsulation and Release Characteristics in Self-Assembled Peptide Hydrogels; University of Delaware: Newark, DE, USA, 2015. [Google Scholar]
- Gin, H.; Dupuy, B.; Baquey, A.; Baquey, C.; Ducassou, D. Lack of responsiveness to glucose of microencapsulated islets of Langerhans after three weeks’ implantation in the rat—Influence of the complement. J. Microencapsul. 1990, 7, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Matthew, H.W.; Salley, S.O.; Peterson, W.D.; Klein, M.D. Complex coacervate microcapsules for mammalian cell culture and artificial organ development. Biotechnol. Prog. 1993, 9, 510–519. [Google Scholar] [CrossRef] [PubMed]
- Hsu, F.; Tsai, S.; Wang, F.; Wang, Y. The collagen-containing alginate/poly(L-lysine)/alginate microcapsules. Artif. Cells Blood Substit. Biotechnol. 2000, 28, 147–154. [Google Scholar] [CrossRef]
- Sefton, M.; May, M.; Lahooti, S.; Babensee, J. Making microencapsulation work: conformal coating, immobilization gels and in vivo performance. J. Control. Release 2000, 65, 173–186. [Google Scholar] [CrossRef]
- Dillon, G.P.; Yu, X.; Sridharan, A.; Ranieri, J.P.; Bellamkonda, R.V. The influence of physical structure and charge on neurite extension in a 3D hydrogel scaffold. J. Biomater. Sci. Polym. Ed. 1998, 9, 1049–1069. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Rodriguez, A.; Vacanti, M.; Ibarra, C.; Arevalo, C.; Vacanti, C.A. Comparative study of the use of poly (glycolic acid), calcium alginate and pluronics in the engineering of autologous porcine cartilage. J. Biomater. Sci. Polym. Ed. 1998, 9, 475–487. [Google Scholar] [PubMed]
- Gupta, P.; Vermani, K.; Garg, S. Hydrogels: From controlled release to pH-responsive drug delivery. Drug Discov. Today 2002, 7, 569–579. [Google Scholar] [CrossRef]
- Kumar, C.S.; Mohammad, F. Magnetic nanomaterials for hyperthermia-based therapy and controlled drug delivery. Adv. Drug Deliv. Rev. 2011, 63, 789–808. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Banerjee, H.; Ren, H. Optimizing double-network hydrogel for biomedical soft robots. Soft Robot. 2017, 4, 191–201. [Google Scholar] [CrossRef] [PubMed]
- Muscat-Fenech, C.; Atkins, A. Elastoplastic trouser tear testing of sheet materials. Int. J. Fract. 1994, 67, 69–80. [Google Scholar] [CrossRef]
- Birchall, J.; Howard, A.; Kendall, K. Flexural strength and porosity of cements. Nature 1981, 289, 388. [Google Scholar] [CrossRef]
- Hassan, C.M.; Peppas, N.A. Structure and applications of poly(vinyl alcohol) hydrogels produced by conventional crosslinking or by freezing/thawing methods. In Biopolymers PVA Hydrogels, Anionic Polymerisation Nanocomposites; Springer: Berlin/Heidelberg, Germany, 2000; pp. 37–65. [Google Scholar]
- Friedrich, K. Application of Fracture Mechanics to Composite Materials; Elsevier: New York, NY, USA, 2012; Volume 6. [Google Scholar]
- Kabir, M.E.; Saha, M.; Jeelani, S. Tensile and fracture behavior of polymer foams. Mater. Sci. Eng. A 2006, 429, 225–235. [Google Scholar] [CrossRef]
- Hartwig, G. Fracture behavior of polymers. In Polymer Properties at Room and Cryogenic Temperatures; Springer: Berlin, Germany, 1994; pp. 187–218. [Google Scholar]
- Nicolas, J.; Charleux, B.; Guerret, O.; Magnet, S. Nitroxide-Mediated Controlled Free-Radical Emulsion Polymerization of Styrene and n-Butyl Acrylate with a Water-Soluble Alkoxyamine as Initiator. Angew. Chem. Int. Ed. 2004, 43, 6186–6189. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Zhu, L.; Zhao, C.; Wang, Q.; Zheng, J. A robust, one-pot synthesis of highly mechanical and recoverable double network hydrogels using thermoreversible sol-gel polysaccharide. Adv. Mater. 2013, 25, 4171–4176. [Google Scholar] [CrossRef] [PubMed]
- Pacelli, S.; Paolicelli, P.; Pepi, F.; Garzoli, S.; Polini, A.; Tita, B.; Vitalone, A.; Casadei, M.A. Gellan gum and polyethylene glycol dimethacrylate double network hydrogels with improved mechanical properties. J. Polym. Res. 2014, 21, 409. [Google Scholar] [CrossRef]
- Xu, X.; Lü, S.; Gao, C.; Wang, X.; Bai, X.; Gao, N.; Liu, M. One-pot facile synthesis of silica reinforced double network hydrogels based on triple interactions. Chem. Eng. J. 2014, 240, 331–337. [Google Scholar] [CrossRef]
- Ahmed, S.; Nakajima, T.; Kurokawa, T.; Haque, M.A.; Gong, J.P. Brittle–ductile transition of double network hydrogels: Mechanical balance of two networks as the key factor. Polymer 2014, 55, 914–923. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Guo, X.; Yang, S.; Tan, S.; Li, X.; Dai, H.; Yu, X.; Zhang, X.; Weng, N.; Jian, B.; et al. Double-network hydrogel with high mechanical strength prepared from two biocompatible polymers. J. Appl. Polym. Sci. 2009, 112, 3063–3070. [Google Scholar] [CrossRef]
- Geng, L.; Wong, Y.; Hutmacher, D.; Feng, W.; Loh, H.; Fuh, J. Rapid Prototyping of 3D Scaffolds for Tissue Engineering Using a Four-Axis Multiple-Dispenser Robotic System; National University of Singapore: Singapore, 2003. [Google Scholar]
- Ding, Y.; Lan, H.; Hong, J.; Wu, D. An integrated manufacturing system for rapid tooling based on rapid prototyping. Robot. Comput.-Integr. Manuf. 2004, 20, 281–288. [Google Scholar] [CrossRef]
- Unger, M.A.; Chou, H.P.; Thorsen, T.; Scherer, A.; Quake, S.R. Monolithic microfabricated valves and pumps by multilayer soft lithography. Science 2000, 288, 113–116. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhu, L.; Hickner, M.; Bai, B. Molecular Engineering Mechanically Programmable Hydrogels with Orthogonal Functionalization. Chem. Mater. 2017, 29, 9981–9989. [Google Scholar] [CrossRef]
- Creton, C. 50th Anniversary Perspective: Networks and Gels: Soft but Dynamic and Tough. Macromolecules 2017, 50, 8297–8316. [Google Scholar] [CrossRef]
- Lake, G.; Thomas, A. The strength of highly elastic materials. Proc. R. Soc. Lond. A 1967, 300, 108–119. [Google Scholar] [CrossRef]
- Huang, T.; Xu, H.; Jiao, K.; Zhu, L.; Brown, H.R.; Wang, H. A novel hydrogel with high mechanical strength: a macromolecular microsphere composite hydrogel. Adv. Mater. 2007, 19, 1622–1626. [Google Scholar] [CrossRef]
- Sakai, T.; Matsunaga, T.; Yamamoto, Y.; Ito, C.; Yoshida, R.; Suzuki, S.; Sasaki, N.; Shibayama, M.; Chung, U.I. Design and fabrication of a high-strength hydrogel with ideally homogeneous network structure from tetrahedron-like macromonomers. Macromolecules 2008, 41, 5379–5384. [Google Scholar] [CrossRef]
- Seitz, M.E.; Martina, D.; Baumberger, T.; Krishnan, V.R.; Hui, C.Y.; Shull, K.R. Fracture and large strain behavior of self-assembled triblock copolymer gels. Soft Matter 2009, 5, 447–456. [Google Scholar] [CrossRef]
- Lin, W.C.; Fan, W.; Marcellan, A.; Hourdet, D.; Creton, C. Large strain and fracture properties of poly(dimethylacrylamide)/silica hybrid hydrogels. Macromolecules 2010, 43, 2554–2563. [Google Scholar] [CrossRef]
- Wang, Q.; Mynar, J.L.; Yoshida, M.; Lee, E.; Lee, M.; Okuro, K.; Kinbara, K.; Aida, T. High-water-content mouldable hydrogels by mixing clay and a dendritic molecular binder. Nature 2010, 463, 339. [Google Scholar] [CrossRef] [PubMed]
- Haque, M.A.; Kurokawa, T.; Kamita, G.; Gong, J.P. Lamellar bilayers as reversible sacrificial bonds to toughen hydrogel: Hysteresis, self-recovery, fatigue resistance, and crack blunting. Macromolecules 2011, 44, 8916–8924. [Google Scholar] [CrossRef]
- Tuncaboylu, D.C.; Sari, M.; Oppermann, W.; Okay, O. Tough and self-healing hydrogels formed via hydrophobic interactions. Macromolecules 2011, 44, 4997–5005. [Google Scholar] [CrossRef]
- Haider, H.; Yang, C.H.; Zheng, W.J.; Yang, J.H.; Wang, M.X.; Yang, S.; Zrínyi, M.; Osada, Y.; Suo, Z.; Zhang, Q.; et al. Exceptionally tough and notch-insensitive magnetic hydrogels. Soft Matter 2015, 11, 8253–8261. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.X.; Yang, C.H.; Liu, Z.Q.; Zhou, J.; Xu, F.; Suo, Z.; Yang, J.H.; Chen, Y.M. Tough photoluminescent hydrogels doped with lanthanide. Macromol. Rapid Commun. 2015, 36, 465–471. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Illeperuma, W.R.; Suo, Z.; Vlassak, J.J. Hybrid hydrogels with extremely high stiffness and toughness. ACS Macro Lett. 2014, 3, 520–523. [Google Scholar] [CrossRef] [Green Version]
- Bai, R.; Yang, J.; Morelle, X.P.; Yang, C.; Suo, Z. Fatigue Fracture of Self-Recovery Hydrogels. ACS Macro Lett. 2018, 7, 312–317. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, X.; Wang, J.; Tang, J.; Hu, J.; Lu, T.; Suo, Z. Fatigue of double-network hydrogels. Eng. Fract. Mech. 2017, 187, 74–93. [Google Scholar] [CrossRef]
- Bai, R.; Yang, Q.; Tang, J.; Morelle, X.P.; Vlassak, J.; Suo, Z. Fatigue fracture of tough hydrogels. Extreme Mech. Lett. 2017, 15, 91–96. [Google Scholar] [CrossRef] [Green Version]
- Tang, J.; Li, J.; Vlassak, J.J.; Suo, Z. Fatigue fracture of hydrogels. Extreme Mech. Lett. 2017, 10, 24–31. [Google Scholar] [CrossRef]
- Illeperuma, W.R.; Rothemund, P.; Suo, Z.; Vlassak, J.J. Fire-resistant hydrogel-fabric laminates: A simple concept that may save lives. ACS Appl. Mater. Interfaces 2016, 8, 2071–2077. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Chen, B.; Xiang, F.; Zhou, J.; Wang, H.; Suo, Z. Transparent hydrogel with enhanced water retention capacity by introducing highly hydratable salt. Appl. Phys. Lett. 2014, 105, 151903. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Suo, Z.; Vlassak, J.J. Stiff, strong, and tough hydrogels with good chemical stability. J. Mater. Chem. B 2014, 2, 6708–6713. [Google Scholar] [CrossRef] [Green Version]
- Chen, B.; Bai, Y.; Xiang, F.; Sun, J.Y.; Mei Chen, Y.; Wang, H.; Zhou, J.; Suo, Z. Stretchable and transparent hydrogels as soft conductors for dielectric elastomer actuators. J. Polym. Sci. Part B Polym. Phys. 2014, 52, 1055–1060. [Google Scholar] [CrossRef] [Green Version]
- Bai, Y.; Jiang, Y.; Chen, B.; Chiang Foo, C.; Zhou, Y.; Xiang, F.; Zhou, J.; Wang, H.; Suo, Z. Cyclic performance of viscoelastic dielectric elastomers with solid hydrogel electrodes. Appl. Phys. Lett. 2014, 104, 062902. [Google Scholar] [CrossRef] [Green Version]
- Huebsch, N.; Kearney, C.J.; Zhao, X.; Kim, J.; Cezar, C.A.; Suo, Z.; Mooney, D.J. Ultrasound-triggered disruption and self-healing of reversibly cross-linked hydrogels for drug delivery and enhanced chemotherapy. Proc. Natl. Acad. Sci. USA 2014, 111, 9762–9767. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parada, G.A.; Yuk, H.; Liu, X.; Hsieh, A.J.; Zhao, X. Impermeable Robust Hydrogels via Hybrid Lamination. Adv. Healthc. Mater. 2017, 6. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.; Lin, S.; Zhao, X.; Anand, L. A large deformation viscoelastic model for double-network hydrogels. J. Mech. Phys. Solids 2017, 100, 103–130. [Google Scholar] [CrossRef]
- Guo, J.; Liu, X.; Jiang, N.; Yetisen, A.K.; Yuk, H.; Yang, C.; Khademhosseini, A.; Zhao, X.; Yun, S.H. Highly stretchable, strain sensing hydrogel optical fibers. Adv. Mater. 2016, 28, 10244–10249. [Google Scholar] [CrossRef] [PubMed]
- Yuk, H.; Zhang, T.; Parada, G.A.; Liu, X.; Zhao, X. Skin-inspired hydrogel–elastomer hybrids with robust interfaces and functional microstructures. Nat. Commun. 2016, 7, 12028. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gonzalez, M.A.; Simon, J.R.; Ghoorchian, A.; Scholl, Z.; Lin, S.; Rubinstein, M.; Marszalek, P.; Chilkoti, A.; López, G.P.; Zhao, X. Strong, tough, stretchable, and self-adhesive hydrogels from intrinsically unstructured proteins. Adv. Mater. 2017, 29. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Yuk, H.; Lin, S.; Parada, G.A.; Zhao, X. Tough and tunable adhesion of hydrogels: Experiments and models. Acta Mech. Sin. 2017, 33, 543–554. [Google Scholar] [CrossRef]
- Lin, S.; Yuk, H.; Zhang, T.; Parada, G.A.; Koo, H.; Yu, C.; Zhao, X. Stretchable hydrogel electronics and devices. Adv. Mater. 2016, 28, 4497–4505. [Google Scholar] [CrossRef] [PubMed]
- Yuk, H.; Zhang, T.; Lin, S.; Parada, G.A.; Zhao, X. Tough bonding of hydrogels to diverse non-porous surfaces. Nat. Mater. 2016, 15, 190. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.; Sycks, D.; Chan, H.F.; Lin, S.; Lopez, G.P.; Guilak, F.; Leong, K.W.; Zhao, X. 3D printing of highly stretchable and tough hydrogels into complex, cellularized structures. Adv. Mater. 2015, 27, 4035–4040. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Zhou, Y.; Zhao, X. Designing extremely resilient and tough hydrogels via delayed dissipation. Extreme Mech. Lett. 2014, 1, 70–75. [Google Scholar] [CrossRef]
- Majidi, C. Soft robotics: A perspective—Current trends and prospects for the future. Soft Robot. 2014, 1, 5–11. [Google Scholar] [CrossRef]
- Autumn, K.; Majidi, C.; Groff, R.; Dittmore, A.; Fearing, R. Effective elastic modulus of isolated gecko setal arrays. J. Exp. Biol. 2006, 209, 3558–3568. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mirfakhrai, T.; Madden, J.D.; Baughman, R.H. Polymer artificial muscles. Mater. Today 2007, 10, 30–38. [Google Scholar] [CrossRef]
- White, T.J.; Broer, D.J. Programmable and adaptive mechanics with liquid crystal polymer networks and elastomers. Nat. Mater. 2015, 14, 1087. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Qin, H.; Mather, P. Review of progress in shape-memory polymers. J. Mater. Chem. 2007, 17, 1543–1558. [Google Scholar] [CrossRef]
- Keller, T.; Mao, Z.; Spengler, D. Young’s modulus, bending strength, and tissue physical properties of human compact bone. J. Orthop. Res. 1990, 8, 592–603. [Google Scholar] [CrossRef] [PubMed]
- Guz, N.; Dokukin, M.; Kalaparthi, V.; Sokolov, I. If cell mechanics can be described by elastic modulus: Study of different models and probes used in indentation experiments. Biophys. J. 2014, 107, 564–575. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X. Designing toughness and strength for soft materials. Proc. Natl. Acad. Sci. USA 2017, 114, 8138–8140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bertotti, G. General properties of power losses in soft ferromagnetic materials. IEEE Trans. Magn. 1988, 24, 621–630. [Google Scholar] [CrossRef]
- Beyer, M.K. The mechanical strength of a covalent bond calculated by density functional theory. J. Chem. Phys. 2000, 112, 7307–7312. [Google Scholar] [CrossRef]
- Gao, H.; Ji, B.; Jäger, I.L.; Arzt, E.; Fratzl, P. Materials become insensitive to flaws at nanoscale: Lessons from nature. Proc. Natl. Acad. Sci. USA 2003, 100, 5597–5600. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, C.; Wang, Z.; Suo, Z. Flaw sensitivity of highly stretchable materials. Extreme Mech. Lett. 2017, 10, 50–57. [Google Scholar] [CrossRef] [Green Version]
- Takamizawa, K.; Hayashi, K. Strain energy density function and uniform strain hypothesis for arterial mechanics. J. Biomech. 1987, 20, 7–17. [Google Scholar] [CrossRef]
- James, H.M.; Guth, E. Theory of the elastic properties of rubber. J. Chem. Phys. 1943, 11, 455–481. [Google Scholar] [CrossRef]
- Okay, O.; Durmaz, S. Charge density dependence of elastic modulus of strong polyelectrolyte hydrogels. Polymer 2002, 43, 1215–1221. [Google Scholar] [CrossRef]
- Huglin, M.B.; Rehab, M.M.M. Mechanical and thermodynamic properties of butyl acrylate-N-vinylpyrrolidone hydrogels. Polymer 1987, 28, 2200–2206. [Google Scholar] [CrossRef]
- Dubrovskii, S. Compressional and shear behaviour of weakly ionic polyacrylamide gels. Polym. Gels Netw. 1996, 4, 467–480. [Google Scholar] [CrossRef]
- Horkay, F.; Han, M.H.; Han, I.S.; Bang, I.S.; Magda, J.J. Separation of the effects of pH and polymer concentration on the swelling pressure and elastic modulus of a pH-responsive hydrogel. Polymer 2006, 47, 7335–7338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Henderson, K.J.; Zhou, T.C.; Otim, K.J.; Shull, K.R. Ionically cross-linked triblock copolymer hydrogels with high strength. Macromolecules 2010, 43, 6193–6201. [Google Scholar] [CrossRef]
- Hu, X.; Vatankhah-Varnoosfaderani, M.; Zhou, J.; Li, Q.; Sheiko, S.S. Weak hydrogen bonding enables hard, strong, tough, and elastic hydrogels. Adv. Mater. 2015, 27, 6899–6905. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wang, X.; Yang, F.; Shen, H.; Wu, D. A Universal Soaking Strategy to Convert Composite Hydrogels into Extremely Tough and Rapidly Recoverable Double-Network Hydrogels. Adv. Mater. 2016, 28, 7178–7184. [Google Scholar] [CrossRef] [PubMed]
- Phadke, A.; Zhang, C.; Arman, B.; Hsu, C.C.; Mashelkar, R.A.; Lele, A.K.; Tauber, M.J.; Arya, G.; Varghese, S. Rapid self-healing hydrogels. Proc. Natl. Acad. Sci. USA 2012, 109, 4383–4388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haque, M.A.; Kurokawa, T.; Gong, J.P. Anisotropic hydrogel based on bilayers: Color, strength, toughness, and fatigue resistance. Soft Matter 2012, 8, 8008–8016. [Google Scholar] [CrossRef]
- Jeon, I.; Cui, J.; Illeperuma, W.R.; Aizenberg, J.; Vlassak, J.J. Extremely Stretchable and Fast Self-Healing Hydrogels. Adv. Mater. 2016, 28, 4678–4683. [Google Scholar] [CrossRef] [PubMed]
- Tuncaboylu, D.C.; Argun, A.; Algi, M.P.; Okay, O. Autonomic self-healing in covalently crosslinked hydrogels containing hydrophobic domains. Polymer 2013, 54, 6381–6388. [Google Scholar] [CrossRef]
- Li, T.; Wang, J.; Zhang, L.; Yang, J.; Yang, M.; Zhu, D.; Zhou, X.; Handschuh-Wang, S.; Liu, Y.; Zhou, X. “Freezing”, morphing, and folding of stretchy tough hydrogels. J. Mater. Chem. B 2017, 5, 5726–5732. [Google Scholar] [CrossRef]
- Malda, J.; Visser, J.; Melchels, F.P.; Jüngst, T.; Hennink, W.E.; Dhert, W.J.; Groll, J.; Hutmacher, D.W. 25th anniversary article: Engineering hydrogels for biofabrication. Adv. Mater. 2013, 25, 5011–5028. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Yang, F.; Zhao, H.; Gao, Q.; Xia, B.; Fu, J. Research on the printability of hydrogels in 3D bioprinting. Sci. Rep. 2016, 6, 29977. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hutmacher, D.W. Scaffolds in tissue engineering bone and cartilage. In The Biomaterials: Silver Jubilee Compendium; Elsevier Science: Amsterdam, The Netherlands, 2006; pp. 175–189. [Google Scholar]
- De Mori, A.; Peña Fernández, M.; Blunn, G.; Tozzi, G.; Roldo, M. 3D printing and electrospinning of composite hydrogels for cartilage and bone tissue engineering. Polymers 2018, 10, 285. [Google Scholar] [CrossRef]
- Yang, F.; Tadepalli, V.; Wiley, B.J. 3D printing of a double network hydrogel with a compression strength and elastic modulus greater than those of cartilage. ACS Biomater. Sci. Eng. 2017, 3, 863–869. [Google Scholar] [CrossRef]
- Chimene, D.; Lennox, K.K.; Kaunas, R.R.; Gaharwar, A.K. Advanced bioinks for 3D printing: A materials science perspective. Ann. Biomed. Eng. 2016, 44, 2090–2102. [Google Scholar] [CrossRef] [PubMed]
- Lorson, T.; Jaksch, S.; Lübtow, M.M.; Jüngst, T.; Groll, J.; Lühmann, T.; Luxenhofer, R. A Thermogelling Supramolecular Hydrogel with Sponge-Like Morphology as a Cytocompatible Bioink. Biomacromolecules 2017, 18, 2161–2171. [Google Scholar] [CrossRef] [PubMed]
- Bakarich, S.E.; Gorkin, R., III; Gately, R.; Naficy, S.; Spinks, G.M. 3D printing of tough hydrogel composites with spatially varying materials properties. Addit. Manuf. 2017, 14, 24–30. [Google Scholar] [CrossRef]
- Fischer, B.; Schulz, A.; Gepp, M.M.; Neubauer, J.; Gentile, L.; Zimmermann, H. 3D printing of hydrogels in a temperature controlled environment with high spatial resolution. Curr. Dir. Biomed. Eng. 2016, 2, 109–112. [Google Scholar] [CrossRef]
- Tan, Z.; Parisi, C.; Di Silvio, L.; Dini, D.; Forte, A.E. Cryogenic 3D Printing of Super Soft Hydrogels. Sci. Rep. 2017, 7, 16293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Müller, M.; Becher, J.; Schnabelrauch, M.; Zenobi-Wong, M. Printing thermoresponsive reverse molds for the creation of patterned two-component hydrogels for 3D cell culture. J. Vis. Exp. 2013. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.J.; An, N.; Yang, J.H.; Zhou, J.; Chen, Y.M. Tough al-alginate/poly(N-isopropylacrylamide) hydrogel with tunable lcst for soft robotics. ACS Appl. Mater. Interfaces 2015, 7, 1758–1764. [Google Scholar] [CrossRef] [PubMed]
- Stroganov, V.; Al-Hussein, M.; Sommer, J.U.; Janke, A.; Zakharchenko, S.; Ionov, L. Reversible thermosensitive biodegradable polymeric actuators based on confined crystallization. Nano Lett. 2015, 15, 1786–1790. [Google Scholar] [CrossRef] [PubMed]
- Kuckling, D.; Harmon, M.E.; Frank, C.W. Photo-cross-linkable PNIPAAm copolymers. 1. Synthesis and characterization of constrained temperature-responsive hydrogel layers. Macromolecules 2002, 35, 6377–6383. [Google Scholar] [CrossRef]
- Albrecht, D.R.; Tsang, V.L.; Sah, R.L.; Bhatia, S.N. Photo-and electropatterning of hydrogel-encapsulated living cell arrays. Lab Chip 2005, 5, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Lo, C.W.; Zhu, D.; Jiang, H. An infrared-light responsive graphene-oxide incorporated poly(N-isopropylacrylamide) hydrogel nanocomposite. Soft Matter 2011, 7, 5604–5609. [Google Scholar] [CrossRef]
- Lv, C.; Sun, X.C.; Xia, H.; Yu, Y.H.; Wang, G.; Cao, X.W.; Li, S.X.; Wang, Y.S.; Chen, Q.D.; Yu, Y.D.; et al. Humidity-responsive actuation of programmable hydrogel microstructures based on 3D printing. Sens. Actuators B Chem. 2018, 259, 736–744. [Google Scholar] [CrossRef]
- Han, D.; Lu, Z.; Chester, S.A.; Lee, H. Micro 3D Printing of a Temperature-Responsive Hydrogel Using Projection Micro-Stereolithography. Sci. Rep. 2018, 8, 1963. [Google Scholar] [CrossRef] [PubMed]
- Cvetkovic, C.; Raman, R.; Chan, V.; Williams, B.J.; Tolish, M.; Bajaj, P.; Sakar, M.S.; Asada, H.H.; Saif, M.T.A.; Bashir, R. Three-dimensionally printed biological machines powered by skeletal muscle. Proc. Natl. Acad. Sci. USA 2014, 111, 10125–10130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ni, Y.; Ji, R.; Long, K.; Bu, T.; Chen, K.; Zhuang, S. A review of 3D-printed sensors. Appl. Spectrosc. Rev. 2017, 52, 623–652. [Google Scholar] [CrossRef]
- Odent, J.; Wallin, T.J.; Pan, W.; Kruemplestaedter, K.; Shepherd, R.F.; Giannelis, E.P. Highly Elastic, Transparent, and Conductive 3D-Printed Ionic Composite Hydrogels. Adv. Funct. Mater. 2017, 27. [Google Scholar] [CrossRef]
- Appel, E.A.; Tibbitt, M.W.; Webber, M.J.; Mattix, B.A.; Veiseh, O.; Langer, R. Self-assembled hydrogels utilizing polymer–nanoparticle interactions. Nat. Commun. 2015, 6, 6295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, B.; Kang, S.Y.; Akthakul, A.; Ramadurai, N.; Pilkenton, M.; Patel, A.; Nashat, A.; Anderson, D.G.; Sakamoto, F.H.; Gilchrest, B.A.; et al. An elastic second skin. Nat. Mater. 2016, 15, 911. [Google Scholar] [CrossRef] [PubMed]
- Lei, Z.; Wang, Q.; Wu, P. A multifunctional skin-like sensor based on a 3D printed thermo-responsive hydrogel. Mater. Horiz. 2017, 4, 694–700. [Google Scholar] [CrossRef]
- Algi, M.P.; Okay, O. Highly stretchable self-healing poly(N,N-dimethylacrylamide) hydrogels. Eur. Polym. J. 2014, 59, 113–121. [Google Scholar] [CrossRef]
- Li, L.; Yan, B.; Yang, J.; Chen, L.; Zeng, H. Novel Mussel-Inspired Injectable Self-Healing Hydrogel with Anti-Biofouling Property. Adv. Mater. 2015, 27, 1294–1299. [Google Scholar] [CrossRef] [PubMed]
- Cong, H.P.; Wang, P.; Yu, S.H. Stretchable and self-healing graphene oxide–polymer composite hydrogels: A dual-network design. Chem. Mater. 2013, 25, 3357–3362. [Google Scholar] [CrossRef]
- Wu, D.Y.; Meure, S.; Solomon, D. Self-healing polymeric materials: A review of recent developments. Prog. Polym. Sci. 2008, 33, 479–522. [Google Scholar] [CrossRef] [Green Version]
- Gulyuz, U.; Okay, O. Self-healing poly(acrylic acid) hydrogels with shape memory behavior of high mechanical strength. Macromolecules 2014, 47, 6889–6899. [Google Scholar] [CrossRef]
- Li, G.; Wu, J.; Wang, B.; Yan, S.; Zhang, K.; Ding, J.; Yin, J. Self-healing supramolecular self-assembled hydrogels based on poly(l-glutamic acid). Biomacromolecules 2015, 16, 3508–3518. [Google Scholar] [CrossRef] [PubMed]
- Cerqueira, M.T.; da Silva, L.P.; Santos, T.C.; Pirraco, R.P.; Correlo, V.M.; Marques, A.P.; Reis, R.L. Human skin cell fractions fail to self-organize within a gellan gum/hyaluronic acid matrix but positively influence early wound healing. Tissue Eng. Part A 2014, 20, 1369–1378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, Z.; He, J.; Liang, T.; Oh, K.; Athas, J.; Tong, Z.; Wang, C.; Nie, Z. Autonomous self-healing of poly(acrylic acid) hydrogels induced by the migration of ferric ions. Polym. Chem. 2013, 4, 4601–4605. [Google Scholar] [CrossRef]















| Hydrogels | Silicone Elastomers |
|---|---|
| Excellent water-absorbing properties | Excellent elastic properties |
| Volume changes with external stimuli | Less responsive to external stimuli |
| Preferred as biomaterials, e.g., dressing wounds | Chemically inert and more toxic |
| Absorb body fluids; very soft and bendable | Good absorbers of gases |
| Excellent transport properties, e.g., controlling drugs and nutrient release | Generally hydrophobic; cannot retain fluids |
| Semi-solid/liquid state; better relaxation behavior | Mixed with hydrogels give better hydrophilic properties |
| Mostly water; hence better fire-retardant skin-based devices | Better oxygen permeability and transport |
| Gelatin is hemostatic; hence faster wound healing | Highly viscous and hydrophobic |
| Better mechanical strength in composite, better self-healing time, etc. | Less responsive |
| Resist protein adsorption; good wettability; can be used in soft contact lenses | Less likely to be used |
| Adaptable to small, delicate structures | Inability to adapt to small scale |
| Relatively complex preparation protocol | Ease of preparation |
| Actuator | Energy Density (J cm) | Elongation (%) | Pressure (MPa) | Response Time (ms) |
|---|---|---|---|---|
| Solenoids | 0.025 | 50 | 0.1 | 5 |
| Piezo-actuators | 0.05 | 0.2 | 110 | 0.5 |
| Magnetostrictive | 0.025 | 0.2 | 70 | 0.4 |
| Electrostrictive | 0.17 | 32 | 2 | 2 |
| Shape-memory alloy | 10 | 8 | 900 | 300 |
| Hydrogels | 0.35 | 90 | 4 | 300 |
| Electrochemical | 0.14 | 50 | 25 | 16 |
| Electrostatic | 0.0015 | 50 | 0.03 | 0.003 |
| Muscle | 0.59 | 70 | 1.18 | 0.03 |
| Stimulus | Advantages | Limitations | Reference |
|---|---|---|---|
| Thermal | Ease of operation | Slow response | [143,144] |
| Electrical | Faster response | Electrolyte layer formation | [145,146] |
| pH | Faster response, reversible volume change | Complex pH solution formation | [147,148] |
| Magnetic | Wireless, remote-controlled | Brittle structure, difficult to print | [147,149] |
| Light | Wireless, remote-controlled | Difficult to control and penetrate | [80] |
| Hydrogel | Transduction Strategy | Specification | Reference |
|---|---|---|---|
| Polyaniline | Electrochemical | RT: 3 s; LR: 0.01–8 mM | [179] |
| Polyaniline–PEG | Electrochemical | - | [180] |
| PEG | Optical | RT: 10 min; LR: 0–600 mg/dL | [166] |
| PVA–vinyl pyridine | Electrochemical | RT: 11 s | [181] |
| Chitosan | Electrochemical | RT: 7 s; LR: 5 M to 2.5 nM | [182] |
| Chitosan–graphene oxide | Electrochemical | LR: 0.02–6.78 mM | [183] |
| Polypyrrole | Electrochemical | LR: up to 15 mM | [184] |
| PEG (injectable) | Optical | RT: 11 min; LR: up to 370 mg dL | [185] |
| Polyvinylpyrrolidone | Optical | - | [186] |
| Alginate | Optical | LR: 2.6–350 mg/dL | [187] |
| HEMA | Electrochemical | LR: 10 M to 40 mM | [188] |
| Chemicals | Stimulus | Reference |
|---|---|---|
| Alginate/poly(N-isopropylacrylamide) (pNIPAM) Polycaprolactone Alginate/poly(acrylamideionic covalent entanglement) (Alg/PAAmICE) Pluronic F127 Gelatinmethacryloyl (GelMa) | Thermal | [143,147,316,317,318] |
| Poly(2-acrylamido-2-methylpropanesulfonic acid) (PAMPS) 4-Hydroxybutyl acrylate (4-HBA) Acrylamide (AAm)/sodium acrylate (NaAc) | Electrical | [52,145,146] |
| Poly(methacrylic acid) (PMAA) Collagen Keratin Polyacrylic acid (PAA) | pH | [147,148] |
| Magnetic nanoparticles such as FeO, FeO, FePt, and CoFeO in polymers such as poly(2-hydroxyethyl methacrylate) (pHEMA) | Magnetic | [110,147] |
| Gellan gum methacrylate (GGMA) Graphene oxide (GO)/pNIPAM | Light | [319,320,321] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Banerjee, H.; Suhail, M.; Ren, H. Hydrogel Actuators and Sensors for Biomedical Soft Robots: Brief Overview with Impending Challenges. Biomimetics 2018, 3, 15. https://doi.org/10.3390/biomimetics3030015
Banerjee H, Suhail M, Ren H. Hydrogel Actuators and Sensors for Biomedical Soft Robots: Brief Overview with Impending Challenges. Biomimetics. 2018; 3(3):15. https://doi.org/10.3390/biomimetics3030015
Chicago/Turabian StyleBanerjee, Hritwick, Mohamed Suhail, and Hongliang Ren. 2018. "Hydrogel Actuators and Sensors for Biomedical Soft Robots: Brief Overview with Impending Challenges" Biomimetics 3, no. 3: 15. https://doi.org/10.3390/biomimetics3030015